- *Corresponding Author:
- V. Madhavan
Departments of Pharmacognosy and Pharmaceutical Chemistry, M. S. Ramaiah College of Pharmacy, Bangalore-560 054, India
E-mail: profvmadhavan@vsnl.net
| Date of Submission | 25 August 2007 |
| Date of Revision | 23 May 2008 |
| Date of Acceptance | 25 August 2007 |
| Indian J. Pharm. Sci., 2008, 70 (6): 798-800 |
Abstract
HPTLC fingerprinting profile of the alcohol and aqueous extracts of Drosera burmannii is described. Seven components have been detected in the alcohol extract. Further, plumbagin, an useful antifertility agent, was also detected by comparison with the reference standard. The aqueous extract revealed two spots with no spot corresponding to plumbagin.
Keywords
Drosera burmannii, HPTLC fi ngerprinting, marker component, plumbagin
Drosera burmannii Vahl (Droseraceae) is an insectivorous, glandular, hairy herb, with rose coloured flowers occurring throughout India up to 2666 m [1] and is reported to have rubefacient property [1,2]. It contains 1,4-naphthoquinones, plumbagin, ramantaceon and its glucoside rossoliside [3], flavonoids like quercetin and hyperoside [4]. Plumbagin is 5-hydroxy-2-methyl-1,4-naphthoquinone, a yellow colour pigment found in Plumbaginaceae and Droseraceae [5,6]. Plumbagin possesses antifertility [7], antimalarial [8], antiviral [9], antimicrobial [10], anticancer [11] and leishmanicidal [12] activity. Different species of Drosera L. like D. rotundifolia L. are used in whooping cough, fever, mental and stomach disorders, skin diseases in homeopathic system of medicine2. The objective of the present study was to develop HPTLC-aided fingerprint profile of D. burmannii, which may be used as markers for quality evaluation, and standardization of the drug.
D. burmannii was collected from forests of Savanadurga, Bangalore during February 2006 and was authenticated. A voucher herbarium specimen (Hema Basnett 005) along with a voucher drug sample is preserved at this College herbarium and crude drug museum. The material was washed, shade dried, powdered, passed through sieve no. 60 and stored in airtight containers in day light for three months at room temperature (±20o). Plumbagin reference standard was procured from HiMedia, Mumbai. The air dried powder was successively extracted with 95% ethanol in a Soxhlet apparatus and finally the marc was macerated with chloroform water (0.25%) for 24 h to obtain the aqueous extract. The extracts were further concentrated under vacuum using a rotary flash evaporator and dried in a desiccator.
Camag HPTLC system equipped with Linomat V sample applicator, Camag TLC Scanner 3 and WinCATS 4 software for interpretation of the data was used. An aluminium plate (20×10 cm) precoated with silica gel 60F254 (E. Merck) was used as adsorbent. The plates were developed using toluene:glacial acetic acid (55:1) and toluene:chloroform:glacial acetic acid (1:1:0.1) as mobile phase for alcohol and aqueous extracts respectively in a Camag twin trough chamber to a distance of 8 cm each.
Solution of plumbagin reference standard (1 mg/ml) was prepared in alcohol as stock solution. Solution of the alcohol extract was prepared by dissolving the extract in alcohol. The aqueous extract was dissolved in alcohol, filtered, the filtrate used as aqueous extract solution. The TLC plates were activated by heating at 1150 for about 30 min prior to use. The standard plumbagin solution and alcohol extract solution or aqueous extract solution were applied as 6 mm bands on two different precoated silica gel 60 F254 TLC plates, and the plates were developed in appropriate mobile phase. No prewashing of plates was carried out. Chamber saturation time was maintained at 1 h. The developed plates were allowed to dry and scanned at a wavelength of 425 nm, slit dimension 6.00×3.00 nm, scanning speed 20 nm/sec and the source of radiation was tungsten lamp. The Rf and peak area of the standard and the extracts were interpreted by using the software. The developed plates were photo documented using Camag Reprostar-3, equipped with a 12bit CCD camera, under 254, 366 nm and white light.
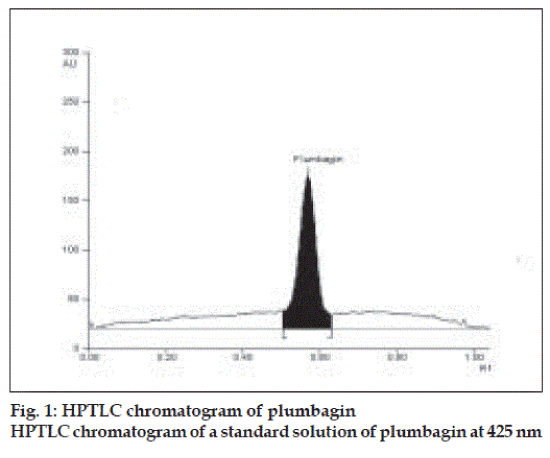
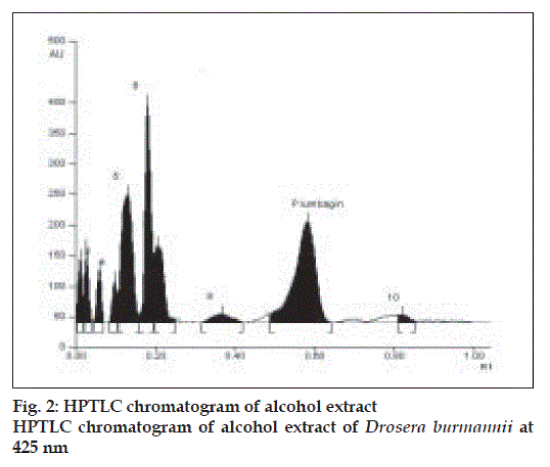
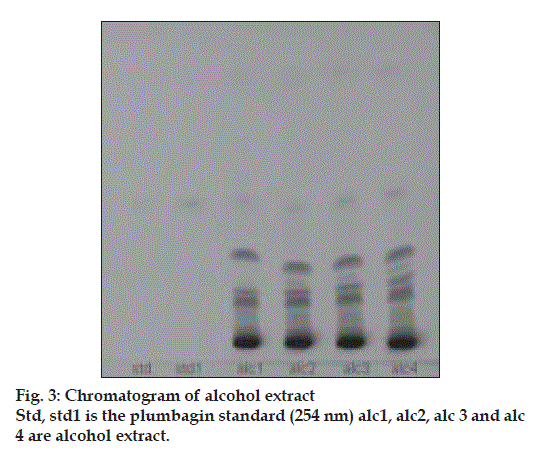
Plumbagin reference standard shows an Rf of 0.56 (fig. 1) and 0.66 in the mobile phase adopted for alcohol and aqueous extracts respectively. HPTLC analysis of alcohol and aqueous extracts of D. burmannii revealed different chromatographic profiles. In this study the alcohol extract revealed seven components at Rf 0.12, 0.18, 0.21, 0.24, 0.29, 0.56, 0.81 (figs. 2 and 3). Out of these, the most pronounced spot of maximum area was at Rf 0.56, corresponding to that of marker compound plumbagin. Three other spots at Rf 0.12, 0.18 and 0.21 were also prominent.
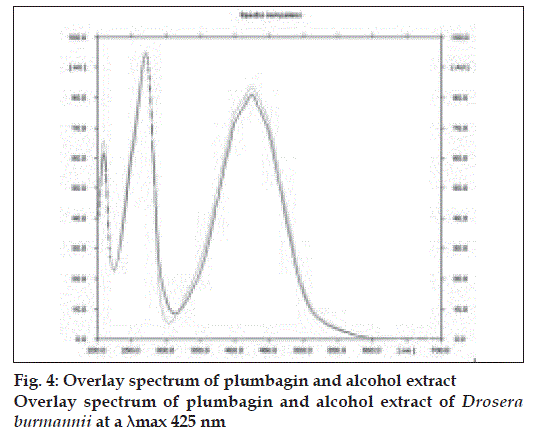
The method is specific for plumbagin in alcohol extract since it resolves the peak of plumbagin in the mobile phase proposed for the alcohol extracts to an Rf of 0.56 in the presence of other components. The specificity was confirmed by overlaying the spectra of plumbagin in reference standard (λmax 425 nm), with the absorption spectrum obtained from the corresponding band in the track of alcohol extract (fig. 4). The aqueous extract revealed spots at Rf 0.15 and 0.24 with no spot corresponding to that of plumbagin reference standard (Rf 0.66). This is due to the hydrophobic nature of plumbagin [13]. The study is the first report on HPTLC profile of D. burmannii, which reveals components useful for quality evaluation, and standardization of the drug. Further it confirms the presence of plumbagin as marker compound in D. burmannii.
Acknowledgements
The authors are thankful to the Gokula Education Foundation for providing facilities, encouragement, and financial support throughout this work.
References
- Santapau H, Henry AN. A Dictionary of the Flowering Plants in India. New Delhi: Publication and Information Directorate; 1976.
- Anonymous. The Wealth of India: A Dictionary of Indian Raw Materials and Industrial Products. Vol. 3, New Delhi: Council of Scientific and Industrial Research; 1989.
- Wagner H, Baldt S, Zgainski EM. Plant Drug Analysis. Tokyo:Springer- Verlag; 1984.
- Wang Q, Su J, Zeng. The isolation and identification of flavonoids from Droseraburmanni. Zhong Yao Cai 1998;21:401.
- Botanical Dermatology Database. Available from: http://bodd.cf.ac.uk/ BotDermFolder/BottDermP/DROS.html. [cited in 1999b], Droseraceae (Sundew family) (date of last modification, 1984).
- Available from: http://bodd.cf.ac.uk/BotDermFolder/Bot DermP/DROS. html, Botanical Dermatology Database [cited in 1999b], Droseraceae (Sundew family) (date of last modification, 1984).
- Premakumari P, Rathinam K, Santhakumari. Antifertility activity of plumbagin. Indian J Med Res 1977;65:829.
- Suraveratum N, Krungkrai, SR, Leangaramgul P, Prapunwattana P, Krugkai J. Purification and characterization of Plasmodium falciparum succinate dehydrogenase. MolBiochemParasitol 2000;105:215.
- Min BS, Kim YH, Toomiyama M, Nakamura N, Miyashiro H, Otake T, et al. Inhibitory effects of Korean plants on HIV-1 activities. Phytother Res 2001;15:481.
- Ahmed I, Mahmood Z, Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol 1998;62:83.
- MohanaKrishnaswamy, Purushothaman KK. Plumbagin: A study of its anticancer, antibacterial and antifungal properties. Indian J ExpBiol 1980;18:876.
- Iwu MM, Jackson JE, Schuster BG. Medicinal plants in the fight against leishmaniasis. Parasitol Today 1994;10:65.
- Nayana SK, Shalini AI, Mamta BS. A simple method for isolation of plumbagin from roots of Plumbagorosea. Pharm Biol 2005;43:551.