- *Corresponding Author:
- Aruna P. Jadhav
Department of Quality Assurance, Bharati Vidyapeeth’s College of Pharmacy, Navi Mumbai, Maharashtra 400614, India
E-mail: aruna.jadhav@bvcop.in
| Date of Received | 18 July 2022 |
| Date of Revision | 28 August 2024 |
| Date of Acceptance | 20 December 2024 |
| Indian J Pharm Sci 2024;86(6):2000-2003 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Neohesperidin is a bitter-tasting flavanone glycoside found in citrus fruits. Neohesperidin dihydrochalcone is a non-nutritive artificial sweeter derived from neohesperidin. In the present, a forced degradation study of neohesperidin by using high-peRformance thin-layer chromatography through various stability parameters like acid hydrolysis, base hydrolysis, oxidative stress degradation, hydrolytic induced degradation, photolytic degradation, and dry heat degradation was carried out. Significant degradation was found to occur by acid hydrolysis, base hydrolysis, and to a lesser extent, under hydrolytic induced degradation and oxidative induced degradation. The percentage recovery of neohesperidin was found to be lower in acid-induced degradation (0 %) and base-induced degradation (0 %) and more in water-induced degradation (97.6 %) and oxidative degradation (88.1 %). Forced degradation studies on neohesperidin provide information on its intrinsic stability and storage conditions.
Keywords
Neohesperidin, stress testing, stability, high-peRformance thin layer chromatography
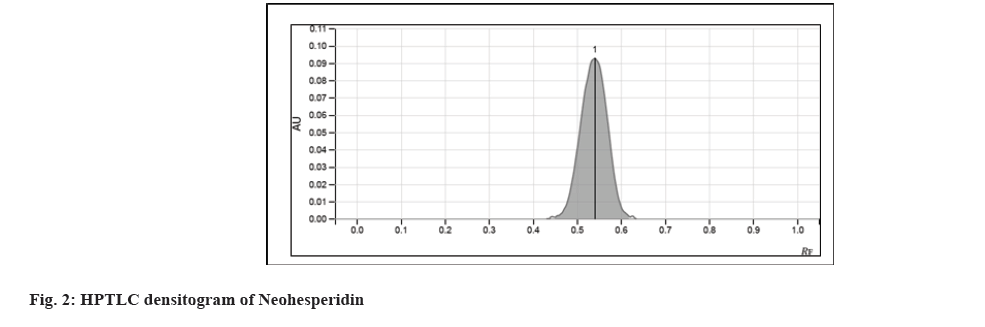
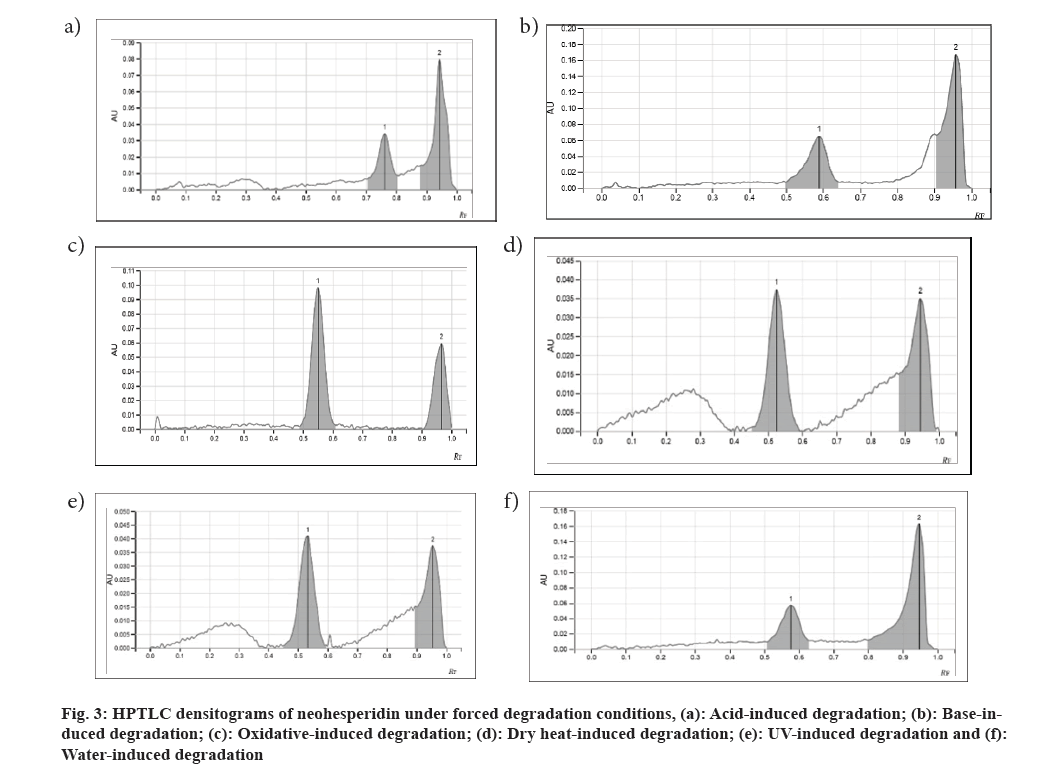
The chemical stability of pharmaceutical compounds is a major concern since it impacts the drug's safety and efficacy. According to Food and Drug Administration (FDA) and International Council for Harmonisation (ICH) recommendations, stability testing data is required to understand how the quality of a drug substance and drug product changes over time as a result of numerous environmental conditions. Forced degradation is a process in which drug products and drug substances are degraded under conditions that are more severe than accelerated settings, resulting in degradation products that may be analyzed to determine a molecule's stability[1]. The stability testing guideline Q1A (R2) of the ICH states the following goal of stress testing for new drug substances in section 2.1.2: Stress testing of the drug substance can help identify the likely degradation products, which may successively help establish the degradation pathways and therefore the intrinsic stability of the molecule and validate the stability-indicating power of the analytical procedures used. The nature of the strain testing will depend upon the individual drug substance and therefore the sort of drug product involved[2]. Neohesperidin (C28H34O15) (fig. 1), a citrus flavanone glycoside, is chemically known as (S)-4′-Methoxy-3′,5,7-trihydroxyflavanone-7-[2-O-(α-L-rhamnopyranosyl)-β-D-glucopyranoside]. This compound (C28H34O15) is present in various citrus species[3]. It is present in Huyou peel (Citrus Paradisi (C. Paradisi) cv. Changshanhuyou)[4], Citrus limon var. pompia[5], C. paradisi, Citrus sinensis[6], bergamot (Citrus bergamia Risso)[7], and Chinotto (Citrus myrtifolia Raf.)[8]. Neohesperidin has been investigated for various pharmacological effects including anti-inflammatory, neuroprotective activity, anti-proliferative[9], cardiovascular protection, and suppression of osteoclast differentiation[10]. In the present work, stress testing was carried out on neohesperidin and its evaluation was carried out by High-peRformance thin-layer chromatography (HPTLC) [2,11]. The reference standard for neohesperidin was procured from Yucca Enterprises, Mumbai. Analytical grade reagents and solvents, including ethyl acetate, methanol, and formic acid, were procured from SD Fine-Chem Ltd. (Mumbai, India). Chromatographic analysis was peRformed on Merck Thin Layer Chromatography (TLC) plates pre-coated with silica gel 60F254. A 15 μl sample was applied in triplicate as 8 mm wide bands using a CAMAG® automatic TLC sample applicator (ATS-4), fitted with a 100 μl applicator syringe (Hamilton, Bonaduz, Switzerland). The distance between adjacent bands was 10 mm. The CAMAG® twin-trough glass chamber (20×10 cm) saturated with mobile phase was used for the development of the plates. The previously developed mobile phase by authors consisting of ethyl acetate:methanol:formic acid:water (7.1:1.4:1:0.5 v/v/v/v) was used for chromatogram development in the linear ascending mode to the migration distance of 70 mm. Before the study, the chamber was saturated with the mobile phase for 20 min at room temperature. The slit dimension was kept at 6 mm×0.45 mm and a scanning speed of 20 mm/s. Analysis was carried out using CAMAG® TLC scanner 4 for scanning at 254 mm, which was found to be the wavelength of Neohesperidin. CAMAG® vision Computer Aided Textile Supervision (CATS) software was used for application and scanning. A standard stock solution of neohesperidin was prepared by dissolving 10 mg of the marker in 10 ml of methanol in order to compare the Rf of degradants with the Rf of standard. The stock solution was used for the development of the standard curve. For acid-induced and base-induced degradation studies, 10 mg of neohesperidin was refluxed separately in 10 ml of 0.1 N Hydrochloric acid (HCl) and 0.1 N Sodium Hydroxide (NaOH) solution respectively for 2 h. The solutions were diluted 10 times with methanol and subjected to chromatographic analysis. For oxidative degradation studies, 10 ml of 6 % w/w Hydrogen Peroxide (H2O2) solution was added to 10 mg of neohesperidin. Then the solution was kept aside for 4 h. The solution was diluted 10 times with methanol and subjected to chromatographic analysis. For dry heat-induced degradation studies 10 mg of neohesperidin was placed in an oven for about 8 h at 105°. After 8 h it was dissolved in 100 ml of methanol and this solution was subjected to chromatographic analysis. For water-induced degradation studies, 10 mg of neohesperidin was refluxed in 10 ml of water for 8 h. The solution was then diluted 10 times with methanol and subjected to chromatographic analysis. For the photochemical stability study, 10 mg neohesperidin was placed inside the Ultraviolet (UV) cabinet at 254 nm for 8 h. After exposing it for 8 h, neohesperidin was transferred to a 100 ml volumetric flask and the volume was made up of methanol. This solution was then used for the chromatographic analysis. An accurate, precise, robust HPTLC method has been developed. A good symmetric peak for neohesperidin was observed with Rf value 0.54±0.02 (fig. 2). The linear relationship was found to be in the concentration range 1000-3000 ng/spot (y=1.88x+732) with r2=0.9922. Neohesperidin was found to be highly susceptible to acid and base degradation with no recovery of neohesperidin at given conditions. Degradant peaks were observed at Rf 0.76 and 0.95 in acid-induced and Rf 0.58 and 0.95 for base-induced conditions (fig. 3a and fig. 3b). Neohesperidin was less susceptible to oxidative degradation (88.1 % recovery of neohesperidin), and a degradant peak was observed at Rf 0.96 (fig. 3c). Neohesperidin showed moderate degradation when exposed to dry heat (48.8 % recovery of neohesperidin) and UV radiation (59.9 % recovery of neohesperidin) with degradant peaks at Rf 0.94 and Rf 0.95 respectively (fig. 3d and fig. 3e). Neohesperidin was less susceptible to hydrolytic degradation (97.6 % recovery of neohesperidin) with a degradant peak at Rf 0.97 (fig. 3f). Similar degradant peak was observed at Rf 0.94-0.97 in all conditions. Therefore identification of this degradant is essential. In conclusion, forced degradation studies were carried out on neohesperidin. These findings offer valuable insights into the storage and inherent stability conditions of neohesperidin, which will be helpful during its formulation or derivatization. These degradation studies are useful in accelerated stability studies of prepared formulations and annual stability commitments of marketed formulations of neohespiridin.
Acknowledgement:
The authors were thankful to Anchrom Enterprises, Mumbai, Maharashtra, India for providing the facilities for performing the HPTLC method.
Conflict of interests:
The authors declared no conflict of interests.
References
- Blessy MR, Patel RD, Prajapati PN, Agrawal YK. Development of forced degradation and stability indicating studies of drugs-A review. J Pharm Anal 2014;4(3):159-65.
[Crossref] [Google Scholar] [PubMed]
- Guideline IH. Stability testing of new drug substances and products. Q1A (R2), current step. 2003;4:1-24.
- Lu Y, Zhang C, Bucheli P, Wei D. Citrus flavonoids in fruit and traditional Chinese medicinal food ingredients in China. Plant Foods Hum Nutr 2006;61:55-63.
[Crossref] [Google Scholar] [PubMed]
- Manconi M, Manca ML, Marongiu F, Caddeo C, Castangia I, Petretto GL, et al. Chemical characterization of Citrus limon var. pompia and incorporation in phospholipid vesicles for skin delivery. Int J Pharm 2016;506(1-2):449-57.
[Crossref] [Google Scholar] [PubMed]
- Widmer W, Collaborators. Determination of naringin and neohesperidin in orange juice by liquid chromatography with UV detection to detect the presence of grapefruit juice: Collaborative study. J AOAC Int 2000;83(5):1155-66.
[Crossref] [Google Scholar] [PubMed]
- Russo M, Arigo A, Calabro ML, Farnetti S, Mondello L, Dugo P. Bergamot (Citrus bergamia Risso) as a source of nutraceuticals: Limonoids and flavonoids. J Funct Foods 2016;20:10-9.
- Barreca D, Bellocco E, Caristi C, Leuzzi UG, Gattuso G. Flavonoid composition and antioxidant activity of juices from chinotto (Citrus × myrtifolia Raf.) fruits at different ripening stages. J Agric Food Chem 2010;58(5):3031-6.
[Crossref] [Google Scholar] [PubMed]
- Xia N, Wan W, Zhu S, Liu Q. Synthesis of hydrophobic propionyl neohesperidin ester using an immobilied enzyme and description of its anti-proliferative and pro-apoptotic effects on MCF-7 human breast cancer cells. Front Bioeng Biotechnol 2020;8:1025.
[Crossref] [Google Scholar] [PubMed]
- Gong Y, Dong R, Gao X, Li J, Jiang L, Zheng J, et al. Neohesperidin prevents colorectal tumorigenesis by altering the gut microbiota. Pharmacol Res 2019;148:104460.
[Crossref] [Google Scholar] [PubMed]
- Clapham D. Stability testing: Photostability testing of new drug substances and products ICH Q1B. ICH quality guidelines: An implementation guide. 2017:45-72.
- Tatkare PC, Jadhav AP. Development and validation of a novel high-performance thin-layer chromatography method for the quantitative estimation of neohesperidin from Citrus aurantium peel extract. JPC-J Planar Chromat 2022;35(6):579-84.