- *Corresponding Author:
- P. Jadhav
Department of Quality Assurance, Bharati Vidyapeeth’s College of Pharmacy, Navi Mumbai, Maharashtra 400614, India
E-mail: aruna.jadhav@bvcop.in
| Date of Received | 18 March 2022 |
| Date of Revision | 10 July 2024 |
| Date of Acceptance | 02 October 2024 |
| Indian J Pharm Sci 2024;86(5):1900-1903 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Stress degradation of mangiferin and gallic acid was induced to establish the stability indicating power of the validated high performance thin layer chromatography method. Mangiferin is a C-glycosyl xanthone derivative with many health-endorsing biological activities such as hypoglycaemic, antioxidant, and anti-inflammatory. Similarly, gallic acid a phenolic compound possesses antioxidant, antimicrobial, anti-inflammatory, anticancer, cardioprotective, gastroprotective, and neuroprotective effects. To elucidate the intrinsic stability, both molecules were subjected to a forced degradation process under conditions more severe than accelerated ones, generating degradation products for further study. Forced degradation studies were performed according to stability guidelines by International Council for Harmonisation of Technical Requirements of Pharmaceuticals for Human Use Q1(R2) and Q1B. Accordingly, mangiferin and gallic acid were exposed to oxidation, acid hydrolysis, alkaline hydrolysis, photolysis, as well as hydrolytic and thermal degradation experiments. Forced degradation results show that mangiferin was less susceptible to acidic and hydrolytic conditions and moderately susceptible to dry heat and photostability conditions. Mangiferin was highly susceptible to alkali and oxidative conditions. Gallic acid was less susceptible to photostability conditions and moderately susceptible to alkali, hydrolytic, and dry heat conditions. Gallic acid was highly susceptible to acid and oxidative conditions.
Keywords
Mangiferin, gallic acid, stress testing, high performance thin layer chromatography
Mangiferin (1,3,6,7-tetrahydroxyxanthen-9-one) is a C-glycosyl xanthone present in considerable levels in higher plants and different parts of the mango plant, such as peels, stalk, leaves, barks, kernels, and stone[1]. It has a role as a hypoglycaemic agent, an antioxidant, an anti-inflammatory agent, and a plant metabolite[2]. Gallic acid (3,4,5-trihydroxybenzoic acid) is a phenolic compound, a naturally occurring secondary metabolite found in various plants, vegetables, nuts, and fruits[3]. It has a role as an antioxidant, antimicrobial, anti-inflammatory, anticancer, cardioprotective, gastroprotective, and neuroprotective effects[4]. There is a constant need for the qualitative and quantitative analysis of various phytoconstituents in the pharmaceutical, cosmetic, and food industries, as well as in medicine, biology, organic chemistry, and biochemical analysis. Among these, mangiferin and gallic acid are two important molecules, which are commonly explored for a variety of applications. The study was aimed to identify degradation products, assess the stability of markers, and determine their susceptibility to different degradation pathways, thereby providing insights into its intrinsic stability and informing storage and formulation considerations. The objective of the work was investigating the stability and degradation behaviour of mangiferin and gallic acid. The forced degradation studies of the drug substance were carried out under various stress conditions such as the effect of light, elevated temperature, oxidation agents, and acid-base degradation according to stability guidelines by International Council for Harmonisation of Technical Requirements of Pharmaceuticals for Human Use (ICH) Q1A(R2) [5] and ICH Q1B[6]. The effect of various force degradation conditions was evaluated using High Performance Thin Layer Chromatography (HPTLC).
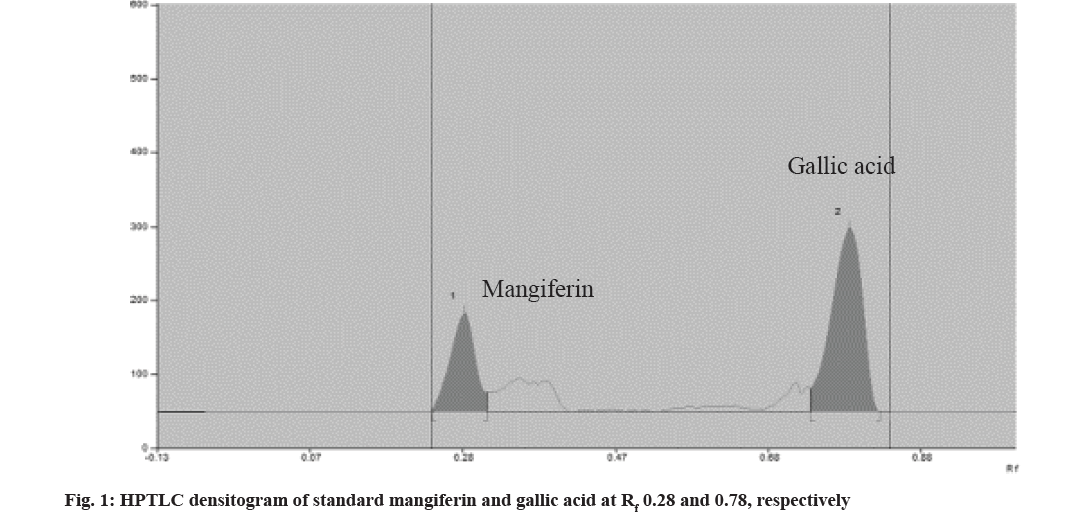
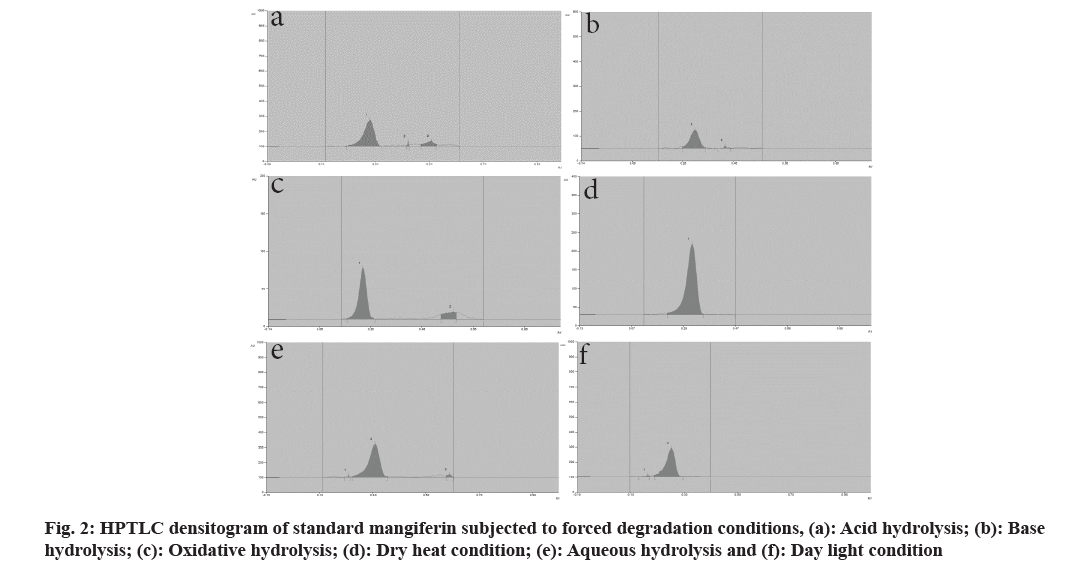
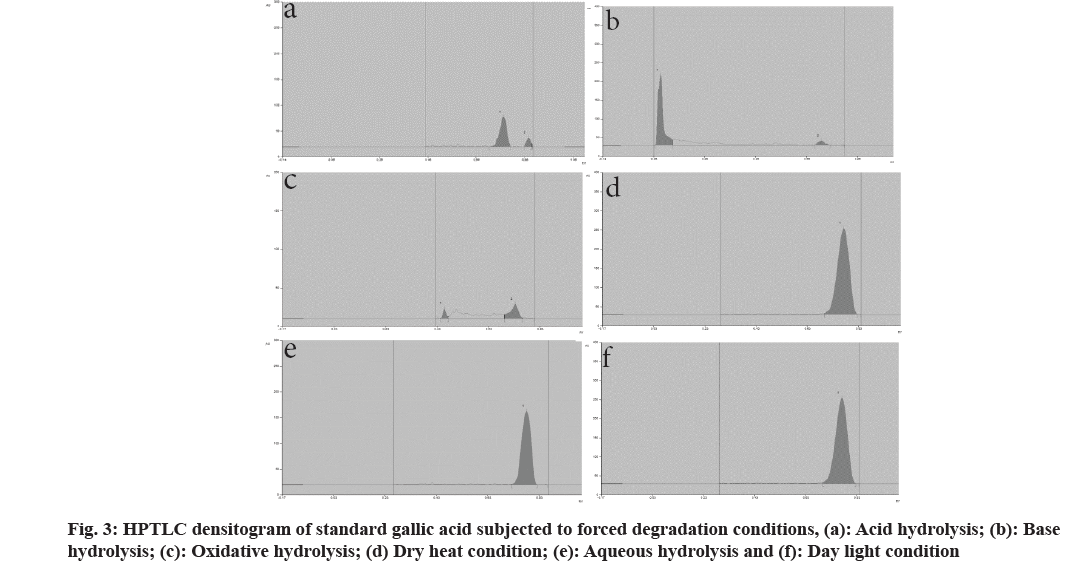
Analytical grade solvents and reagents, methanol, toluene, acetone, and formic acid were purchased from S D Fine Chem Ltd., Mumbai, India. Standards of mangiferin and gallic acid were procured from Yucca Enterprises, Mumbai. In a volumetric flask, stock solutions of mangiferin and gallic acid were prepared by dissolving 10 mg of each marker in 10 ml of methanol. CAMAG® Linomat 5 sample applicator with 100 μl Hamilton syringe was used and a Thin Layer Chromatography (TLC) plate precoated with silica gel 60F254 was used as a stationary phase. Standard solutions were applied as a 6 mm wide band to the 10×10 cm plate. Development of the plate was done ascending to a distance of 90 mm with the mobile phase at room temperature (24±2º) in the CAMAG® glass twins trough chamber. The chamber was saturated for 20 min previously and the plates were scanned with CAMAG® TLC scanner 3 using a mercury lamp. Mangiferin and gallic acid were subjected to various stressful conditions to establish their inherent stability[7,8]. For acid hydrolysis, 10 mg each of mangiferin and gallic acid were refluxed separately in 10 ml of 0.1 N Hydrochloric acid (HCl) solution for 3 h. The solutions were diluted 10 times with methanol and subjected to chromatographic analysis. Base hydrolysis was carried out in 0.1 N Sodium Hydroxide (NaOH) solutions for 2 h. Similarly, water induced degradation was carried out by refluxing in the distilled water for 8 h. For oxidative hydrolysis, 10 ml of 6 % w/w Hydrogen peroxide (H2O2) solution was added separately in 10 mg of mangiferin and gallic acid. Then the solutions were kept aside for 3 h. The solutions were diluted 10 times with methanol and subjected to chromatographic analysis. For both dry heat and daylight-induced degradation, 10 mg of each marker was placed separately in an oven at 105° and under daylight. After 8 h, the markers were dissolved separately in 100 ml of methanol, and the solutions were subjected to chromatographic analysis. The overlain spectrum of mangiferin and gallic acid indicated an isosbestic points at 299 nm, which was used as the scanning wavelength. Previously developed and validated HPTLC method by authors, with toluene:ethyl acetate:formic acid:methanol, 4:6:0.8:2 (v/v/v/v) as mobile phase has shown good resolution for mangiferin and gallic acid with the Rf values at 0.28±0.03 and 0.78±0.03, respectively (fig. 1). Linear relationship was found to be in concentration range 300-800 ng/spot (y=10.759x+234.5 with r2=0.9931) for mangiferin and 500-900 ng/spot (y=6.7733x+293.5 with r2=0.9901) for gallic acid[9]. Fig. 2a-fig. 2f and fig. 3a-fig. 3f represent densitograms of mangiferin and gallic acid under forced degradation conditions. Table 1 represents details of forced degradation studies of mangiferin and gallic acid. The concentration of recovered mangiferin and gallic acid was calculated under several forced degradation conditions based on area under the curve. Forced degradation results show that mangiferin was less susceptible to acidic, hydrolytic conditions and moderately susceptible to dry heat and photostability conditions.
| Exposure conditions | Time (h) | Mangiferin | Gallic acid | ||
|---|---|---|---|---|---|
| Recovery | Rf of degradation products | Recovery | Rf of degradation products | ||
| Acid (0.1 N HCl) reflux | 3 | 80.5 % | 0.43, 0.52 | 16.5 % | 0.88 |
| Base (0.1 N NaOH) reflux | 2 | 18.2 % | 0.42 | 34.2 % | 0.09 |
| H2O2 (6 % v/v) | 3 | 13.6 % | 0.58 | 17.5 % | 0.46 |
| Dry heat (105°) | 8 | 61.7 % | 0.19 | 39.2 % | No peak observed |
| Water, reflux | 8 | 93.7 % | 0.21, 0.59 | 49.3 % | No peak observed |
| Photostability-daylight | 8 | 70.4 % | 0.19 | 87.8 % | No peak observed |
Table 1: Forced Degradation Data of Mangiferin and Gallic Acid
Mangiferin was highly susceptible to alkaline and oxidative conditions. Similar degradant peaks were observed for mangiferin under various conditions. Therefore identification of these degradants is essential. Gallic acid was less susceptible to photostability conditions and moderately susceptible to alkali, hydrolytic and dry heat conditions. Gallic acid was highly susceptible to acid and oxidative conditions. The HPTLC technique developed for both drugs resolved the degradation products thus providing information on the intrinsic stability of mangiferin and gallic acid. Care should be taken when storing or exposing these molecules to the mentioned conditions, as both undergo rapid degradation. These degradation studies are useful in accelerated stability studies of prepared formulations and annual stability commitments of marketed formulations. A stability-indicating validated HPTLC method was successfully developed which can be applied for quantitative estimation of mangiferin and gallic acid and could be recommended for their marketed formulations analysis.
Acknowledgments:
The authors are thankful to the Anchrom Laboratory, Mumbai for giving training on HPTLC instruments.
Conflict of interest:
The authors declared no conflict of interests.
References
- Imran M, Arshad MS, Butt MS, Kwon JH, Arshad MU, Sultan MT. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis 2017;16:1-7.
[Crossref] [Google Scholar] [PubMed]
- Mangiferin. PubChem.
- Bai J, Zhang Y, Tang C, Hou Y, Ai X, Chen X, et al. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed Pharmacother 2021;133:110985.
[Crossref] [Google Scholar] [PubMed]
- Kahkeshani N, Farzaei F, Fotouhi M, Alavi SS, Bahramsoltani R, Naseri R, et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J Basic Med Sci 2019;22(3):225.
[Crossref] [Google Scholar] [PubMed]
- Guideline IH. Stability testing of new drug substances and products. Q1A (R2), current step. 2003;4:1-24.
- International Conference on Harmonisation-ICH harmonised tripartite guideline: Stability testing-photostability of new drug substances and products Q1B. 1996:1-8.
- Blessy MR, Patel RD, Prajapati PN, Agrawal YK. Development of forced degradation and stability indicating studies of drugs-a review. J Pharm Anal 2014;4(3):159-65.
[Crossref] [Google Scholar] [PubMed]
- Mokal RR, Jadhav AP. Forced degradation studies of mangiferin and berberine by high-performance thin-layer chromatography. J Planar Chromatogr 2021;34(6):543-8.
- Hage A, Mokal R, Jadhav A. Development and validation of high performance thin layer chromatography method for standardization of polyherbal formulation using mangiferin and gallic acid as markers. Indian J Pharm Sci 2022;84(4):1083-88.