- *Corresponding Author:
- Y. Xiong
Department of Infection and Immunity, Jiangxi Provincial Chest Hospital, Nanchang, Jiangxi 330006, China
E-mail: 2534530920@qq.com
| This article was originally published in a special issue, “Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(1) Spl Issue “67-71” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
We aimed to investigate the expression and significance of Epstein-Barr virus-latent membrane protein 1 in human immunodeficiency virus-related diffuse large B-cell lymphomas. 22 cases of human immunodeficiency virus-related diffuse large B-cell lymphomas samples were collected. The control group consisted of 7 cases of non-human immunodeficiency virus-related diffuse large B-cell lymphomas, 6 cases of human immunodeficiency virus-reactive hyperplasia and 6 cases of non-human immunodeficiency virus. The expression of Epstein-Barr virus-latent membrane protein 1 was detected by immunohistochemistry and analyzed in combination with clinicopathology characteristics. The total positive rate of Epstein-Barr virus-latent membrane protein 1 was 77.3 % in human immunodeficiency virus-related diffuse large B-cell lymphomas and 71.4 % in non-human immunodeficiency virus-related diffuse large B-cell lymphomas. Most related diffuse large B-cell lymphomas cells showed nuclear staining pattern and the nuclear positive rate was 82.4 % in human immunodeficiency virus-related diffuse large B-cell lymphomas and 60 % in non-human immunodeficiency virus-related diffuse large B-cell lymphomas. There was no significant difference between human immunodeficiency virus-related diffuse large B-cell lymphomas and non-human immunodeficiency virus-related diffuse large B-cell lymphomas. The expression of Epstein-Barr virus-latent membrane protein 1 was significant between human immunodeficiency virus-related diffuse large B-cell lymphomas and benign lesion. Epstein-Barr virus-latent membrane protein 1 expression in non-germinal center B cell human immunodeficiency virus-related diffuse large B-cell lymphomas was significantly higher than that in germinal center B cell human immunodeficiency virus-related diffuse large B-cell lymphomas. However, Epstein-Barr virus-latent membrane protein 1 expression had no correlation with sex, age, location of tumor and clinical stage (p>0.05). Epstein-Barr virus-latent membrane protein 1 was mainly located in nuclear and overexpressed in human immunodeficiency virus-related diffuse large B-cell lymphomas and non-human immunodeficiency virus-related diffuse large B-cell lymphomas, suggesting that Epstein-Barr virus-latent membrane protein 1 overexpression was involved in tumourogenesis of related diffuse large B-cell lymphomas. Epstein-Barr virus-latent membrane protein 1 expression was correlated with immunophenotype of related diffuse large B-cell lymphomas. As the prognosis was different in different immunophenotype subgroups, Epstein-Barr virus-latent membrane protein 1 may be the potential marker of prognosis for human immunodeficiency virus-related diffuse large B-cell lymphomas.
Keywords
Acquired immune deficiency syndrome, B-cell lymphoma, Epstein-Barr virus, lymphoma, immunophenotype
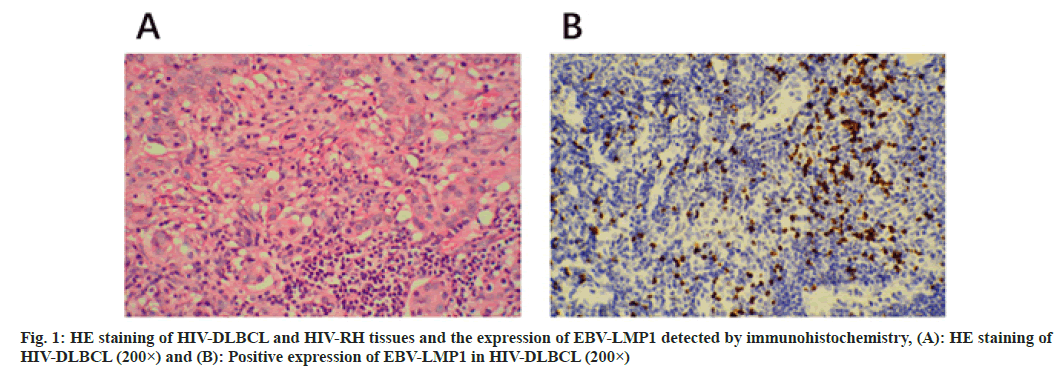
Acquired Immune Deficiency Syndrome (AIDS) is a chronic immune deficiency disease and patients are infected with Human Immunodeficiency Virus (HIV)[1]. Individuals with AIDS disorders can become vulnerable to ubiquitous viruses and neoplasms ensue[2]. HIVpositive patients have a 60 to 200 fold increase incidence of non-Hodgkin’s lymphomas compared to healthy people[3]. There is approximately 10 % of AIDS patients with malignancy is non-Hodgkin’s lymphoma. Furthermore, approximately 75 % of non-Hodgkin's lymphoma is invasive Diffuse Large B Cell Lymphoma (DLBCL) in HIV-positive patients[4]. DLBCL is identified into two types as Activated B Cell-like (ABC) and Germinal Center B Cell-like (GCB) due to arise by distinct mechanisms[5]. Epstein-Barr Virus (EBV) is composed of linear double stranded Deoxyribonucleic Acid (DNA) molecules encoding more than 85 genes, which is related to different subtypes of lymphoma[6]. The pathogenesis of EBV associated lymphomas is considered to be a complex interaction between different cellular genetic changes and viral gene expression profiles[7]. Although chemotherapy is effective enough for EBV related malignancies, as many as half of Hodgkin's lymphoma survivors die of complications caused by chemotherapy, secondary malignancies or recurrence[8]. Latent Membrane Proteins (LMP) are essential for the transformation and survival of infected cells into permanently proliferating cells[9]. The cytoplasmic fragment of LMP-1 contains three domains and their induction involves Nuclear Factor-kappa B (NF-κB) pathway[10]. EBV-LMP1 is necessary for the transformation of B lymphocytes into proliferative lymphoblastic cell line[11]. The high expression of EBV is associated with cancer and lymphoma[12]. In addition, EBV-LMP1 transfection led to transient cell proliferation of primary B cells in vitro. It suggested that up-regulation of EBV-LMP1 may be caused by HIV-1 integration in B cell lymphoma. In this study, the expression of EBV-LMP1 protein in AIDS-related DLBCL tissues was detected by immunohistochemical method. We aimed to explore whether there is a correlation between EBV-LMP1 and AIDS-related DLBCL and provide mechanism for further research on the pathogenesis of ARL. In this work, we collected paraffin specimens from several hospitals in Jiangxi Provincial Chest Hospital province from January 2020 to July 2021. A total of 22 cases of HIV-DLBCL as the experiment group and 18 cases into 3 groups as our control groups, group A consisted of 7 cases of HIV-negative DLBCL (non-HIV-DLBCL), group B consisted of 5 cases of HIV-Reactive Hyperplasia (HIV-RH) and group C consisted of 6 cases of HIV-RH. Among them, the experiment group and the group A patients had never received any HIV-related treatment. The HE slices were checked by senior according to the World Health Organization (WHO) classification of tumors hematopoietic and lymphoid tissues. The 22 cases of patients in our experiment group contained 16 males and 6 females, ranged from 29 to 66, including of 5 cases more than 60, 17 cases of patients less than 60 y old. Sixteen patients of which were located in lymph nodes and 6 cases were in extra nodal areas. With 6 of them in GCB, the others were non-GCB. There were 8 of which distributed in the stage I~II, 14 in III~IV. In the control group, patients consist of 10 males and 5 females ranged from 27 y to 72 y old. Tissues preserved and paraffin embedding was suitable for use. The tissues were cut into sections of 4 μm, mounted onto slides and at 65° for 1 h to dry. Prior to staining, tissue slides must be deparaffinized to remove embedding medium and rehydrated at room temperature (20°-25°). Avoid incomplete removal of paraffin; residual embedding medium would result in increased nonspecific staining. Heat mediated antigen retrieval at pH 6 (citrate) in 0.01 mol/l. This study was optimized with the Envision™ for IHC. EBV-LMP1 (Abcam), horseradish peroxidase-conjugated secondary antibodies, blocking serum and DAB color development kit were purchased from Dako. Xylene, ethanol, sodium chloride, disodium hydrogen phosphate, potassium dihydrogen phosphate, potassium chloride, trisodium citrate, citric acid, formaldehyde, hydrogen peroxide and other reagents were purchased from Fengchuan chemical reagent technology Co. Ltd. (Tianjin). PBS and the positive EBV-LMP1 of pancreas sections were arranged for negative control and positive control. When observed, the clear background with brown particles in the cytoplasm and nucleus view of the staining in a microscope (magnification 400×) were identified as the EBV-LMP1 in positive, the number of cells in positive was quantified by manual counting and photographed with a microscopy in five non-overlapping views. The positive results determined by the percentage of positive (≥5 %). All the experiment were performed in triplicate. All statistical analyses were performed using IBM Statistical Package for the Social Sciences (SPSS) statistics software. Grouped data were expressed as the mean±Standard Error of the Mean (SEM). The significance was analyzed by one-way Analysis of Variance (ANOVA). These symbols represent the levels of statistical significance within each analysis; *p value 0.01e0.05, **p value 0.001e0.01 and ***p<0.001. The positive expression rate of EBV-LMP1 in HIV-DLBCL was 77.3 % and the positive expression rate of EBV-LMP1 in non-HIV-DLBCL of 7 cases in control group A was 71.4 %. The results indicated that EBV-LMP1 was activated and overexpressed in HIV-DLBCL and non-HIV-DLBCL, but the expression difference was not statistically significant (p>0.05) (Table 1). The expression difference of EBV-LMP1 between HIV-DLBCL and RH of benign lesions in the two groups was statistically significant (p<0.05, p<0.01) as shown in fig. 1 and Table 1. In the positive cases of EBV-LMP1 in HIV-DLBCL, 82.4 % were mainly expressed in the nucleus of tumor cells, 11.8 % in the cytoplasm and 17.6 % in the cytoplasm and nucleus simultaneously. In the non-HIV-DLBCL cases with EBV-LMP1 positive, 60 % of them expressed mainly in the nucleus of tumor cells and 40 % expressed both in the cytoplasm and nucleus. EBV-LMP1 was mainly localized in the nucleus of DLBCL in both groups; there was no significant difference in the nuclear positive rate as shown in Table 2. The expression of EBV-LMP1 was related to the immunophenotype of HIV-DLBCL (p<0.01). The expression rate of EBV-LMP1 in non- GCB-DLBCL was significantly higher than that in GCB-DLBCL, but the expression of EBV-LMP1 was not significantly related to the sex, age, location of disease and clinical stage of tumor in HIV-DLBCL patients (p>0.05) as shown in Table 3. DLBCL is a heterogeneous disease, including intermediate and high-grade B lymphoma with different molecular background, clinical course and therapeutic response[13]. The molecular pathogenesis of DLBCL is complex comprising chromosome translocation, somatic mutation, proto-oncogene amplification, tumor suppressor gene inactivation[14]. In addition, DLBCL is resistant to chemotherapy, and has the characteristics of preventing and treating relapse/refractory[15]. EBV is a critical carcinogen promoting angiogenesis in many human tumors[16]. Up regulation of virus induced by angiogenic factors in virus mediated malignant tumors play an important role in tumor progression. LMP1 is expressed in many neoplasias-like nasopharyngeal cancer and Burkitt’s lymphoma cases, which play a role by inducing the expression of angiogenic factors such as Vascular Endothelial Growth Factor (VEGF), Interleukin (IL)-6 and IL-8 in potentially infected tumor cells[17,18]. Furthermore, EBV would cause epithelial cytolytic infection and B lymphocyte latent infection[16]. EBV-LMP1 is overexpressed in many types of lymphoma, but the mechanism in HIV-related DLBCL is still unclear. It has been reported that in 177 patients, 14 % subjects had the EBV positivity; 61 % subjects had VEGF-A expression. The results indicated a consistence between the expression of EBV and the VEGF-A level[16]. In addition, the adverse outcomes of primary central nervous system lymphomas patients are lack the expression of EBV-LMP1 which is similar to those reported for DLBCL[19,20]. Our results found that the positive expression rate of EBV-LMP1 in non- HIV-DLBCL patients was 77.3 % and the nuclear expression was 60 %, which was consistent with previous studies. In this study, we found that the positive expression rate of EBV-LMP1 in HIV-related DLBCL was 77.3 % and the expression rate of EBVLMP1 in nucleus was 82.4 %, which was significantly different from that in benign lesions. The expression rate of EBV-LMP1 in non-GCB was significantly higher than that in GCB type of HIV-related DLBCL. Our results indicated that targeting EBV-LMP1 signaling pathway has beneficial effects on DLBCL therapy. These results indicate that EBV-LMP1 is critical to maintain the pathophysiology of B cell-like DLBCL and EBV-LMP1 inhibition may provide a promising method in DLBCL treatment. In this study, EBV-LMP1 is highly expressed in both HIV-related DLBCL and non-HIV-related DLBCL, and mainly expressed in the nucleus, suggesting that EBV-LMP1 activation is related to the occurrence of DLBCL. In addition, the targeted inhibition of EBV-LMP1 may be a promising approach for the therapy of HIV-related DLBCL. However, a limitation of this study is that the HIV-related DLBCL patients is relatively small, which limits the analysis of EBV-LMP1 expression in HIV positive and negative tumors. Therefore, further investigations are warranted to analyze the molecular level of EBV-LMP1 in the pathogenesis of HIV-related DLBCL and non-HIV-related DLBCL.
| n | Histologic subtype n (%) | Positive rate (%) | p value | ||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| HIV-DLBCL | 22 | 17 | 6 | 77.3 | |
| Non-HIV-DLBCL | 7 | 5 | 3 | 71.4 | 0.69 |
| HIV-RH | 5 | 0 | 5 | 0 | 0.01 |
| Non-HIV-RH | 6 | 0 | 6 | 0 | 0.003 |
Table 1: The Expression of EBV-LMP1 in HIV-DLBCL and Control Samples
| n | Positive signal positioning | Positive rate (%) | p value | |||
|---|---|---|---|---|---|---|
| Nucleus | Cytoplasm | Nucleus+cytoplasm | ||||
| HIV-DLBCL | 17 | 14 | 2 | 3 | 82.4 | 0.259 |
| Non-HIV-DLBCL | 5 | 3 | 0 | 3 | 60 | |
Table 2: The Localization of EBV-LMP1 in Positive Cells
| n | EBV-LMP1 expression | p value | ||
|---|---|---|---|---|
| Positive | Negative | |||
| Sex | 0.584 | |||
| Male | 16 | 13 | 3 | |
| Female | 6 | 4 | 2 | |
| Age | 1 | |||
| >60 | 5 | 3 | 2 | |
| ≤60 | 17 | 13 | 4 | |
| Pathogenic site | 0.624 | |||
| Nodal | 16 | 12 | 4 | |
| Extra nodal | 6 | 4 | 2 | |
| Immunophenotype | 0.004 | |||
| GCB | 6 | 2 | 5 | |
| Non-GCB | 16 | 15 | 1 | |
| Clinical stages | 0.626 | |||
| Phase I~II | 8 | 7 | 1 | |
| Phase III~IV | 14 | 9 | 5 | |
Table 3: The Correlation between EBV-LMP1 Expression and Clinicopathological Factors of HIV-DLBCL
Funding:
This study was supported by the Natural Science Foundation of Jiangxi Province (20202BABL206085).
Conflict of interests:
The authors declared no conflict of interests.
References
- Wang J, Lin HS, Liu MY, Li Y. Immune reconstitution of acquired immune deficiency syndrome. Chin J Integr Med 2010;16(6):557-64.
[Crossref] [Google Scholar] [PubMed]
- Purtilo DT, Manolov G, Manolova Y, Harada S, Lipscomb H. Squamous-cell carcinoma, Kaposi's sarcoma and Burkitt's lymphoma are consequences of impaired immune surveillance of ubiquitous viruses in acquired immune deficiency syndrome, allograft recipients and tropical African patients. IARC Sci Publ 1984;(63):749-70.
[Google Scholar] [PubMed]
- Castelli R, Schiavon R, Preti C, Ferraris L. HIV-related lymphoproliferative diseases in the era of combination antiretroviral therapy. Cardiovasc Haematol Dis Drug Targets 2020;20(3):175-80.
[Crossref] [Google Scholar] [PubMed]
- Nganga EC, Gitau S. Spectrum of imaging findings in AIDS-related diffuse large B cell lymphoma. Insights Imaging 2020;11(1):67.
[Crossref] [Google Scholar] [PubMed]
- Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015;21(8):922-6.
[Crossref] [Google Scholar] [PubMed]
- Abusalah MA, Gan SH, Al-Hatamleh MA, Irekeola AA, Shueb RH, and Yean YC. Recent advances in diagnostic approaches for Epstein–Barr virus. Pathogens 2020;9(3):226.
[Crossref] [Google Scholar] [PubMed]
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70(3):145-64.
[Crossref] [Google Scholar] [PubMed]
- Cohen JI, Fauci AS, Varmus H, Nabel GJ. Epstein-Barr virus: An important vaccine target for cancer prevention. Sci Transl Med 2011;3(107):107fs7.
[Crossref] [Google Scholar] [PubMed]
- Ayee R, Ofori ME, Wright E, Quaye O. Epstein Barr virus associated lymphomas and epithelia cancers in humans. J Cancer 2020;11(7):1737-50.
[Crossref] [Google Scholar] [PubMed]
- Sun L, Zhao Y, Shi H, Ma C, Wei L. LMP-1 induces survivin expression to inhibit cell apoptosis through the NF-κB and PI3K/Akt signaling pathways in nasal NK/T-cell lymphoma. Oncol Rep 2015;33(5):2253-60.
[Crossref] [Google Scholar] [PubMed]
- Kim SY, Kim JE, Won J, Song YJ. Characterization of the rapamycin-inducible EBV LMP1 activation system. J Microbiol 2015;53(10):732-8.
[Crossref] [Google Scholar] [PubMed]
- Huang YC, Lin SJ, Lin KM, Chou YC, Lin CW, Yu SC, et al. Regulation of EBV LMP1-triggered EphA4 downregulation in EBV-associated B lymphoma and its impact on patients’ survival. Blood 2016;128(12):1578-89.
[Crossref] [Google Scholar] [PubMed]
- Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: An analysis of the surveillance, epidemiology and end results database. Am J Hematol 2014;89(3):310-4.
[Crossref] [Google Scholar] [PubMed]
- Ollila TA, Olszewski AJ. Extranodal diffuse large B cell lymphoma: Molecular features, prognosis and risk of central nervous system recurrence. Curr Treat Options Oncol 2018;19(8):1-20.
[Crossref] [Google Scholar] [PubMed]
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Am Soc Hematol Educ Program 2011;2011(1):498-505.
- Paydas S, Ergin M, Erdogan S, Seydaoglu G. Prognostic significance of EBV-LMP1 and VEGF-A expressions in non-Hodgkin's lymphomas. Leukemia Res 2008;32(9):1424-30.
[Crossref] [Google Scholar] [PubMed]
- Wakisaka N, Kondo S, Yoshizaki T, Murono S, Furukawa M, Pagano JS. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1α. Mol Cell Biol 2004;24(12):5223-34.
[Crossref] [Google Scholar] [PubMed]
- Hong GK, Kumar P, Wang L, Damania B, Gulley ML, Delecluse HJ, et al. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J Virol 2005;79(22):13984-92.
[Crossref] [Google Scholar] [PubMed]
- Petrilli G, Lorenzi L, Paracchini R, Ubiali A, Schumacher RF, Cabassa P, et al. Epstein–Barr virus–associated adrenal smooth muscle tumors and disseminated diffuse large B-cell lymphoma in a child with common variable immunodeficiency: A case report and review of the literature. Int J Surg Pathol 2014;22(8):712-21.
[Crossref] [Google Scholar] [PubMed]
- Radotra BD, Parkhi M, Chatterjee D, Yadav BS, Ballari NR, Prakash G, et al. Clinicopathological features of primary central nervous system diffuse large B cell lymphoma: Experience from a Tertiary Center in North India. Surg Neurol Int 2020;11:424.
[Crossref] [Google Scholar] [PubMed]