- *Corresponding Author:
- Shucai Yang
Department of Clinical Laboratory, Pingshan Hospital, Southern Medical University (Pingshan District People’s Hospital of Shenzhen), Shenzhen, Guangdong 518118, People's Republic of China
E-mail: 1155045115@link.cuhk.edu.hk
| Date of Received | 12 August 2022 |
| Date of Revision | 04 June 2023 |
| Date of Acceptance | 27 May 2024 |
| Indian J Pharm Sci 2024;86(3):1132-1137 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study investigates the expression of long non-coding RNA hypoxia-inducible factor-1 alpha-antisense 1 in albino rats with type 2 diabetes mellitus and its correlation with bone mineral density and bone metabolism markers. The research included comparing type 2 diabetes mellitus albino rats with healthy controls. The diabetic model was confirmed by significant weight loss and elevated blood glucose levels after a high-fat diet and streptozotocin injection. Serum analysis showed that diabetic rats had significantly lower calcium levels and higher alkaline phosphatase levels compared to controls. Bone mineral density was measured using dual-energy X-ray absorptiometry before and after ovariectomy in two groups of rats. Post-ovariectomy, control group exhibited a significant reduction in bone mineral density, confirming the establishment of an osteoporosis model. Reverse transcription polymerase chain reaction analysis revealed that long non-coding RNA hypoxia-inducible factor-1 alpha-antisense 1 expression was significantly lower in diabetic rats compared to controls. These findings suggest that long non-coding RNA hypoxia-inducible factor-1 alpha-antisense 1 is inhibited in diabetic osteoporotic rats and may play a role in the bone metabolism alterations observed in type 2 diabetes mellitus.
Keywords
Long non-coding RNA hypoxia-inducible factor-1 alpha-antisense 1, type 2 diabetes mellitus, bone mineral density, osteoporosis, reverse transcription, polymerase chain reaction
Despite growing awareness, the prevalence of Diabetes Mellitus (DM) continues to rise, posing a significant global health challenge. According to Saeedi et al.[1], about 463 million individuals were affected by DM in 2019, with forecasts representing a rush to 700 million persons by 2045[1,2]. Type 2 DM (T2DM), instituting over 90 % of all diabetes cases, signifies a main hormonal metabolic disorder categorized by raised blood glucose levels due to insulin deficiency and dysfunction[3]. Diabetes Induced Osteoporosis (DOP) develops as a DM complication, primarily stemming from long-term disruptions in carbohydrate, fat, and protein metabolism[4]. DOP reveals through lessened bone mass, reduced trabecular connectivity, compromised microstructural integrity, cortical thinning, and sensitive fracture susceptibility[5]. Present management tactics for DOP comprise of dietary alterations and physical activity involvements with a combination of anti-osteoporosis agents and hypoglycemic medications[6].
Growing evidence recommends that T2DM patients display compromised cortical bone microarchitecture and trabecular, influencing them to ruptures or fractures or fissures risk by influencing hormonal variations, osteogenic difference, and Calcium (Ca) imbalance[7,8]. Accordingly, there is a pressing requirement to report fissure prevention and osteoporosis in diabetic patients[9]. However, the particular mechanisms underlying DOP and the interaction between bone metabolism and raised sugar levels remain vague.
Hypoxia-Inducible Factor-1 (HIF-1), a potent transcriptional activator, has been associated in controlling osteogenic variation and contributing to the pathogenesis of DM and its complications[10-13]. Emerging studies have identified long non-coding RNA HIF-1 Alpha-Antisense 1 (lncRNA HIF1AAS1), a lncRNA regulated by HIF-1α, which plays a role in osteogenic differentiation[14]. However, there is a dearth of research on the relevance of lncRNA HIF1A-AS1 in rodent models of T2DM combined with DOP. Therefore, our study aims to investigate the expression levels of lncRNA HIF1A-AS1 in albino rats with T2DM compared to healthy controls and its correlation with Bone Mineral Density (BMD) and bone metabolism markers.
Materials and Methods
Animals:
The research involved 20 adult albino rats, with an initial weight ranging from (180-220) g, accommodated under standard boarding conditions. These conditions included a room temperature maintained at 22°-25°, a regular light/ dark cycle, and unrestricted access to food and water ad libitum. The study received approval from the Ethical Committee of Pingshan District Peoples’s Hospital of Shenzhen.
Induction of T2DM:
Twenty albino rats were randomly allocated into two groups, with each group consisting of ten rats. The control group received a standard rat diet and intraperitoneal injections of 2 ml of 0.05 M citrate buffer. In contrast, the diabetic group underwent overnight fasting and induction of diabetes via a single intraperitoneal injection of Streptozotocin (STZ) (Sigma, United States of America (USA)) at a dose of 40 mg/kg body weight, dissolved in 2 ml of 0.05 M citrate buffer[15]. STZ, a glucosaminenitrosourea compound, acts as a chemotherapeutic agent and irreversibly damages pancreatic β-cells through Deoxyribonucleic Acid (DNA) alkylation and free radical generation, leading to the onset of T2DM[16,17]. Following STZ administration, the rats were provided ad libitum access to food and water, along with a 5 % glucose solution after 6 h to prevent hypoglycemic shock. The diabetic condition was confirmed by measuring fasting blood glucose levels from the rat tail 72 h post-STZ injection, with rats exhibiting blood glucose levels exceeding 150 mg/dl classified as diabetic [18]. Subsequently, the rats were maintained on a standard rat diet for duration of 8 w.
Establishment of DOP rat model:
Prior to modeling, BMD assessments were conducted using dual-energy X-ray absorptiometry (NORLAND Corporation, USA)[19]. Subsequently, ovariectomy was performed on rats in the control group to induce the DOP model. Briefly, rats were anesthetized with 3 % pentobarbital sodium (30 mg/kg; Sigma-Aldrich, USA) prior to the operation and immobilized. The surgical area was prepared by shaving the hair and making an incision in the skin, followed by removal of the ovaries through a retroperitoneal approach. Upon ligating blood vessels for hemostasis, the incision was closed layer by layer. Postoperatively, 80×104 U penicillin (Batch No: S100824, Shanghai Xianfeng Pharmaceutical Co., Ltd., China) was administered intramuscularly twice daily for 3 consecutive days to prevent infection. Conversely, rats in the diabetic group did not undergo any surgical intervention. After an 8 w period, BMD measurements were repeated using dual-energy X-ray absorptiometry to verify the successful establishment of the DOP model in the rats.
Biochemical analysis:
Biochemical analysis was conducted following the manufacturer’s protocols using a spectrophotometer (Biosystems, USA). The commercial kits utilized included those for assessing serum levels of Alkaline Phosphatase (ALP) and Ca.
Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) for lncRNA HIF1AAS1:
Femoral tissues from both groups were initially treated with 2-3 ml of TRIzol (Invitrogen, Carlsbad, California, USA), then cut into pieces and thoroughly ground into homogenate powder. The resulting samples were transferred into 1.5 ml Eppendorf tubes (EP) (Hamburg, Germany) and incubated at room temperature for 5 min to ensure complete lysis. Total Ribonucleic Acid (RNA) extraction was performed using the following procedure.
Nucleic acid extraction: Serum RNA was extracted utilizing a blood RNA extraction kit and an automated nucleic acid extractor.
Complementary DNA (cDNA) synthesis: A 10 μl reverse transcription reaction system was prepared on ice, comprising 2.0 μl of 5× Prime Script RT Master Mix, 400 ng of RNA, and Diethyl Pyrocarbonate (DEPC) water. RT was conducted at 37° for 15 min, followed by 85° for 1 min.
qRT-PCR reaction: The reaction mixture was prepared on ice, and 20 μl was dispensed into a 96-well plate. Each reaction contained cDNA (2 μl), qPCR SYBR green mix (10 μl), upstream and downstream primers (0.8 μl each), and doubledistilled Water (ddH2O) (6.4 μl). The Applied Biosystems PCR machine was employed for the reaction under the following conditions; predenaturation at 95° for 1 min, followed by 40 cycles of denaturation at 95° for 15 s, annealing at 60° for 30 s and extension at 72° for 40 s.
Data analysis: Following the reaction, a melt curve analysis was conducted, and the Cycle threshold (Ct) value was determined as the cycle number at which the signal reached the set threshold. Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) expression served as an internal reference to establish a standard curve. LncRNA HIF1A-AS1 expression was calculated using the ΔCt method, where ΔCt=ΔCt (LncRNA HIF1AAS1)- ΔCt (GAPDH).
Statistical analysis:
Data processing and analysis for this study were conducted using Statistical Package for the Social Sciences (SPSS) 22.0 and GraphPad Prism 5.0 software. Normal distribution tests were performed on all continuous variables, and the results were presented as "x±SD" (mean±Standard Deviation).
Results and Discussion
After randomly selecting 20 albino rats, their BMD was measured using dual-energy X-ray absorptiometry (Table 1). Subsequently, all rats were divided randomly into two groups; the control group (n=10) and the diabetic group (n=10). Rats in the control group underwent ovariectomy, while those in the diabetic group did not receive any operative treatment. Following an 8 w period, BMD was measured again using dual-energy X-ray absorptiometry (Table 1). The findings indicated that BMD in the control group after ovariectomy was significantly lower than that before the procedure. Furthermore, BMD in the control group post-ovariectomy was notably lower than that of the diabetic group, with statistically significant differences observed (p<0.05). These results suggest the successful establishment of the osteoporosis model in rats.
| Group | BMD before ovariectomy (g/cm2) | BMD at 8 w after ovariectomy (g/cm2) |
|---|---|---|
| Control | 0.40±0.002 | 0.40±0.003 |
| Diabetic | 0.40±0.004 | 0.21±0.008* |
Note: *p<0.05 in comparison of BMD in control group after ovariectomy with diabetic group
Table 1: Measurement of BMD Before and After Ovariectomy in Both Groups
In the T2DM model group, rats exhibited a significant reduction in body weight compared to the control group from 4 w to 8 w, indicative of diabetic weight loss (Table 2). Additionally, the blood glucose levels of rats in the diabetic group were higher than those in the control group after 4 w of high-fat diet feeding and STZ injection (Table 2).
| Group | Body weight (g) | Blood glucose levels (mg/dl) | ||||
|---|---|---|---|---|---|---|
| 0 w | 4 w | 8 w | 0 w | 4 w | 8 w | |
| Control | 205.20±10.58 | 210.65±14.10 | 230.14±10.42 | 80.34±2.55 | 85.67±3.54 | 88.84±5.78 |
| Diabetic | 208.55±12.22 | 195.24±16.05* | 171.21±13.18* | 180.67±6.58* | 210.50±8.99* | 230.34±10.48* |
Note: *p<0.05 vs. control group
Table 2: Measurement of Body Weight and Blood Glucose Levels in Both Groups
Evaluation of serum levels of Ca and ALP revealed that diabetic rats had significantly (p<0.05) lower serum Ca levels and significantly (p<0.05) higher ALP levels compared to control rats (Table 3).
| Group | Ca levels (mol/l) | ALP (U/l) |
|---|---|---|
| Control | 11.28±0.14 | 101.31±3.20 |
| Diabetic | 6.55±0.12* | 142.66±9.52* |
Note: *p<0.05 vs. control group
Table 3: Biochemical Parameters Assessed in Both Groups on 8 W
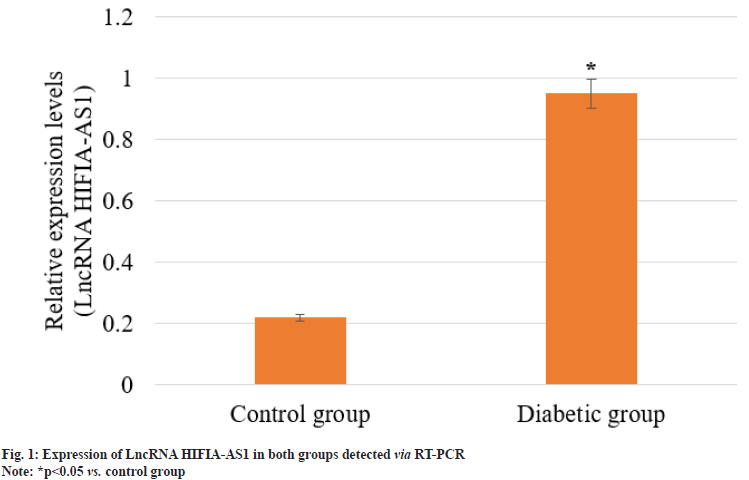
Femoral tissues were extracted from 4 rats in each group, smashed and mixed evenly, followed by extraction of total RNA. The expression of lncRNA HIFIA-AS1 in control group and diabetic group was detected via RT-PCR. As shown in fig. 1, the expression of lncRNA HIFIA-AS1 in control group was significantly declined when compared with diabetic group, and the difference was statistically significant (p<0.05). This indicated that the expression of lncRNA HIFIA-AS1 was significantly inhibited in DOP rats.
DOP, a severe complication affecting the skeletal system, represents a form of secondary osteoporosis characterized by diminished bone mass, compromised microstructure and increased susceptibility to fractures. Studies indicate that individuals with diabetes face a heightened risk of reduced bone mass and osteoporosis, a trend exacerbated by improving living standards and dietary changes. The multifaceted pathogenesis of DOP involves factors such as hyperglycemia, oxidative stress, and the accumulation of advanced glycation end-products, compounded by adverse effects of certain antidiabetic medications on bone formation. Additionally, due to subtle early symptoms and limited treatment options, DOP awareness among patient’s remains low, leading to significant disability rates and financial problems[20]. Existing diagnostic procedures for DOP, chiefly depend on DXA and CT scans, present challenges due to operational complexities, and equipment costs, potential measurement variations, that established an urgent need to find reliable biomarkers [21].
Sustaining bone homeostasis relies on a gentle balance between osteoclastic bone formation and resorption. The elevated serum sugar levels in DM can interrupt this balance and contribute to osteoporosis[21]. LncRNAs are decisive regulators of various cellular processes, which gathered much attention for their contribution in DOP particularly in osteoblast mineralization and proliferation[22-24]. A key transcriptional regulator, HIF-1α has been widely studied in orthopedics, influencing osteoblast variation in numerous bone-related ailments. In addition, lncRNA HIF1A-AS1 originating from the 5' region of the HIF-1α gene[25], displays ability in understanding osteoporosis[26-28]. In this study, a statistically significant (p<0.05) higher expression levels of lncRNA HIF1A-AS1 exhibited in the diabetic group, compared to control group (fig. 1). BMD and serum bone metabolism markers, including Ca and ALP, are critical indicators of bone health. In our present study observation, we noticed that the diabetic group showed significantly (p<0.05) lesser BMD and Ca levels (Table 1 and Table 3), with ALP levels standing higher (Table 3). Further investigation specified positive correlations between lncRNA HIF1AAS1 and ALP (p<0.05) and negative correlations with BMD and Ca (p<0.05). These findings suggest that overexpression of lncRNA HIF1AAS1 in T2DM patients may prevent osteoblastic mineralization induced by high blood sugar levels, possibly leading to reduced BMD, improved bone resorption, and heightened osteoporosis risk. Hence, lncRNA HIF1A-AS1 appears as a crucial factor in preventing osteoblastic mineralization under diabetic conditions, with diagnostic implications justifying further examination and application.
In conclusion, our present study validates the expression of lncRNA HIF1A-AS1 in T2DM albino rats and its correlation with BMD and bone metabolism markers. A statistically significant higher expression level of lncRNA HIF1A-AS1 exhibited in the diabetic group, compared to control group, indicating its possible association in the altered bone metabolism related with T2DM. Also, the diabetic group showed significantly (p<0.05) lesser BMD and Ca levels (Table 1-Table 3), with ALP levels standing higher. Overall, these results shed light on the mechanisms underlying bone health in T2DM complications, including DOP, and may cover the approach for future therapeutic interventions targeting LncRNA HIF1A-AS1 to alleviate diabetic osteoporosis.
Funding:
This study was supported by grants from the Scientific Research Project of Shenzhen Pingshan District Health System (No: 202061 to Li Zhang).
Conflict of interests:
The authors declared no conflict of interests.
References
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract 2019;157:107843.
[Crossref] [Google Scholar] [PubMed]
- Tatipamula VB, Annam SS, Nguyen HT, Polimati H, Yejella RP. Sekikaic acid modulates pancreatic β-cells in streptozotocin-induced type 2 diabetic rats by inhibiting digestive enzymes. Nat Prod Res 2021;35(23):5420-4.
[Crossref] [Google Scholar] [PubMed]
- Liu C, Li C, Deng Z, Du E, Xu C. Long non-coding RNA BC168687 is involved in TRPV1-mediated diabetic neuropathic pain in rats. Neuroscience 2018;374:214-22.
[Crossref] [Google Scholar] [PubMed]
- Si Y, Wang C, Guo Y, Yin H, Yong MA. Prevalence of osteoporosis in patients with type 2 diabetes mellitus in the Chinese mainland: A protocol of systematic review and meta-analysis. Medicine 2020;99(16):e19762.
[Crossref] [Google Scholar] [PubMed]
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: Now and the future. Lancet 2011;377(9773):1276-87.
[Crossref] [Google Scholar] [PubMed]
- Ebeling PR, Nguyen HH, Aleksova J, Vincent AJ, Wong P, Milat F. Secondary osteoporosis. Endocr Rev 2022;43(2):240-313.
- Sheu A, Greenfield JR, White CP, Center JR. Contributors to impaired bone health in type 2 diabetes. Trends Endocrinol Metab 2023;34(1):34-48.
[Crossref] [Google Scholar] [PubMed]
- Murray CE, Coleman CM. Impact of diabetes mellitus on bone health. Int J Mol Sci 2019;20(19):4873.
[Crossref] [Google Scholar] [PubMed]
- Kurra S, Fink DA, Siris ES. Osteoporosis-associated fracture and diabetes. Endocrinol Metab Clin 2014;43(1):233-43.
[Crossref] [Google Scholar] [PubMed]
- Lee SH, Golinska M, Griffiths JR. HIF-1-independent mechanisms regulating metabolic adaptation in hypoxic cancer cells. Cells 2021;10(9):2371.
[Crossref] [Google Scholar] [PubMed]
- Prabhakar NR, Peng YJ, Nanduri J. Hypoxia-inducible factors and obstructive sleep apnea. J Clin Invest 2020;130(10):5042-51.
[Crossref] [Google Scholar] [PubMed]
- Catrina SB, Zheng X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021;64:709-16.
[Crossref] [Google Scholar] [PubMed]
- Zhu Y, Liu Q, Liao M, Diao L, Wu T, Liao W, et al. Overexpression of lncRNA EPB41L4A-AS1 induces metabolic reprogramming in trophoblast cells and placenta tissue of miscarriage. Mol Ther Nucl Acids 2019;18:518-32.
[Crossref] [Google Scholar] [PubMed]
- Xu J, Zhang Y, Chu L, Chen W, Du Y, Gu J. Long non‑coding RNA HIF1A‑AS1 is upregulated in intracranial aneurysms and participates in the regulation of proliferation of vascular smooth muscle cells by upregulating TGF‑β1. Exp Ther Med 2019;17(3):1797-801.
[Crossref] [Google Scholar] [PubMed]
- Killari KN, Polimati H, Prasanth DS, Singh G, Panda SP, Vedula GS, et al. Salazinic acid attenuates male sexual dysfunction and testicular oxidative damage in streptozotocin-induced diabetic albino rats. RSC Adv 2023;13(19):12991-3005.
- Talluri MR, Ketha A, Battu GR, Tadi RS, Tatipamula VB. Protective effect of Aurelia aurita against free radicals and streptozotocin-induced diabetes. Bangladesh J Pharmacol 2018;13(3):287-95.
- Tatipamula VB. Seaweed Chara baltica: Isolation, characterization and in vivo antidiabetic study. Brazil J Pharm Sci 2022;58:e19323.
- Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 2015;70(1):5-47.
[Crossref] [Google Scholar] [PubMed]
- Zheng S, Wang YB, Yang YL, Chen BP, Wang CX, Li RH, et al. LncRNA MALAT1 inhibits osteogenic differentiation of mesenchymal stem cells in osteoporosis rats through MAPK signaling pathway. Eur Rev Med Pharmacol Sci 2019;23(11):4609-17.
[Crossref] [Google Scholar] [PubMed]
- Aspray TJ, Hill TR. Osteoporosis and the ageing skeleton. Subcell Biochem 2019;91:453-76.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Yujiao W, Fang W, Linhui Y, Ziqi G, Zhichen W, et al. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol Res 2020;53(1):40.
- Liu X, Chen M, Liu Q, Li G, Yang P, Zhang G. LncRNA PTCSC3 is upregulated in osteoporosis and negatively regulates osteoblast apoptosis. BMC Med Genom 2022;15(1):57.
[Crossref] [Google Scholar] [PubMed]
- Chen T, Huo K, Kong D, Su S, Yang T, Zhang W, et al. Comprehensive analysis of lncRNA expression profiles in postmenopausal osteoporosis. Genomics 2022;114(5):110452.
[Crossref] [Google Scholar] [PubMed]
- Qian G, Yu Y, Dong Y, Hong Y, Wang M. LncRNA AWPPH is downregulated in osteoporosis and regulates type I collagen α1 and α2 ratio. Arch Physiol Biochem 2022;128(5):1297-301.
[Crossref] [Google Scholar] [PubMed]
- Hong F, Gao Y, Li Y, Zheng L, Xu F, Li X. Inhibition of HIF1A-AS1 promoted starvation-induced hepatocellular carcinoma cell apoptosis by reducing HIF-1α/mTOR-mediated autophagy. World J Surg Oncol 2020;18:1-8.
[Crossref] [Google Scholar] [PubMed]
- Xu F, Huang M, Chen Q, Niu Y, Hu Y, Hu P, et al. LncRNA HIF1A-AS1 promotes gemcitabine resistance of pancreatic cancer by enhancing glycolysis through modulating the AKT/YB1/HIF1α pathway. Cancer Res 2021;81(22):5678-91.
[Crossref] [Google Scholar] [PubMed]
- Li D, Weng S, Zeng K, Xu H, Wang W, Shi J, et al. Long non-coding RNAs and tyrosine kinase-mediated drug resistance in pancreatic cancer. Gene 2024;895:148007.
[Crossref] [Google Scholar] [PubMed]
- Gao J, Cao R, Mu H. Long non-coding RNA UCA1 may be a novel diagnostic and predictive biomarker in plasma for early gastric cancer. Int Clin Exp Pathol 2015;8(10):12936.
[Google Scholar] [PubMed]