- *Corresponding Author:
- M. M. Mahmoud
Cancer Biology Unit, King Fahd Medical Research Center, King Abdulaziz University,King Fahd Medical Research Center, King Abdulaziz University, Jeddah 21589, Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah 22252, Saudi Arabia,Department of Molecular Genetics and Enzymology, Human Genetics and Genome Research Institute, National Research Centre, Cairo 12622, Egypt
E-mail: mamostafa@kau.edu.sa
| This article was originally published in a special issue,“New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “65-73” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Matrix metalloproteinase-10 is an extracellular matrix degradation enzyme that facilitates cell invasion in many cancers and is associated with poor clinical outcomes. In urinary bladder cancer, the clinical importance of matrix metalloproteinase-10 remains inadequately explored and poorly related to the patients clinicopathological factors and survival status. Hence, this study was conducted to explore matrix metalloproteinase-10 prognostic value in bladder cancer using a cohort of patients in Saudi Arabia. A total of 170 primary transitional cell carcinoma tissue sections of consent patients were prepared and arranged as tissue microarray, and the expression of matrix metalloproteinase-10 was analysed by immunohistochemistry. The potential clinical value of matrix metalloproteinase-10 was assessed by a correlation analysis of its expression with the patient’s clinicopathological features. The present data revealed that moderate (+2) and high (+3) matrix metalloproteinase-10 expression was detected in 47 % of our bladder cancer patient’s cohort. The expression was strongly associated with low-grade non-invasive tumours. The analysis also displayed a significant positive association of matrix metalloproteinase-10 expression with better overall survival and a low recurrence rate (p=0.023). The study showed an association of matrix metalloproteinase-10 expression with less invasive tumorigenic profiles and a better overall survival rate. This finding may suggest matrix metalloproteinase-10 as a biomarker for favourable treatment response. Further prospective investigations are needed to profoundly evaluate matrix metalloproteinase-10 functional role in bladder cancer.
Keywords
Bladder cancer, matrix metalloproteinase-10, immunohistochemistry, tissue microarray
Urinary bladder cancer is a common malignancy with an estimate of more than 570 000 new cases and 200 000 deaths worldwide[1]. In Saudi Arabia, Bladder Cancer (BC) is ranked as the eleventh most deadly cancer type with an estimate of more than 880 new cases and over 350 deaths only in 2020[1]. Despite laudable studies and efforts, BC continues to pose a great challenge for patients and their families as well as the healthcare system. About 50 %-70 % of BC in Saudi Arabia recur within 5 y with a higher potential of progression to the metastatic stage[2,3]. Based on the pathological analysis, BC can be classified into two genetically and clinically disparate categories: Non-Muscle-Invasive Bladder Cancer (NMIBC) and Muscle-Invasive Bladder Cancer (MIBC). NMIBC is a low-grade tumour, characterized by gain?of?function mutations which frequently affects Fibroblast Growth Factor Receptor 3 (FGFR3) and is suitable for localized therapy[4]. Yet, the low-grade non-invasive tumour often progresses rapidly to MIBC which is often characterized by loss-of-function mutations that inactivate tumour suppressors p53 and retinoblastoma[5].
So far, the management of BC largely has relied on patient’s clinicopathological parameters such as tumour grade and the Tumour-Node-Metastasis (TNM) stage, which are implemented as prognostic indicators of the disease outcomes[6]. However, these parameters are inadequate to predict the treatment outcomes and often exhibit discrepancies, especially within the same grade/stage due to the high heterogeneity of the tumour cells[7].
In the precision oncology era, considerable efforts are being made to identify novel diagnostic and prognostic biomarkers to diagnose BC at an earlier stage, enhance clinical management and improve the stratification of high-risk patients[8]. Despite some promising outcomes, most of the biomarkers are lacking adequate sensitivity or specificity which necessitates the identification of additional prognostic biomarkers that could be more accurately used in BC prognosis and treatment.
In this context, Matrix Metalloproteinases (MMPs) is a family of more than 25 zinc-dependent proteolytic enzymes involved in a variety of normal physiological processes including cell proliferation, differentiation and migration[9]. Recently, increasing evidence showed the implication of MMPs in several cancer pathogeneses such as Extracellular Matrix (ECM) degradation, loss of cellular adhesion, Epithelialto- Mesenchymal Transitions (EMT), angiogenesis, cell invasion and distant migration[10]. Hence, several MMPs have been suggested as potential diagnostic or prognostic biomarkers for cancer which could reinforce the currently available diagnostic testing. Particularly, Matrix Metalloproteinase-10 (MMP10) (also known as stromelysin-2) has been recently associated with the increased malignancy of epithelial origin cancers such as skin, oesophageal, gastric, bladder and nonsmall cell lung cancer[11]. MMP10 has been reported to degrade fibronectin collagen (type IV, V, IX and X), laminin, gelatine, elastin and proteoglycan core proteins[12]. The ability of MMP10 to degrade the ECM increases the tumour cells motility, which may allow the development of metastatic disease. Unlike many MMPs, which are localized mostly in stromal cells, MMP10 appears to be expressed by tumour cells[13].
Several studies have reported the overexpression of MMP10 transcripts in BC and the presence of MMP10 protein in urine samples of patients with more aggressive tumours[11,14-16]. A previous study also reported a moderate to high MMP10 protein expression in approximately 57 % and 35 % in patients with MIBC and NMIBC, respectively[11]. This may reveal a trend of association between malignancy and MMP10 expression. Hence, these findings incited us to investigate its prognostic value in our BC cohort. This study aimed to investigate the expression of MMP10 in BC using Tissue Microarray (TMA) analysis. A special focus was given to the correlation of MMP10 protein expression pattern with patient’s clinicopathological parameters, and to assessing the MMP10 as a potential prognosticator of BC.
Materials and Methods
Subjects:
170 Formalin-Fixed and Paraffin-Embedded (FFPE) tissue samples of primary Transitional Cell Carcinoma (TCC) of BC were retrieved from consent patient’s materials of the Pathology Department archives at King Abdulaziz University Hospital (KAUH), Jeddah, Saudi Arabia. Patients were diagnosed and treated mainly at the Departments of Pathology and Urology, KAUH and King Faisal Specialist Hospital and Research Centre (KFSHRC), between 1996 and 2012. Only specimens containing more than 80 % tumour cells were used for analysis. The histopathological features of the carcinoma specimens were classified according to the TNM classification system. All clinical and pathological data of the patients were collected from the patient’s medical records. The key clinicopathological data of the patients are shown in (Table 1).
| Feature | Number of cases (%) | Low expression (0, 1+) (%) | High expression (2+, 3+) (%) | p value (0,1 vs. 2,3) (Chi-square) |
|---|---|---|---|---|
| Age | ||||
| <60 | 77 (45.3 %) | 36 (46.8 %) | 41 (53.2 %) | 0.073 |
| >60 | 89 (52.4 %) | 54 (60.7 %) | 35 (39.3 %) | |
| Missing | 4 (2.3 %) | - | - | |
| Tumour type | ||||
| Transitional | 144 (84.7 %) | 76 (52.8 %) | 68 (47.2 %) | 0.176 |
| Non-transitional | 22 (12.9 %) | 15 (68.2 %) | 7 (31.8 %) | |
| Missing | 4 (2.4 %) | - | - | |
| Tumour stage | ||||
| Low stage | 96 (56.5 %) | 46 (47.9 %) | 50 (52.1 %) | 0.001 |
| High stage | 40 (23.5 %) | 32 (80.0 %) | 8 (20.0 %) | |
| Missing | 34 (20.0 %) | - | - | |
| Tumour grade | ||||
| Low grade | 67 (39.4 %) | 24 (35.8 %) | 43 (64.2 %) | 0.001 |
| High grade | 83 (48.8 %) | 57 (68.7 %) | 26 (31.3 %) | |
| Missing | 20 (11.8 %) | - | - | |
| LN status | ||||
| Positive | 22 (12.9 %) | 49 (53.8 %) | 42 (46.2 %) | 0.005 |
| Negative | 91 (53.5 %) | 19 (86.4 %) | 3 (13.6 %) | |
| Missing | 57 (33.6 %) | - | - | |
| Vascular invasion | ||||
| Positive | 21 (12.4 %) | 51 (54.3 %) | 43 (45.7 %) | 0.008 |
| Negative | 94 (55.3 %) | 18 (85.7 %) | 3 (14.3 %) | |
| Missing | 55 (32.3 %) | - | - | |
| Metastasis | ||||
| No | 88 (51.8 %) | 49 (55.7 %) | 39 (44.3 %) | 0.605 |
| Yes | 21 (12.4 %) | 13 (61.9 %) | 8 (38.1 %) | |
| Missing | 61 (35.8 %) | - | - | |
| Smoking | ||||
| No | 23 (13.5 %) | 16 (69.6 %) | 7 (30.4 %) | 0.018 |
| Yes | 39 (23.0 %) | 15 (38.5 %) | 24 (61.5 %) | |
| Missing | 108 (63.5 %) | - | - | |
| DSS | ||||
| Living | 115 (67.6 %) | 34 (65.4 %) | 18 (34.6 %) | 0.027 |
| Dead | 52 (30.6 %) | 54 (47.0 %) | 61 (53.0 %) | |
| Missing | 3 (1.8 %) | - | - | |
| DFS | ||||
| No recurrence | 121 (71.2 %) | 63 (52.1 %) | 58 (47.9 %) | 0.55 |
| Recurrence | 40 (23.5 %) | 23 (57.5 %) | 17 (42.5 %) | |
| Missing | 9 (5.3 %) | - | - | |
Table 1: Correlation between MMP10 Expression and Clinicopathological Features of BC Patients
TMA construction:
TMA protocol previously described by Al-Maghrabi and his colleagues[17] was followed to construct and validate TMA slides of approximately 170 BC blocks to evaluate and analyze the expression pattern of MMP10.
Immunohistochemistry (IHC):
MMP10 rabbit monoclonal antibody (NCL-MMP10, Leica) was used to detect MMP10 protein expression and the colour was developed using Ventana iView 3,3′-Diaminobenzidine (DAB) detection kit on a Ventana BenchMark XT automated immunostaining system. Briefly, the protocol included the following steps using Ventana reagents: Deparaffinization with EZ Prep at 75°, heating, pre-treatment in Cell Conditioning (CC1) buffer for 8 min, treatment with mild CC1 for 30 min for antigen retrieval at 100°, then incubation with the anti-MMP10 primary antibody for 16 min at 37°. Slides were counterstained with haematoxylin II for 4 min and treated with bluing reagent for 4 min. Following the staining step, slides were removed from the automated slide stainer then those with a residual buffer were rinsed with a mild detergent followed by rinsing in water until complete removal of the soap. The slides were dehydrated through ascending grades of alcohol buffers (70 %, 95 % and 100 %) for 3 min in each concentration. Sections were mounted with Tissue-Tek mounting medium and covered with a glass coverslip.
Scoring and evaluation of MMP10 expression:
The staining intensity of the tissue array sections was scored manually to check the expression levels of MMP10 using a Nikon brightfield upright microscope at 40× magnification. Scoring of MMP10 protein expression was carried out by two certified pathologists in a blind fashion to the clinicopathological parameters of the patients. The intensity of the staining was categorized into two groups: Low (no/weak) expression and High (moderate/strong) expression. The intensity of staining and the fraction of positively stained cells were used to calculate the staining index score by applying the following formula:
I=0xf0+1xf1+2xf2+3xf3
Where (I) is the staining index and (f0 to f3) are the fractions of the cells showing a level of staining intensity (from 0 to +3)[18].
Statistical analysis:
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) (IBM New York, United States of America) software packages (Predictive Analytics Software (PASW) Statistics for Windows, version 19). Frequency tables were analysed using the Chi-square test, with Likelihood Ratio (LR) or Fisher’s exact test to assess the significance of the correlation between the categorical variables. Odds Ratios (OR) and their 95 % Confidence Intervals (95 % CI) were calculated, where it is appropriate using the exact method. Univariate survival analysis for the outcome measure (Disease-Specific Survival (DSS) and Disease-Free Survival (DFS)) was based on the Kaplan-Meier method, with log-rank (Mantel-Cox) comparison test. In all tests, the values of p<0.05 were considered statistically significant.
Results and Discussion
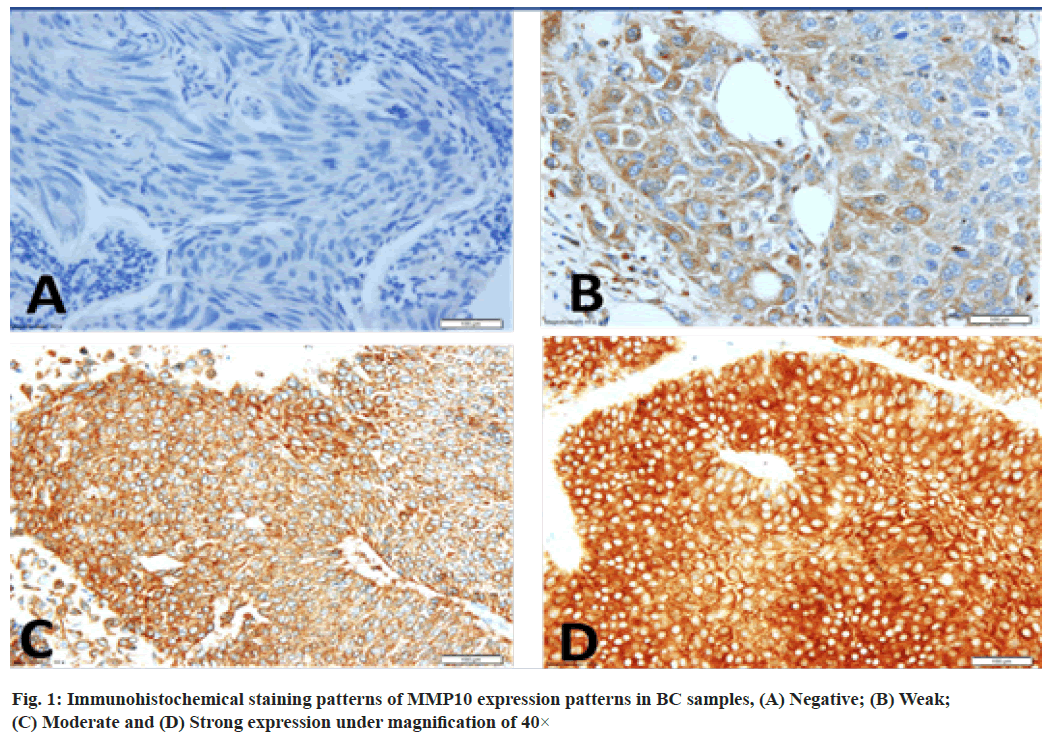
Expression pattern of MMP10 protein profiling in urinary BC was shown below. Our cohort consisted of 170 cases with 71 % of low stage BC tumours, 45 % low grade, 20 % presented with lymphovascular invasion. Smokers represented 63 % of the cohort. High expression of MMP10 protein was observed in 47 % of the cases, as evaluated by the TMA analysis, the majority of them were low-grade non-invasive tumours. The frequencies of the MMP10 expression pattern in 170 of BC samples were: No expression (0 %, 15 %), weak expression (+1, 39 %), moderate expression (+2, 35 %) and strong expression (+3, 11 %) (fig. 1).
Concordance between MMP10 expression and the clinicopathological features of BC patients was shown below. Our data revealed a significant association between low expression of MMP10 with high BC stage (Table 1). Around 80 % of high stage tumours (III, IV) showed low cytoplasmic MMP10 expression compared to those at lower stages (I, II) (p<0.0005). Similarly, 86 % of lymph node-positive BC showed a statistically significant low MMP10 protein expression compared to non-invasive tumours (p<0.005) (Table 1). Interestingly, low MMP10 expression was also observed in 78 % of transitional cases and 49 % of metastatic tumours. Interestingly, the data displayed a significant increase in MMP10 expression in BC among smokers (p<0.018), however, the age and gender of BC patients did not show any significant relationship with MMP10 expression pattern.
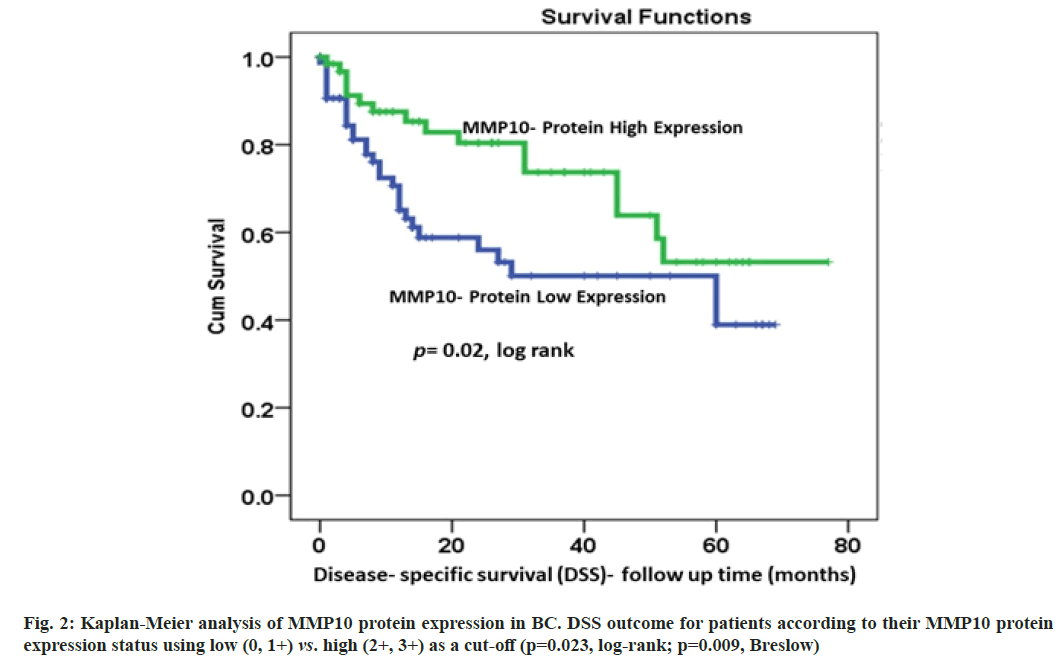
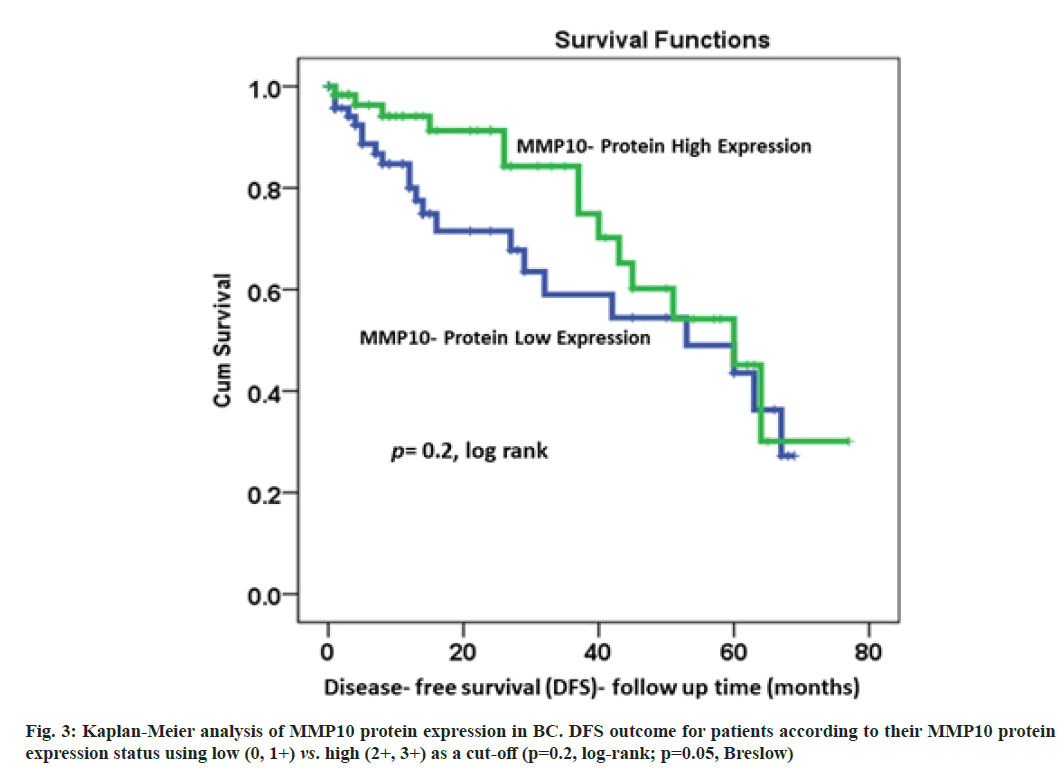
Correlations of MMP10 protein expression pattern with the survival outcomes was described here. The data of DSS were available for 170 patients. The results of Kaplan-Meier survival analysis showed a significant (p=0.023, log-rank) correlation between MMP10 expression and DSS (fig. 2). Patients with highly expressed MMP10 survived longer compared to their counterparts with low MMP10 protein expression. At 36 mo follow-up time, about 75 % of patients with elevated MMP10 protein expression were alive compared to only 50 % of their counterparts with no or under-expressed MMP10 (p=0.023, log-rank; p=0.009, Breslow; fig. 2). For DFS, a similar trend with lower recurrence rates for patients with higher MMP10 expression was observed, although it was not statistically significant (p=0.2, logrank; p=0.05, Breslow; fig. 3).
BC is the tenth most common malignancy with a steadily rising incidence and mortality rate worldwide[1]. Clinical management of BC largely depends on assessing several clinicopathological factors including tumour stage, grade and lymphovascular invasion. However, these factors are extremely variable and usually are affected by the high recurrence rate and diverse nature of BC which necessitate developing suitable diagnostic biomarkers to improve disease management. Recent advances in BC research have unveiled several biomarkers that are currently applied in cancer screening, diagnosis, stratification and surveillance of drug responses. Many of them are urinary biomarkers that assisted in decreasing the dependence on cystoscopy for diagnosis yet, most of them showed inconvenient rates of false positive and low sensitivity with low-grade and low stage BC[19]. Thus, more reliable biomarkers are needed for the earlier detection of BC. Several studies have reported the overexpression of MMP10 in many malignancies including BC, however, little is known about the pathophysiological link between MMP10 and the clinical management of BC[11,15,20,21]. This has strongly motivated us to explore the expression pattern of MMP10 to evaluate its diagnostic and prognostic values in our cohort of BC patients for better clinical management.
In the current study, we investigated the expression of MMP10 in a cohort of 170 consented BC patients along with their clinicopathological information. The finding of our study shows significant associations between low MMP10 expression and advanced stages and grades, as well as lymph node status (p=0.001, p=0.001, p=0.005, respectively; Table 1). These findings are consistent with previous studies that showed a negative correlation between MMP10 expression and the stage and grade of BC[11,22]. Based on these results, higher MMP10 protein expression seems to occur more frequently in early BC stages, which may be considered as a favourable indicator of a good prognosis. Other studies, however, reported no significant differences in MMP10 expression between histologically normal vs. MIBC bladder tissues[11,22]. Our results of the Kaplan-Meier analysis showed a significant positive association between MMP10 overexpression and survival outcomes mainly DSS (p=0.023, log-rank). This favourable prognostic value of MMP10 overexpression was marked by lower rates of both disease-specific and disease recurrence (fig. 2 and fig. 3).
Unlike many MMPs suggested to be pro-metastatic factors, the expression of MMP10 in this study appears to be more consistently associated with less malignant behaviour of BC. MMPs were initially characterized as pro-tumorigenic proteases. The results of this study are unique since previous reports used to assume that all MMPs have a role in ECM degradation and cell invasion promotion. However, our results clearly showed that MMP10 is associated with less invasive tumorigenic profiles and better survival outcomes through a still poorly understood mechanism(s). These associations with better survival outcomes were particularly noticeable with the stromelysin, a MMPs subfamily which includes mainly MMP10, MMP3 and MMP11[23,24]. Similar findings reported that MMP10 overexpression in BC promotes both DSS and DFS but did not suggest possible molecular pathways underlying these effects[25,26]. In line with our findings, the downregulation of MMP3 was also reported to promote tumour cell growth and metastasis in the squamous cell carcinoma mouse model[27].
Although MMPs overexpression is the most common feature in cancer compared to control tissues, several discrepancies and differences exist between MMPs subfamilies and/or tumour types. Despite we report here a favourable prognostic value in our BC cohort, MMP10 overexpression in other malignancies including oral, lung, breast, head and neck, prostate, and colorectal cancer showed a positive correlation with advanced staged tumour[13,28,29]. Thus, the expression of MMP10 has been suggested to degrade the ECM and increase the probability of tumour cells migration and metastasis[10,13,18,28]. This pro-metastatic effect of MMP10 overexpression was suggested to be induced via the canonical Wnt Family Member 7a (WNT7a) pathway in BC. In this context, MMP10 expression has been proposed to work collectively with MMP1 to degrade the ECM, promote angiogenesis and facilitate cell invasion and metastasis either in primary or lymph nodes lesions[30]. Some previous studies also suggested MMP10 expression as a potential diagnostic biomarker when used in combination with other approved urinary BC biomarkers[20,31-34]. These findings together with our results suggest different molecular pathophysiological pathways of MMP10 in our BC cohort from Saudi Arabia (and the Arabian Peninsula in general). This could be also explained as a consequence of a specific genomic background combined with particular environmental factors that lead to distinctive molecular roots of tumorigenesis[35]. These molecular events could induce various signalling pathways involved in the onset and progression of cancer in general.
In another context and contrast to the canonical WNT7a pathway, the expression of MMP10 as previously reported is regulated by FGFR3, a receptor that promotes downstream division and differentiation pathways including Mitogen-Activated Protein Kinase (MAPK), Phosphatidylinositol 3-Kinase, Renin- Angiotensin System (RAS) and Signal Transducer and Activator of Transcription 6 (STAT6)[31,36]. Alterations in FGFR3 are among the earliest and most frequently reported events in BC and could have a significant role in malignant progression. Yet, it often does not lead to worse outcomes as shown in our study[36]. The expression of FGFR3, mainly the mutated forms, in BC has been reported to upregulate MMP10 expression through the Extracellular Signal-Regulated Kinase Kinase (MEK)-Mitogen-Activated Protein Kinase (MAPK) pathway[31]. These reports led us to suggest that overexpression of MMP10 could be induced through FGFR3 pathways in early stages and less aggressive BC. This assumption is also consistent with Kaplan- Meier analysis of FGFR3 expression which showed that patients with elevated expression of mutated FGFR3 demonstrated longer DSS compared to those with lower expression[36-39]. Moreover, patients with FGFR3 mutations are more likely to show a better response to anti-FGFR3 therapy[31,40]. Since the overexpression of the wild-type FGFR3 did not show association with a less aggressive phenotype in urothelial carcinoma[40], some BC patients of our cohorts may carry the mutated form of FGFR3, but this requires further investigation.
Single Nucleotide Polymorphisms (SNPs) changes in MMPs, particularly the stromelysin subfamily, have also been reported to drive either the protective or pro-tumorigenic effect of an MMP. For instance, the wild type haplotype of MMP3 and MMP3-Six adenosines (6A) alleles have been associated with protective effects against metastasis in lung cancer and head and neck squamous cell carcinoma, respectively[41,42], whereas the MMP3-A5 allele variant was reported to upregulate gene expression and enhance the pro-tumorigenic and pro-metastatic effects of MMP3 in breast cancer[43-46].
Taken together, this study highlights a positive association between MMP10 protein overexpression and better survival outcomes which are reported for the first time in the Arabic peninsula population. Other reports suggested that some MMPs have some protective roles in cancer[24]. However, the pathophysiological molecular pathways driving these effects seem to encompass several interconnected factors and mutational events that are still poorly understood to explain the discrepancies. Therefore, this interesting prognostic value of our BC cohort should be expanded to confirm these MMP10 intricate effects through mutational screening of relevant upstream and/ or downstream targets. The use of high-throughput technologies and cross-platform validation is warranted to draw meaningful conclusions about the functional role of MMP10 in BC to extract clinically actionable targets towards personalized BC therapeutics.
Author’s contributions:
Maged Mostafa Mahmoud, Abdulelah Saleh Alshawli and Mourad Assidi contributed equally to this work.
Acknowledgements:
This study was funded by the Deputy of Scienti?c Research of King Abdulaziz University (Grant Number: G-501-141-1439). The authors gratefully acknowledge the technical and ?nancial support of the Deanship of Scienti?c Research.
Conflict of interests:
The authors declared no conflict of interest.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Nedjadi T, Al-Maghrabi J, Assidi M, Dallol A, Al-Kattabi H, Chaudhary A, et al. Prognostic value of HER2 status in bladder transitional cell carcinoma revealed by both IHC and BDISH techniques. BMC Cancer 2016;16(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Mokhtar A, Al Alawi MM, Al Taweel WM, Al Othman K, Kattan SA, Al Otaibi MF. Is survival after radical cystectomy for bladder cancer in Saudi patients different from that of Western patients? Ann Saudi Med 2017;37(3):194-200.
[Crossref] [Google Scholar] [PubMed]
- Anastasiadis A, de Reijke TM. Best practice in the treatment of non-muscle invasive bladder cancer. Ther Adv Urol 2012;4(1):13-32.
[Crossref] [Google Scholar] [PubMed]
- Shah JB, McConkey DJ, Dinney CP. New strategies in muscle-invasive bladder cancer: On the road to personalized medicine. Clin Cancer Res 2011;17(9):2608-12.
[Crossref] [Google Scholar] [PubMed]
- Darrell CM, Montironi R, Paner GP. Potential biomarkers and risk assessment models to enhance the tumor-node-metastasis (TNM) staging classification of urologic cancers. Expert Rev Mol Diagn 2020;20(9):921-32.
[Crossref] [Google Scholar] [PubMed]
- Chen C, Qi XJ, Cao YW, Wang YH, Yang XC, Shao SX, et al. Bladder tumor heterogeneity: The impact on clinical treatment. Urol Int 2015;95(1):1-8.
- Knowles MA, Hurst CD. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15(1):25-41.
[Crossref] [Google Scholar] [PubMed]
- Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci 2017;147:1-73.
[Crossref] [Google Scholar] [PubMed]
- Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol 2019;137:57-83.
[Crossref] [Google Scholar] [PubMed]
- Zhang G, Miyake M, Lawton A, Goodison S, Rosser CJ. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer 2014;14(1):1-4.
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011;3(12):a005058.
[Crossref] [Google Scholar] [PubMed]
- Piskór BM, Przylipiak A, D?browska E, Niczyporuk M, ?awicki S. Matrilysins and stromelysins in pathogenesis and diagnostics of cancers. Cancer Manag Res 2020;12:10949-64.
[Crossref] [Google Scholar] [PubMed]
- Urquidi V, Goodison S, Cai Y, Sun Y, Rosser CJ. A candidate molecular biomarker panel for the detection of bladder cancer. Cancer Epidemiol Biomarkers Prev 2012;21(12):2149-58.
[Crossref] [Google Scholar] [PubMed]
- Rosser CJ, Ross S, Chang M, Dai Y, Mengual L, Zhang G, et al. Multiplex protein signature for the detection of bladder cancer in voided urine samples. J Urol 2013;190(6):2257-62.
[Crossref] [Google Scholar] [PubMed]
- Furuya H, Chan OT, Hokutan K, Tsukikawa Y, Chee K, Kozai L, et al. Prognostic significance of lymphocyte infiltration and a stromal immunostaining of a bladder cancer associated diagnostic panel in urothelial carcinoma. Diagnostics 2019;10(1):14.
[Crossref] [Google Scholar] [PubMed]
- Al-Maghrabi J, Mufti S, Gomaa W, Buhmeida A, Al-Qahtani M, Al-Ahwal M. Immunoexpression of cyclin D1 in colorectal carcinomas is not correlated with survival outcome. J Microsc Ultrastruct 2015;3(2):62-7.
[Crossref] [Google Scholar] [PubMed]
- García?Irigoyen O, Latasa MU, Carotti S, Uriarte I, Elizalde M, Urtasun R, et al. Matrix metalloproteinase 10 contributes to hepatocarcinogenesis in a novel crosstalk with the stromal derived factor 1/C?X?C chemokine receptor 4 axis. Hepatology 2015;62(1):166-78.
[Crossref] [Google Scholar] [PubMed]
- Batista R, Vinagre J, Prazeres H, Sampaio C, Peralta P, Conceição P, et al. Validation of a novel, sensitive, and specific urine-based test for recurrence surveillance of patients with non-muscle-invasive bladder cancer in a comprehensive multicenter study. Front Genet 2019:1237.
[Crossref] [Google Scholar] [PubMed]
- Goodison S, Chang M, Dai Y, Urquidi V, Rosser CJ. A multi-analyte assay for the non-invasive detection of bladder cancer. PLoS One 2012;7(10):e47469.
[Crossref] [Google Scholar] [PubMed]
- Mueller J, Steiner C. Stromelysin-3 expression in noninvasive and invasive neoplasms of the urinary bladder. Hum Pathol 2000;31(7):860-5.
[Crossref] [Google Scholar] [PubMed]
- Seargent JM, Loadman PM, Martin SW, Naylor B, Bibby MC, Gill JH. Expression of matrix metalloproteinase-10 in human bladder transitional cell carcinoma. Urology 2005;65(4):815-20.
[Crossref] [Google Scholar] [PubMed]
- Martin MD, Matrisian LM. The other side of MMPs: Protective roles in tumor progression. Cancer Metastasis Rev 2007;26(3):717-24.
[Crossref] [Google Scholar] [PubMed]
- Decock J, Thirkettle S, Wagstaff L, Edwards DR. Matrix metalloproteinases: Protective roles in cancer. J Cell Mol Med 2011;15(6):1254-65.
[Crossref] [Google Scholar] [PubMed]
- Nikkola J, Vihinen P, Vlaykova T, Hahka-Kemppinen M, Kähäri VM, Pyrhönen S. High collagenase-1 expression correlates with a favourable chemoimmunotherapy response in human metastatic melanoma. Melanoma Res 2001;11(2):157-66.
- Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol 2013;34(4):2041-51.
[Crossref] [Google Scholar] [PubMed]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999;98(2):137-46.
[Crossref] [Google Scholar] [PubMed]
- Ren ZH, Wu K, Yang R, Liu ZQ, Cao W. Differential expression of matrix metalloproteinases and miRNAs in the metastasis of oral squamous cell carcinoma. BMC Oral Health 2020;20(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Deraz EM, Kudo Y, Yoshida M, Obayashi M, Tsunematsu T, Tani H, et al. MMP-10/stromelysin-2 promotes invasion of head and neck cancer. PLoS One 2011;6(10):e25438.
[Crossref] [Google Scholar] [PubMed]
- Huang X, Zhu H, Gao Z, Li J, Zhuang J, Dong Y, et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J Biol Chem 2018;293(18):6693-706.
[Crossref] [Google Scholar] [PubMed]
- Du X, Lin BC, Wang QR, Li H, Ingalla E, Tien J, et al. MMP-1 and Pro-MMP-10 as potential urinary pharmacodynamic biomarkers of FGFR3-targeted therapy in patients with bladder cancer. Clin Cancer Res 2014;20(24):6324-35.
[Crossref] [Google Scholar] [PubMed]
- Hirasawa Y, Pagano I, Chen R, Sun Y, Dai Y, Gupta A, et al. Diagnostic performance of Oncuria™, a urinalysis test for bladder cancer. J Transl Med 2021;19(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Zhang G, Gomes-Giacoia E, Dai Y, Lawton A, Miyake M, Furuya H, et al. Validation and clinicopathologic associations of a urine-based bladder cancer biomarker signature. Diagn Pathol 2014;9(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Chen LM, Chang M, Dai Y, Chai KX, Dyrskjøt L, Sanchez-Carbayo M, et al. External validation of a multiplex urinary protein panel for the detection of bladder cancer in a multicenter cohort. Cancer Epidemiol Biomarkers Prev 2014;23(9):1804-12.
[Crossref] [Google Scholar] [PubMed]
- Abu-Elmagd M, Assidi M, Schulten HJ, Dallol A, Pushparaj PN, Ahmed F, et al. Individualized medicine enabled by genomics in Saudi Arabia. BMC Med Genomics 2015;8(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Pandith AA, Shah ZA, Siddiqi MA. Oncogenic role of fibroblast growth factor receptor 3 in tumorigenesis of urinary bladder cancer. Urol Oncol 2013;31(4):398-406.
[Crossref] [Google Scholar] [PubMed]
- Kang HW, Kim YH, Jeong P, Park C, Kim WT, Ryu DH, et al. Expression levels of FGFR3 as a prognostic marker for the progression of primary pT1 bladder cancer and its association with mutation status. Oncol Lett 2017;14(3):3817-24.
[Crossref] [Google Scholar] [PubMed]
- Blinova E, Buzdin A, Enikeev D, Roshchin D, Suntsova M, Samyshina E, et al. Prognostic role of FGFR3 expression status and tumor-related microRNAs level in association with PD-L1 expression in primary luminal non-muscular invasive bladder carcinoma. Life 2020;10(11):305.
[Crossref] [Google Scholar] [PubMed]
- Akanksha M, Sandhya S. Role of FGFR3 in urothelial carcinoma. Iran J Pathol 2019;14(2):148-55.
[Crossref] [Google Scholar] [PubMed]
- van Rhijn BW, Mertens LS, Mayr R, Bostrom PJ, Real FX, Zwarthoff EC, et al. FGFR3 mutation status and FGFR3 expression in a large bladder cancer cohort treated by radical cystectomy: Implications for anti-FGFR3 treatment? Eur Urol 2020;78(5):682-7.
[Crossref] [Google Scholar] [PubMed]
- Sun T, Gao Y, Tan W, Ma S, Zhang X, Wang Y, et al. Haplotypes in matrix metalloproteinase gene cluster on chromosome 11q22 contribute to the risk of lung cancer development and progression. Clin Cancer Res 2006;12(23):7009-17.
[Crossref] [Google Scholar] [PubMed]
- Zinzindohoué F, Blons H, Hans S, Loriot MA, Houllier AM, Brasnu D, et al. Single nucleotide polymorphisms in MMP1 and MMP3 gene promoters as risk factor in head and neck squamous cell carcinoma. Anticancer Res 2004;24(3B):2021-6.
[Google Scholar] [PubMed]
- Krippl P, Langsenlehner U, Renner W, Yazdani-Biuki B, Köppel H, Leithner A, et al. The 5A/6A polymorphism of the matrix metalloproteinase 3 gene promoter and breast cancer. Clin Cancer Res 2004;10(10):3518-20.
[Crossref] [Google Scholar] [PubMed]
- Xiangpo P, Shuzhen L, Jianfeng G, Haiyu L, Haipeng R, Hunanxin W. Current evidence on the association between four polymorphisms in the matrix metalloproteinases (MMP) gene and breast cancer metastasis. J Environ Anal Chem 2015;2(151):2380-91.
- Padala C, Tupurani MA, Puranam K, Gantala S, Shyamala N, Kondapalli MS, et al. Synergistic effect of collagenase-1 (MMP1), stromelysin-1 (MMP3) and gelatinase-B (MMP9) gene polymorphisms in breast cancer. PLoS One 2017;12(9):e0184448.
[Crossref] [Google Scholar] [PubMed]
- Suhaimi SA, Chan SC, Rosli R. Matrix metallopeptidase 3 polymorphisms: Emerging genetic markers in human breast cancer metastasis. J Breast Cancer 2020;23(1):1-9.
[Crossref] [Google Scholar] [PubMed]