- *Corresponding Author:
- Feng Yao
Department of Cardiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong 524013,
China
E-mail: yaofeng0704@163.com
| This article was originally published in a special issue, “Clinical Advancements in Life Sciences and Pharmaceutical Research” |
| Indian J Pharm Sci 2024:86(5) Spl Issue “188-195” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To determine the relative expression of microRNA-499 after anoxia-reoxygenation injury and to investigate the anti-apoptotic regulatory mechanism. The anoxia-reoxygenation injury model of H9C2 cardiomyocytes was developed, including two groups; normoxia and anoxia-reoxygenation. Real-time polymerase chain reaction was used to detect the expression trend of microRNA-499 after hypoxia and anoxia-reoxygenation injury; different concentrations of microRNA-499 mimic were transfected and the cell survival was detected. The target gene of microRNA-499 was confirmed by luciferase reporter gene assay. The expression and effect on apoptosis were detected by real-time polymerase chain reaction and Western blot. The expression of microRNA-499 peaked at 24 h of hypoxia and 9 h of reoxygenation in H9C2 cardiomyocytes. MicroRNA-499 had an optimal protective effect against anoxia-reoxygenation injury when transfected at a concentration of 100 nm. In addition, microRNA-499 down-regulated the expression of target genes Jun N-terminal protein kinase and caspase-3 after anoxia-reoxygenation injury. Overexpression of microRNA-499 reduced anoxiareoxygenation- induced H9C2 cardiomyocyte injury, which may be related to the inhibition of apoptosis in H9C2 cardiomyocytes through the modulation of the Jun N-terminal protein kinase/caspase-3 pathway.
Keywords
Anoxia-reoxygenation injury, microRNA-499, H9C2 cardiomyocytes, apoptosis
Myocardial Ischemia-Reperfusion (I/R) is the restoration of reperfusion of coronary blood flow within a certain critical time after myocardial infarction, and the reperfusion of blood flow is closely related to cardiac tissue damage, which is a very important clinical event. Previous studies have found that Percutaneous Coronary Intervention (PCI) with pharmacological thrombolysis does not improve the prognosis of all patients with reperfusion of blood flow, which may be due to the changes in the structure, function and microenvironment of cardiomyocytes caused by reperfusion of ischaemic myocardium, resulting in arrhythmias, necrosis of cardiomyocytes and cardiac myocardial tonicity[1], and eventually cardiac arrest or death from chronic heart failure[2]. With the introduction and development of precision medicine, new pharmacogenomics technologies for clinical translation to individualized precision medicine will provide new therapeutic targets and strategies for I/R injury.
Recently, microRNA (miRNA)-499 expression was found to be elevated only in cardiomyocytes after Acute Myocardial Infarction (AMI), while the expression level was low in normal cardiomyocytes[3]. Overexpression of miRNA-499 could up-regulate the expression of the target genes, Calcineurin A-Alpha (CnA-α) and CnAβ, which could inhibit the dephosphorylation of Dynamin- Related Protein 1 (DRP1), and then reduce its enrichment in mitochondria and ultimately reduce the level of mitochondrial cleavage mediated by DRP1, which played a role in the inhibition of cardiomyocyte apoptosis. Mitochondrial cleavage inhibit cardiomyocyte apoptosis[4]. Furthermore, during embryonic development, miRNA-499 also encourages the differentiation of cardiac stem cells. Therefore, over-expression of miRNA-499 in cardiomyocyte stem cells down-regulates the expression of its target group Sox6, which promotes the regeneration of cardiomyocytes and improves the ventricular function[5]. However, how the expression level of miRNA-499 will change during myocardial Anoxia/Reoxygenation (A/R) injury has not been clarified. We propose to establish an in vitro A/R injury model, and to deeply explore the role of miRNA-499 and its mechanism in the myocardial A/R model, providing a new experimental basis for the translation of basic medicine to clinical treatment.
Materials and Methods
Main reagents:
Baobao Bioengineering Company supplied the miRNA complementary Deoxyribonucleic Acid (cDNA) first strand synthesis kit and the miRNA fluorescence quantification kit. Shanghai Sangong Biotechnology Company offered the primers. The miRNA transfection kit and enzyme free water were purchased from RuiBo Bio Technology Company. Biyun Tian Bio Technology Company supplied the Cell Counting Kit-8 (CCK-8) kit. Hyclone Company supplied the fetal calf serum. Dulbecco’s Modified Eagle Medium (DMEM) medium was acquired from Gibco, United States of America (USA). Rabbit anti-mouse Mitogen-Activated Protein Kinase 8 (MAPK8) polyclonal antibody, rabbit anti-mouse p-MAPK8 polyclonal antibody, and rabbit antimouse caspase-3 polyclonal antibody were acquired from Abcam, USA.
Cells and cultures:
Cell line of rats H9C2 cardiomyocytes obtained from American Type Culture Collection (ATCC), USA. The cells were cultured in DMEM medium containing 10 % fetal bovine serum in an incubator incubated at 37°, 5 % Carbon dioxide (CO2) and saturated moderately.
Experimental methods:
Establishment of H9C2 cardiomyocyte A/R injury model: Referring to the method for the establishment of cardiomyocyte A/R model, the complete medium of H9C2 cells that needed to be treated with anoxia was discarded, and replaced with the prepared synchronization medium, and then placed in 37°, 5 % CO2 incubator for 24 h; the synchronization medium was discarded and replaced with anoxia medium and then the cells were placed in a 37° low oxygen incubator (1 % O2 and 37°, 5 % CO2) for oxygen replacement; the H9C2 cardiomyocytes were subjected to anoxia for a corresponding period of time and then deoxygenated. 5 % CO2 for O2 replacement for 2 h, at which time it became a anoxia model[6]; H9C2 cardiomyocytes were deoxygenated after anoxia for the corresponding time period. Using a cell pipette, the anoxia solution was removed. The whole medium was then added, and the mixture was placed in an incubator set to 37° with 95 % O2 and 5 % CO2. In reoxygenation time, we further examined the relative changes expression level of miRNA-499 under reoxygenation condition, and established a model of A/R injury of the cells with an optimal time point for the occurrence of reoxygenation injury.
Extraction and purity of total Ribonucleic Acid (RNA) from H9C2 cardiomyocytes: Total cellular RNA was collected from H9C2 cardiomyocytes in the anoxia group at 0 h, 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 18 h and 24 h, whereas H9C2 cells were reoxygenated at 0 h, 3 h, 6 h and 9 h. The precise stages were completed in accordance with the Trizol reagent instructions.
Luciferase reporter gene assay: H9C2 cells in logarithmic growth phase were seeded into 96-well plates with 5×104 cells per well and cultured at 37° for 24 h. 10 μl of OPTI-Minimal Essential Medium (MEM) was used to dilute the miRNA mimic or non-target control, 15 μl of OPTI-MEM medium to dilute the target gene 3’ Untranslated Regions (3’ UTRs) mutant vector, and 25 μl of OPTI-MEM medium to dilute 0.25 μl of LipofectamineTM 2000 reagent. After 5 min, mix the three together and leave for 20 min. OPTI-MEM medium to dilute 0.25 μl LipofectamineTM 2000 reagent, after 5 min the three mixed, a total of 50 μl, gently shaking well and left to stand for 20 min; plasmid, mimic before adding the cells, each well first aspirated 50 μl of medium, and then added to the mixture, and finally the total volume of each well was up to 100 ml. The mimics were transfected at a concentration of 50 nm, the plasmid concentration was 100 ng per well, and each group had three duplicate wells. 100 μl of new medium were introduced after 6 h. After 48 h after transfection, remove the medium, replace it with 35 μl of new media per well, and add 35 μl of luciferase substrate per well. Shake for 10 min and then measure the fluorescence value. Dispense 30 μl of stop reagent, agitate for 10 min, and then quantify the fluorescence value with a fluorimeter.
Real-time Polymerase Chain Reaction (PCR): Shanghai Sangong Biological Co. developed and manufactured the primer sequences for miRNA-499 upstream primer and MAPK8. The miRNA-499 upstream primer sequence is 5 ’ - U U A A G A C U U G C A G U G A U G U U U U - 3 ’ , the MAPK8 upstream primer sequence is 5 ’ - T G A C A G A C G G C G A - A G A G A U - 3 ’ , and the downstream primer sequence is 5’-GGATTTGGAGGACGAACTA-3’. RNA was extracted using Trizol reagent following the instructions, and reverse transcription was performed with an mRNA cDNA first strand synthesis kit using a 10 μl reaction system. The reaction system was amplified using the Real-time PCR instructions. The PCR reaction conditions were as follows; predenaturation at 95° for 30 s, followed by 40 cycles of denaturation at 95° for 5 s and annealing/extension at 60° for 34 s. Each sample underwent three repetitions. Following the completion of the reaction, the specimen was detected and examined using the LightCycler 480 II DNA amplifier, and the recording curve was computed. The data were analyzed by the relative quantification method of 2-ΔΔCt values, ΔCt experimental group=Ct experimental group target gene-Ct experimental group internal reference; ΔCt control group=Ct control group target gene-Ct control group internal reference; ΔΔCt=ΔCt experimental group-ΔCt control group.
Transfection of miRNA-499 mimic and miRNA-499 inhibitor: H9C2 cardiomyocytes with a cell density of 50 %-60 % were synchronized for 24 h and then transfected. 1.25 μl of 20 μl miRNA-499 mimic was diluted with 30 μl of 1×riboFECTTM CP buffer, gently mixed, then 3 μl of riboFECTTM Thermomechanical Control Process (TMCP) reagent was added and gently blown to mix. Incubating at room temperature for 0-15 min, further we added riboFECTTM CP mix to the synchronized cell culture medium, mixed gently and incubated in 37°, 5 % CO2 for 6 h. After that, replacing the medium with completed medium and placing it back in 37°, 5 % CO2 incubator for (8- 10) h. Then the cells were subjected to A/R damage treatment.
CCK-8 assay for cell viability: The normoxic group (miRNA-499 mimic and miRNA-499 inhibitor) and A/R group (miRNA-499 mimic and miRNA-499 inhibitor) were set up, and the cells of each group were transfected with different concentrations, respectively. The cells were inoculated with cell suspensions in 96-well plates (100 μl /well), placed in a 37°, 5 % CO2 incubator, different groups of cells were treated and cultured accordingly. Then, 10 μl CCK solution was added to each well, protecting from light, the 96-well plate was cultured in the incubator for 2 h. The absorbance at 450 nm was measured by an enzyme marker and the results were statistically analyzed to establish the optimal transfection concentration for miRNA-499 to maintain cell activity, and the above experiments were repeated more than 3 times.
Western blot detection: H9C2 cells in exponential growth phase were seeded into 6-well plates at a concentration of 1×106 cells per well and the culture was stopped after 24 h. The proteins were extracted following the precise protocol outlined in the cellular protein separation and extraction kit. The protein concentration for each group was determined using the Bicinchoninic Acid (BCA) method to calculate the required sample concentration for Western blot. The proteins were then separated using Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred to a Polyvinylidene Fluoride (PVDF) membrane five times. 20 μg of sample proteins were separated by SDS-PAGE, electro transferred to a PVDF membrane, washed 5 times, blocked with 5 % skimmed milk powder, and incubated with monoclonal antibodies for Jun N-Terminal Protein Kinase (JNK) and caspase-3, as well as an internal reference antibody for-actin at 4° overnight. The PVDF membrane was then rinsed with Tris-Buffered Saline with 0.1 % Tween® 20 (TBST) detergent 5 times for 5 min each, followed by the addition of the corresponding secondary antibody and further incubation at room temperature for 1 h. The PVDF membrane was washed three times with TBST, followed by incubation with Electrochemiluminescence (ECL) luminous solution. X-ray film was used to capture images in a dark room. The gray value of the target protein bands was analyzed using ImageJ analysis software. The expression of the proteins in each group was determined by calculating the ratio of the gray value of the bands of interest to the gray value of the actin bands.
Results and Discussion
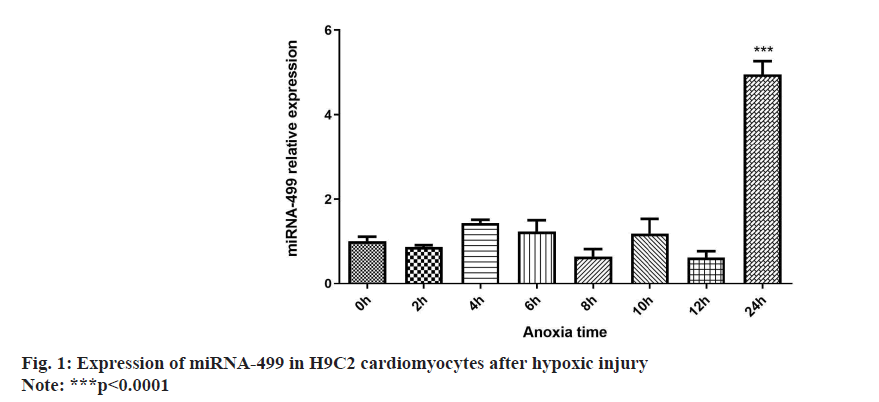
The Real-time PCR experiment revealed a statistically significant change in H9C2 cardiomyocyte’s expression after 24 h of anoxia compared to the other groups as shown in fig. 1.
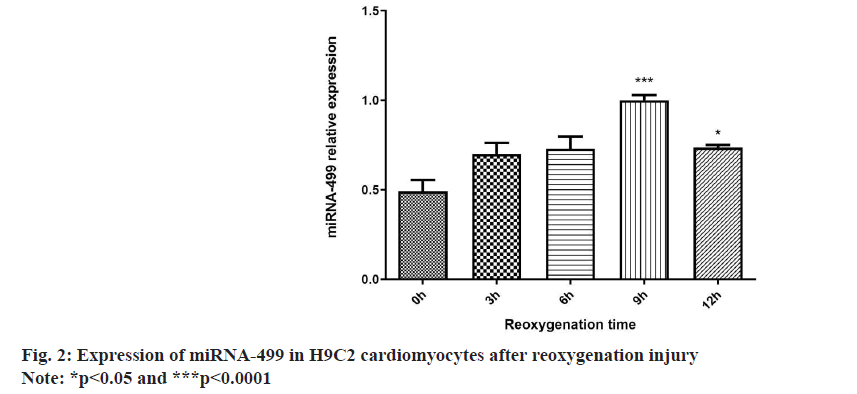
Real-time PCR indicated a substantial increase in miRNA-499 expression after 9 h of reoxygenation compared to 0 h. The reoxygenation 12 h group showed statistically significant differences compared to the reoxygenation 0 h group. However, the miRNA-499 expression decreased remarkably after 12 h of reoxygenation, comparing to reoxygenation after 9 h. Therefore, the optimal time point for reoxygenation injury was set at 9 h after reoxygenation as shown in fig. 2.
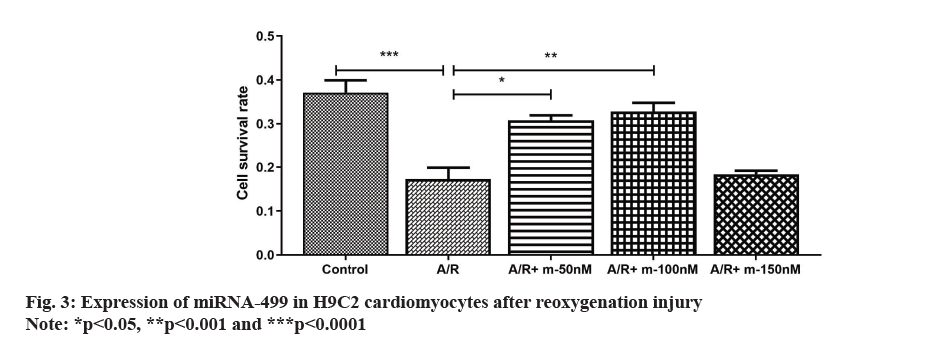
In the CCK-8 experiments, it showed that the cell survival rate of H9C2 cardiomyocytes was significantly reduced after treatment with A/R injury; whereas transfection of H9C2 cells with miRNA-499 mimic at 50 nm and 100 nm significantly increased the survival rate of A/R-injured cardiomyocytes in a dose-dependent manner. When the transfection concentration reached 150 nm, the survival rate of H9C2 cells was significantly reduced after A/R injury. The above results indicate that the best myocardial protective effect was 100 nm as shown in fig. 3.
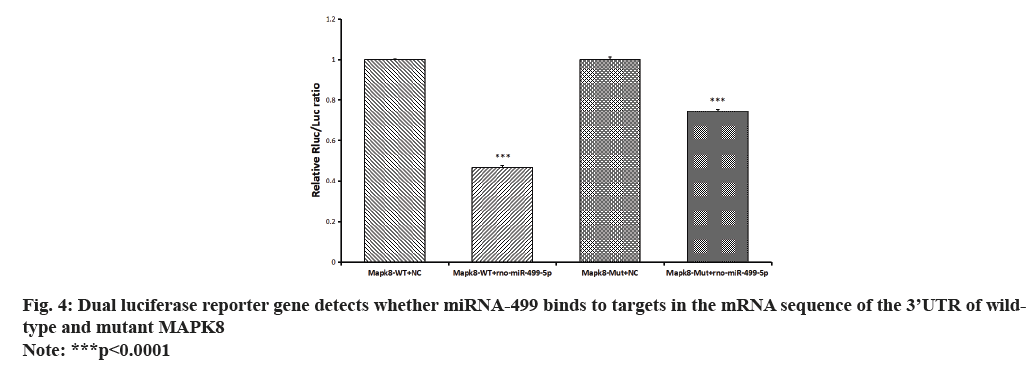
miRNA target gene prediction identified MAPK8 as a potential target gene for validation. Furthermore, the reporter fluorescence of the wild type of MAPK8 was found to be more strongly down-regulated by the rno-miR-499-5p mimic, as revealed by the analysis of dual luciferase reporter gene expression. However, the reporter fluorescence of the mutant vector was restored upon mutation of its predicted target site. According to this finding, the MAPK8 gene’s 3’UTR is most likely the location by which rno-miR-499-5p regulates gene expression as shown in fig. 4.
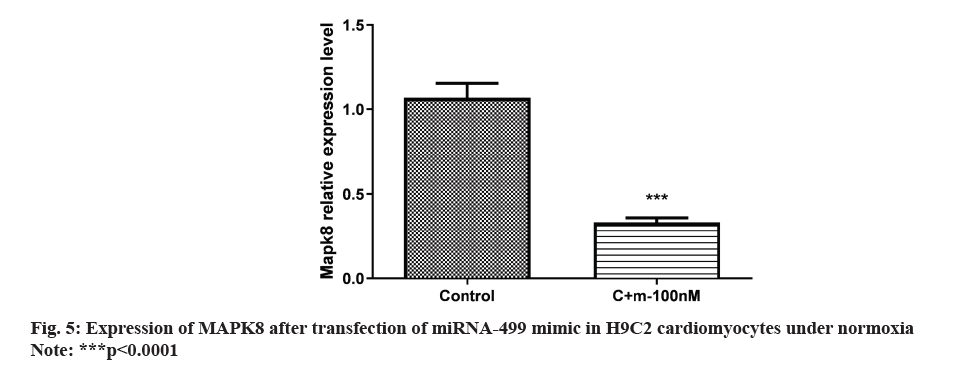
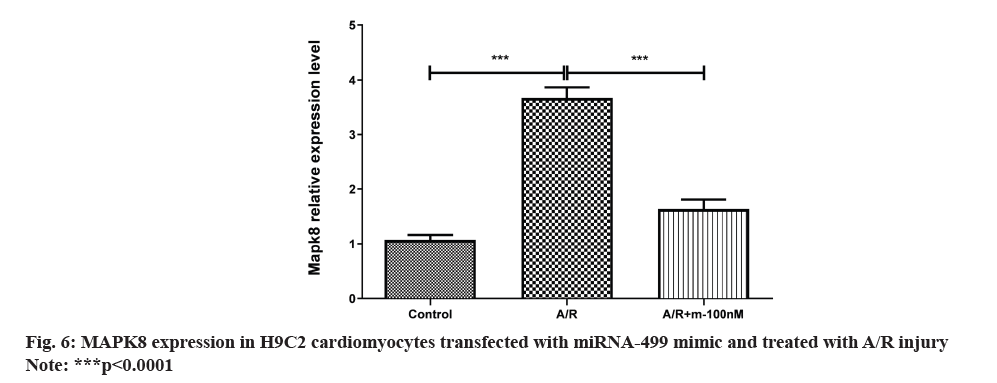
The outcome shown that H9C2 cardiomyocytes were transfected with the miRNA-499 mimic in normoxia. MAPK8 expression was notably decreased in the miRNA-499 mimic group compared to the control group as shown in fig. 5. Following miRNA-499 mimic transfection with A/R therapy, MAPK8 expression was severely repressed as shown in fig. 6.
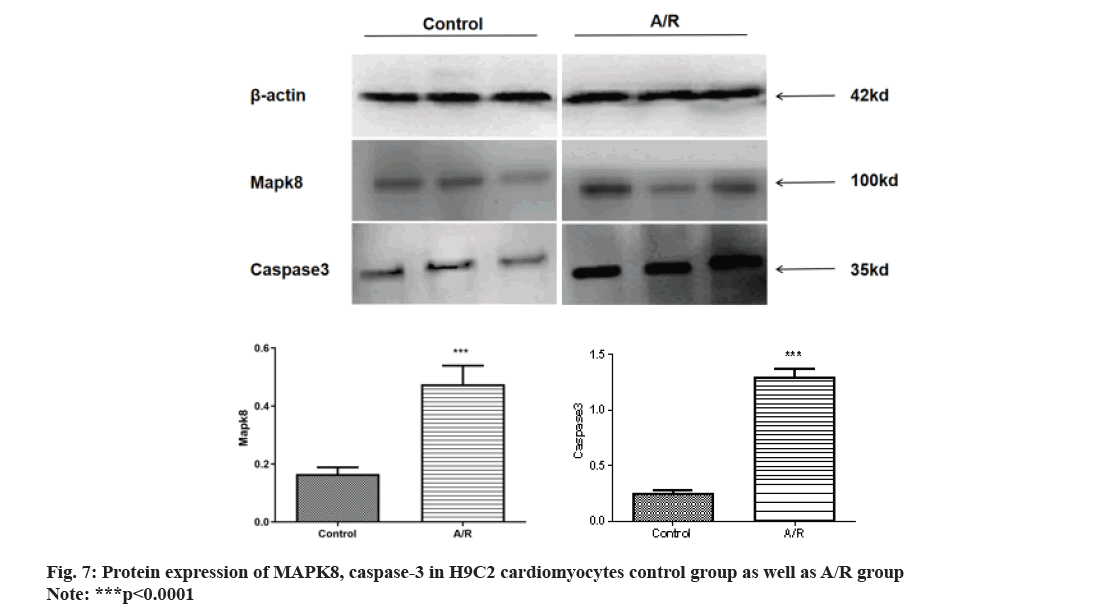
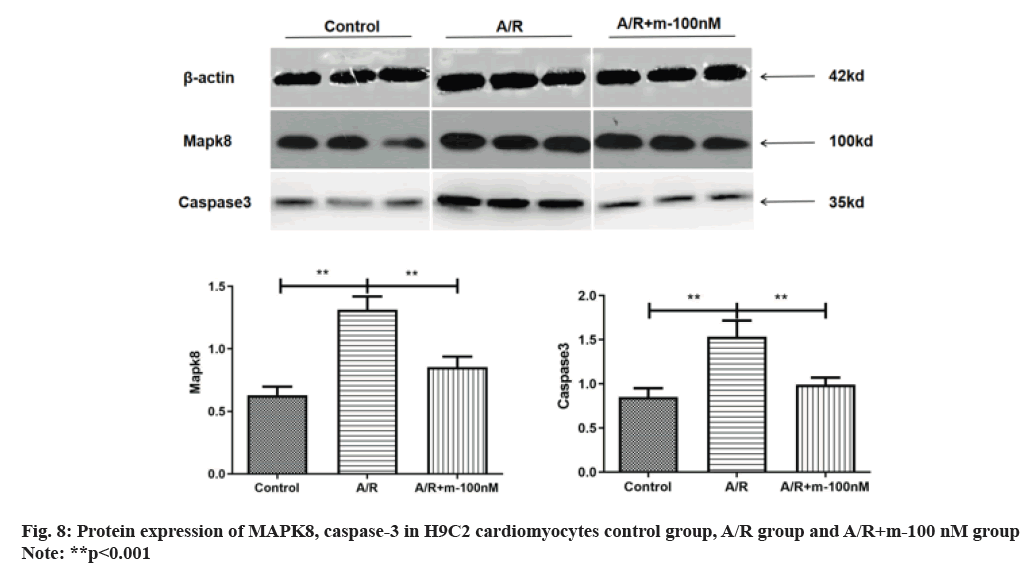
Western blot analysis revealed a substantial increase in MAPK8 levels following A/R injury compared to the control group. The protein expression of caspase-3 increased, suggesting the occurrence of apoptosis in A/R injury as shown in fig. 7. fig. 8 illustrates how MAPK8 was directly involved in the effect of miRNA-499 on cardiomyocyte apoptosis, indicating that after transfection with miRNA-499 mimic, the expression of MAPK8 and caspase-3 was significantly lower in the pure A/R injury group compared to the A/R injury group. This suggests that cardiomyocyte apoptosis occurred in H9C2 cardiomyocytes following A/R injury.
Research on the mechanism of correlation between miRNA-499 and myocardial ischemia and reperfusion has attracted the attention of scholars and achieved preliminary results. It was found that after the occurrence of AMI, the expression of miRNA-499 in cardiomyocytes was elevated, while its expression level was low in normal hearts[3,7]. Agiannitopoulos et al.[8] found that miRNA-499 and miRNA-208 were glued together in AMI patients.
In the control group, miRNA-499 and miRNA-208 were found to be elevated by approximately 100-fold and 1600-fold in AMI patients, with a significant correlation between the elevation and the alteration of cTnI levels. In addition, researchers have found that transfected overexpressed miRNA-499 could upregulate the expression level of target genes CnAα and CnAβ, which could inhibit the dephosphorylation of DRP1 and reduce DRP1-mediated mitochondrial cleavage, and ultimately inhibit the apoptosis of cardiomyocytes[4]. Meanwhile, by evaluating the results of cardiac mass-to-volume ratio, cardiac collagen content, ventricular function and cardiac chamber diameter in miRNA-499 transgenic mice after myocardial infarction, miRNA-499 was found to significantly improve cardiac remodeling and cardiac function after myocardial infarction. Currently, studies have confirmed that miRNA-499 has high specificity and sensitivity for AMI diagnosis and has strong diagnostic value[9], but the interaction mechanism between I/R injury and miRNA-499 has not been clarified. Therefore, we constructed an in vitro model of A/R injury in H9C2 cardiomyocytes and explored the miRNA-499 expression trend, and found that the expression level of miRNA-499 was significantly higher after 24 h of hypoxia compared with the other time groups and after 9 h of A/R injury, the miRNA-499 was most remarkably elevated, and a decrease in expression occurred at 12 h of reoxygenation. This experimental group believed that A/R injury caused a large number of H9C2 cells to die as a result, and that the protective effect of miRNA-499 reached its peak after 9 h of reoxygenation. In addition, the results of this experiment showed that 50 nm and 100 nm miRNA-499 mimic transfection of H9C2 cells significantly increased the survival rate of A/R-injured cardiomyocytes in a dose-dependent way, certifying the best cardio protective effect was exerted at 100 nm miRNA-499 mimic transfection of H9C2 cardiomyocytes, which was similar to that reported by Ban et al.[10]. When the transfection concentration was 150 nm, the survival rate of H9C2 cells after A/R injury was significantly reduced, and it was concluded that miRNA-499 had a toxic effect on the cells after reaching a certain dose. Based on the trend of miRNA-499, the time of hypoxiareoxygenation (24 h of hypoxia/9 h of reoxygenation) was determined to exert the protective effect. At the same time, the optimal transfection concentration of miRNA-499 was determined to be 100 nm, which exerted the best myocardial protective effect.
MAPK8, a protein involved in the inflammatory during A/R injury, activates Nuclear Factor-Kappa B (NF-κB), and then enters the nucleus, by means of releasing of Tumor Necrosis Factor Alpha (TNF-α) and Interleukin-6 (IL-6), eventually promotes the inflammation, apoptosis, infarct size and decrease cardiac function[11,12]. Ferrandi et al.[13] found that AS601245 down-regulated the expression level of MAPK8 in cardiomyocytes after A/R injury, inhibited cardiomyocyte apoptosis and reactive oxygen species production, reduced myocardial infarct size, and protected cardiac function. Importantly, Tien et al.[14] demonstrated that saffron could inhibit lipopolysaccharide-induced cardiomyocyte apoptosis by inhibiting the activities of JNK, one of the important MAPK protein family members, decreasing IκB catabolism and NF-κB activity, and causing the activation of anti-apoptotic proteins B-cell lymphoma-2 (Bcl-2) and Bcl-Extra Large (Bcl-XL), an increase in mitochondrial membrane solidity, and an increase in the intracellular proapoptotic proteins TNF-α, caspase 8, t-Bid caspase 8, t-Bid, Bcl-2-Associated protein X (BAX) and caspase-3. Currently, the research on MAPK8 signaling pathway is deepening, and the important role of MAPK8 in apoptosis is indisputable. Targeting MAPK8 not only provides new ideas and methods for the treatment of apoptosis-induced diseases, but also has an important application prospect for the search of new drug targets and drug development. In this study, MAPK8 was verified as the target gene of miRNA-499 by using informatics prediction software and luciferase reporter gene detection system. At the same time, MAPK8 messenger RNA (mRNA) inflammation was detected by Realtime PCR, and it was found that the expression of MAPK8 in H9C2 cells in the A/R damage group was significantly elevated compared with that in the normoxic control group, whereas in the miRNA-499 mimic transfected and treated with A/R damage, the expression of MAPK8 was significantly suppressed compared with that in the A/R damage group. It was found that MAPK8 and caspase-3 expression was significantly elevated in H9C2 cardiomyocytes after A/R injury compared to normoxic control group. After transfecting miRNA-499 mimic into H9C2 cardiomyocytes, an A/R injury model was established, and then Western blot was applied to detect MAPK8, and caspase-3 protein expression. MAPK8 and caspase-3 proteins were significantly reduced after transfection of miRNA-499 mimic compared with the A/R injury group, suggesting that the apoptosis of cardiomyocytes was reduced, which was similar to the results of the studies conducted by Li et al.[11] and Jiao et al.[15].
Overall, our investigation showed that miRNA-499 is critical for A/R damage of H9C2 cardiomyocytes by controlling the expression of MAPK8/caspase-3, which eventually reduces cardiomyocyte death. Our research team will keep refining the animal model and clinical case model because the mechanism underlying myocardial ischemia/reperfusion injury is extremely intricate. This will provide a solid experimental foundation for the development of miRNA-499 clinical application in treating myocardial ischemia/reperfusion injury.
Conflict of interests:
The authors declared no conflict of interests.
References
- Wang Y, Liu Q, Lu F, Xin J, Dai Q, Wu S, et al. New thoughts in mechanism research of acupuncture for myocardial stunning from κ-opioid receptor signaling pathway. Zhongguo Zhen Jiu 2018;38(1):77-81.
[Crossref] [Google Scholar] [PubMed]
- Ibáñez B, Heusch G, Ovize M, van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 2015;65(14):1454-71.
[Crossref] [Google Scholar] [PubMed]
- Shalaby SM, El-Shal AS, Shoukry A, Khedr MH, Abdelraheim N. Serum miRNA-499 and miRNA-210: A potential role in early diagnosis of acute coronary syndrome. IUBMB Life 2016;68(8):673-82.
[Crossref] [Google Scholar] [PubMed]
- JX W. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med 2011;17:71-8.
[Crossref] [Google Scholar] [PubMed]
- Wang XY, Chen XL, Huang ZQ, Chen DW, Yu B, He J, et al. MicroRNA-499-5p regulates porcine myofiber specification by controlling Sox6 expression. Animal 2017;11(12):2268-74.
[Crossref] [Google Scholar] [PubMed]
- Ekhterae D, Lin Z, Lundberg MS, Crow MT, Brosius III FC, Núñez G. ARC inhibits cytochrome c release from mitochondria and protects against hypoxia-induced apoptosis in heart-derived H9c2 cells. Circ Res 1999;85(12):e70-7.
[Crossref] [Google Scholar] [PubMed]
- Wilson KD, Hu S, Venkatasubrahmanyam S, Fu JD, Sun N, Abilez OJ, et al. Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: Role for miR-499. Circ Cardiovasc Genetic 2010;3(5):426-35.
[Crossref] [Google Scholar] [PubMed]
- Agiannitopoulos K, Pavlopoulou P, Tsamis K, Bampali K, Samara P, Nasioulas G, et al. Expression of miR-208b and miR-499 in Greek patients with acute myocardial infarction. In Vivo 2018;32(2):313-8.
[Crossref] [Google Scholar] [PubMed]
- Wang Q, Junfen MA, Jiang Z. Meta-analysis of the diagnostic value of microRNA-499 for acute myocardial infarction. J Clin Cardiovasc Dis 2017;5:423-6.
- Ban E, Chae DK, Song EJ. Determination of micro-RNA in cardiomyoblast cells using CE with LIF detection. Electrophoresis 2013;34(4):598-604.
- Li Y, Ren X, Lio C, Sun W, Lai K, Liu Y, et al. A chlorogenic acid-phospholipid complex ameliorates post-myocardial infarction inflammatory response mediated by mitochondrial reactive oxygen species in SAMP8 mice. Pharmacol Res 2018;130:110-22.
[Crossref] [Google Scholar] [PubMed]
- Wang ZH, Sun XY, Li CL, Sun YM, Li J, Wang LF, et al. miRNA-21 expression in the serum of elderly patients with acute myocardial infarction. Med Sci Monit 2017;23:5728.
[Crossref] [Google Scholar] [PubMed]
- Ferrandi C, Ballerio R, Gaillard P, Giachetti C, Carboni S, Vitte PA, et al. Inhibition of c-Jun N-terminal kinase decreases cardiomyocyte apoptosis and infarct size after myocardial ischemia and reperfusion in anaesthetized rats. Br J Pharmacol 2004;142(6):953-60.
[Crossref] [Google Scholar] [PubMed]
- Tien YC, Lin JY, Lai CH, Kuo CH, Lin WY, Tsai CH, et al. Carthamus tinctorius L. prevents LPS-induced TNFα signaling activation and cell apoptosis through JNK1/2-NFκB pathway inhibition in H9c2 cardiomyoblast cells. J Ethnopharmacol 2010;130(3):505-13.
[Crossref] [Google Scholar] [PubMed]
- Jiao KU, Chen Xi, Kong T. Deubiquitinating enzyme USP14 regulates H2O2-induced oxidative stress injury in H9C2 cells. Chin J Pathophysiol 2017;33(7):1209-13