- *Corresponding Author:
- S. Harakeh
Yousef Abdul Latif Jameel Scientific Chair of Prophetic Medicine Application, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
E-mail: sharakeh@gmail.com
| Date of Received | 7 September 2023 |
| Date of Revision | 14 March 2024 |
| Date of Acceptance | 29 April 2024 |
| Indian J Pharm Sci 2024;86(5):1575-1589 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Cancer has become one of the most devastating diseases which have become major source of morbidity and mortality. So this has put a lot of burden on the society and the general health sector. The causes of cancer can be attributed to heredity, environment, lifestyle/behavior, viruses and other potential carcinogens including bacteria. Several investigations have attempted to establish the relationship between bacterial infections and cancer development, but conclusive evidence remains elusive. Some investigators believe that bacteria can induce the production of potential carcinogens or genotoxins through the process of oxidation of bile acids and carbohydrates, and through the hydrolysis of other mutagenic precursors. Based on this reasoning, it is possible to note that several strains of bacteria can cause human cancers which include Streptococcus bovis, Escherichia coli, Bartonella, Salmonella typhi, Helicobacter pylori, Chlamydia pneumoniae, Borrelia burgdorferi and Clostridia species belonging to Ruminococcaceae. Current and past findings provide substantial evidence, supporting the etiological role of bacterial pathogens in tumor mutagenesis in humans. Although this area of research is still in its early stages and requires further detailed investigation, this review aims to elucidate various types of cancer associated with these carcinogenic bacterial species and their mutagenic mechanisms.
Keywords
Bacteria, carcinogenesis, colon cancer, tumor, Salmonella, Helicobacter pylori
Cancer has become one of the leading causes of death and morbidity in modern society worldwide[1]. It is known that several traits are attributed to the establishment and development of carcinogenesis[2]. Infectious agents are suspected to be among these factors. Infectious agents are the cause of up to 20 % of cancers worldwide, according to the American Cancer Society (ACS)[3]. The mutagenic interaction among different human cancers and many oncogenic viruses such as papillomavirus, hepatitis B virus, Epstein-Barr virus and bacteria such as Streptococcus bovis (S. bovis), Chlamydia pneumonia (C. pneumoniae), Escherichia coli (E. coli), Helicobacter pylori (H. pylori), Bartonella, Salmonella typhi (S. Typhi), Borrelia burgdorferi (B. burgdorferi) and Clostridia species, belonging to Ruminococcaceae has previously been well documented[4,5].
It has traditionally been believed that bacterial infections make up a small percentage of cancer cases. Bacteria, however have been associated with cancer by two cases, chronic inflammation and synthesis of mutagenic by-products[6]. Various studies have demonstrated that some bacterial species facilitate the process of carcinogenesis by the production of toxic by-products resulting from bile acid and carbohydrates metabolism, as well as through the hydrolysis of other carcinogenic precursors[7].
One of the most well-studied bacterial infections linked to cancer is H. pylori[8]. According to the International Agency for Research on Cancer (IARC), H. pylori is class I human gastric carcinogen. The mechanisms of action of bacterial mediated carcinogenesis are complex and encompass interplay, effecting on the physiology of the host cell, chronic inflammation and alterations in tissue stem cell homeostasis[9].
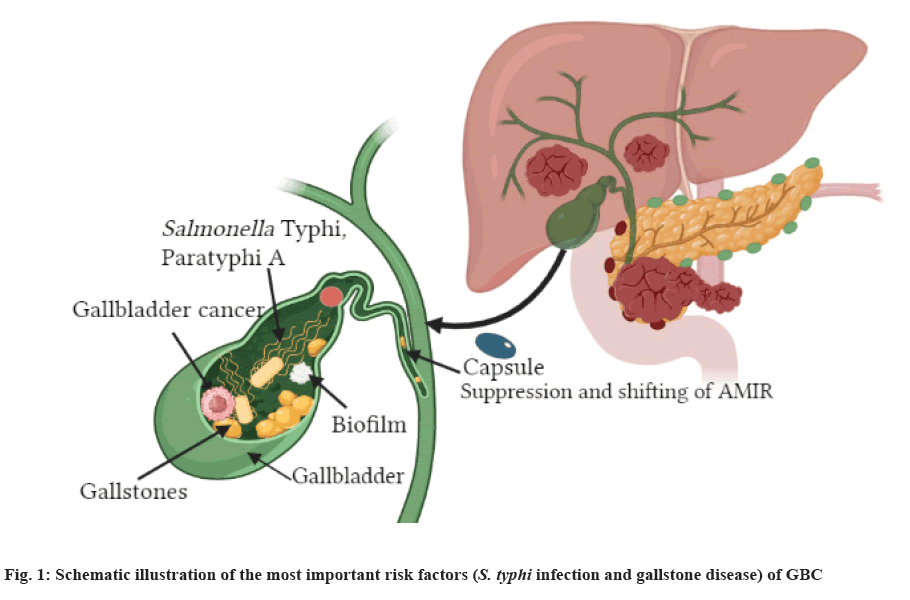
Gallbladder Cancer (GBC) is relatively infrequent in comparison to more common cancers of the colon, lungs, prostate or breast[10]. Bacterial strains like S. Typhi are responsible for GBC. However, the detail mechanism of action of how the organism could cause cancer and the cellular interaction between them is still in its initial stage.
According to the research findings, some people are more vulnerable to bacterial infections that lead to cancer development and certain cancers occur at higher incidence in certain populations. For instance, women are more likely to develop GBC than men in all populations[11]. Despite the fact that lung cancer is more prevalent in smokers, only a small percentage of smokers develop this disease[12]. Colon cancer being the 3rd most common cancer in the United States (US), patients with Inflammatory Bowel Disease (IBD) have far higher risk for Colorectal Cancer (CRC) than those without IBD[13]. In addition, relationship between chronic infectious agent and susceptible host and/or its immune response are thought to attribute to the development of cancer[14].
It has been reported that many bacteria are capable of causing persistent infections or producing toxins that disrupt the cell cycle, altering cell proliferation and development[15]. The resulting disturbance in cell growth and Deoxyribonucleic Acid (DNA) damage is identical to the damage caused by other mutagenic agents[16]. Most of these carcinogenic agents may either directly cause mutations (tumor initiators) or accelerating mutations (promoters). As cancer grows, blood flow to the region boosted, consequently results in the proliferation of blood vessels or angiogenesis. The most dangerous outcome of bacterial mediated tumorigenesis than other form of cancer is metastasis which occurs when cells lyse from tumor and disseminate cancer cells at distant sites[17].
This review explores the association between bacterial infection and different types of cancers. Besides, some of the proposed mechanisms of action of bacterial infections in the development of tumors have also been described. The bacterial pathogens which are particularly discussed in relation to cancer include S. Typhi, Paratyphi A, Typhimurium, S. bovis, Mycoplasma, Fusobacterium nucleatum (F. nucleatum), Chlamydia pneumonia and Bacteroides fragilis (B. fragilis).
Factors Contributing to Carcinogenesis
Bacterial factors:
Several research findings reveal that different bacterial factors such as enzymes, toxins or other metabolic products have been associated with the progression of cancers in humans. According to the report by Luu et al.[18] Clostridia species is known for producing an estrogen-metabolizing enzyme called Beta (β)-glucuronidases. This enzyme was found play substantial role in the development of breast cancer in humans[18]. Similarly, the Cytolethal Distending Toxin (CDT) produced by E. coli[19] and the downregulation of the expression of Cyclin-Dependent Kinase inhibitor 2A (CDKN2) gene by uropathogenic E. coli[20] have been linked with the progression of colorectal and prostate cancer in humans[19]. Further, it has been reported that CDT and Lipopolysaccharide (LPS) of S. Typhi facilitate the development of GBC in humans[19] while lipopeptides, Ribonucleic Acid (RNA) and DNA of E. coli contribute to the occurrence of esophageal cancer[21]. Activation of the host immune response and release of specific cytokines are the mechanisms by which LPS affects cancer development. The LPS mediated cytokines such as Interleukin (IL)-1β, IL-10 and Chemokine Receptor 5 (CCR5) induce chronic inflammatory response which can cause somatic mutations accompanied by higher incidence of carcinogenesis[22]. There are also other bacterial traits that are implicated in causing mutagenesis, including the synthesis of oncogenic bacterial proteins, like the Cytotoxin associated gene A (CagA) protein, encoded by the Cag gene in H. pylori, which leads to gastric adenocarcinoma through increased IL-8 production and proliferation of cells[19].
Moreover, it has been reported that micro (mi) RNAs are known to be the major mediators of the inflammatory process that enhance malignance via their adverse effect on the proliferation of cells, DNA methylation, mutation of DNA and cell death, causing carcinogenesis through host DNA damage[23]. Altogether, induction of chronic inflammatory response by some bacterial mutagenic agents can facilitate genomic transformation and accelerate the development of cancer.
Host factors:
In the last decade, a British microbiologist, proposed the idea that approximately 20 %-25 % of all cancers are caused by bacterial pathogens which arise from chronic inflammatory response associated with persistent infection[24,25].
Regarding factors associated with the host that induces carcinogenesis, it has been realized that constant release of cytokines, growth factors, reactive nitrogen and oxygen species from the inflammatory cells can affect the normal biological activities which eventually lead to genomic instability. The instability of genome in a cell finally results in the development of cancer[26].
The initiation of tumorigenesis by immune cells, inflammatory mediators and Pattern Recognition Receptors (PRRs) like Toll-Like Receptors (TLRs) has globally been reported in which the expression of TLR-1, 2, 3, 6, 7 and 9 in esophageal cancer has enhanced[19]. Besides, raised level of regulatory T cells (Treg) and T helper 17 (Th17) cells is encountered in CRC caused by Enterotoxigenic B. fragilis (ETBF) infection[27]. It has been also reported that pronounced expression of chemokine genes such as Chemokine Ligand 20 (CCL20) and increased activation of Th1 cells are linked with GBC, while increased synthesis of IL-8 and Prostaglandin E2 (PGE2) are linked with CRC caused by S. bovis infection[28].
Another host factor assisting bacterial tumorigenesis is cytokine production by immune cells. In this regard, for instance, cytokines such as Interferon Gamma (INF γ), IL-6, 8, IL-10 and Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF) have been reported in association with ovarian cancer linked with Mycoplasma genitalium infection[19].
Association of Bacterial Agents in Cancer
Bacterial agents (Salmonella typhi, Paratyphi AandTyphimurium):
The incidence of GBC varies widely across the globe[29]. It is also highly lethal with only diagnosis[30]. There are some evidences of chronic biliary infection with S. typhi, the agent that causes typhoid fever, also accounts to increase the risk of GBC[31]. According to a study published by Axelrod et al.[32], an individual with the history of typhoid fever for 30 y was diagnosed with GBC. Later S. typhi was isolated from the gallbladder wall and bile as well. Numerous retrospective investigations in Denmark, US and Scotland have provided evidence that chronic S. typhi disease individuals are at higher risk of death associated with GBC compared with the general population[33-35]. According to Saravanan et al.[36], Indian subcontinent is the place where the incidence of GBC is amongst the highest in the globe whereby Salmonella infection is substantially higher. Currently, numerous studies have stated strong relationship between GBC and S. typhi, where both GBC and typhoid fever are endemic. These relationships are not always correlated with S. typhi infection. Methods used to detect S. typhi in the GBC may not be sensitive enough. The serostatus of Vi antibodies do not necessarily indicate infection with S. typhi. Hence, the association between GBC and S. typhi remains unknown.
Chronic infection caused by S. typhi in GBC is linked with long-term bacteria excretion. Epidemiological investigations conducted in S. typhi endemic area, like Bolivia, Chile, Ecuador and some other areas such as Pakistan, India, Japan and Korea, have displayed that around 90 % of chronically sick individuals and this linkage in turn, infers as a predisposing agent for GBC development in humans[37].
GBC is one of the most common Gastrointestinal Tract (GIT) cancers and denotes one of the most prevalent biliary tracts tumorigeneses[38]. The global yearly prevalence of GBC is around 2 time among 100 000 individuals, with marked geographical and ethnic variations[39]. The uppermost prevalence rates were reported among American Indians, South Americans, Indian subcontinent, Korea and Pakistan. GBC is rare in various North American and European countries and the mortality rate is declining, though comparatively high incidence where mortality rate is still encountered in certain central European regions[38]. The malignancy has been linked with lifestyle and genetics, nevertheless GBC and S. typhi infection represent the prominent risk factors (fig. 1)[40]; size of gallstone increases the risk of GBC. When the size of the stones is >3 cm the risk is 10-fold bigger in comparison with smaller gallstones[41]. Robust epidemiological results of the association between GBC and S. typhi infection arose from retrospective studies performed in the US and Europe, suggesting that chronic S. typhi infected individuals who were exposed to higher risk of mortality from GBC compared with uninfected people[42].
In a study conducted in Scotland, chronically infected typhoid and paratyphoid individuals exhibited superior incidence of GBC and some were exposed to colon, rectum, pancreas and lung cancer coupled with other rarely occurring neoplasms[35]. Consequent investigations confirmed these findings, suggesting typhoid infected individuals with enhanced incidence of the hepatobiliary cancer, though not supported by any serological evidence[43]. On the other hand, serological investigations performed in Northern India, indicated that the frequencies of isolation of S. typhi from bile, gallstones and gallbladder tissue from GBC infected individuals were significantly higher in comparison to those individuals who were suffering from benign gallbladder cancer[44]. The proportion of people having anti-Vi serum antibody titers were 38.5 %, 13.9 % and 9.2 % for GBC patients, benign gallbladder cancer and healthy individuals, respectively. In a study conducted by Tewari et al.[45] hepatobiliary samples from GBC patients contained 67.3 % of S. typhi flagellin gene which was detected using a specific nested Polymerase Chain Reaction (PCR) technique. This percentage was significantly lower in individuals among benign gallbladder disease individuals.
Histologically, over 80 % of GBC are associated with gallstones while 10 % are associated with adenocarcinomas[46]. Studies have shown that chronic S. typhi carrier status is strongly correlated with the presence of gallstones[47].
Although GBC and S. typhi exhibit positive association, the mechanism that facilitate chronic persistence as well as the possible carcinogenic properties of S. typhi remain unclear. The production of biofilms may contribute significantly to the colonization and chronic persistence of S. typhi. This observation is supported by some findings indicating that a lipid-rich bile with antimicrobial characteristics situated in the gallbladder enhances the synthesis of O-antigen that enables the formation of S. typhi biofilm on human gallstones[48]. Hence, gallstones which are embedded with biofilm may represent the most conducive niche for the persistence of bacteria in the gallbladder and may cause recolonization of the intestinal tract and shedding via faeces. This phenomenon will lead to the transmission of bacterial agent to a new host.
On the contrary, non-typhoidal Salmonella species (S. choleraesuis and S. typhimurium) that provoke superior immune response in comparison to the typhoidal species and are associated with systemic sickness which have yet not reported to have any connection with disease of gallbladder or cancer as well[49].
S. bovis:
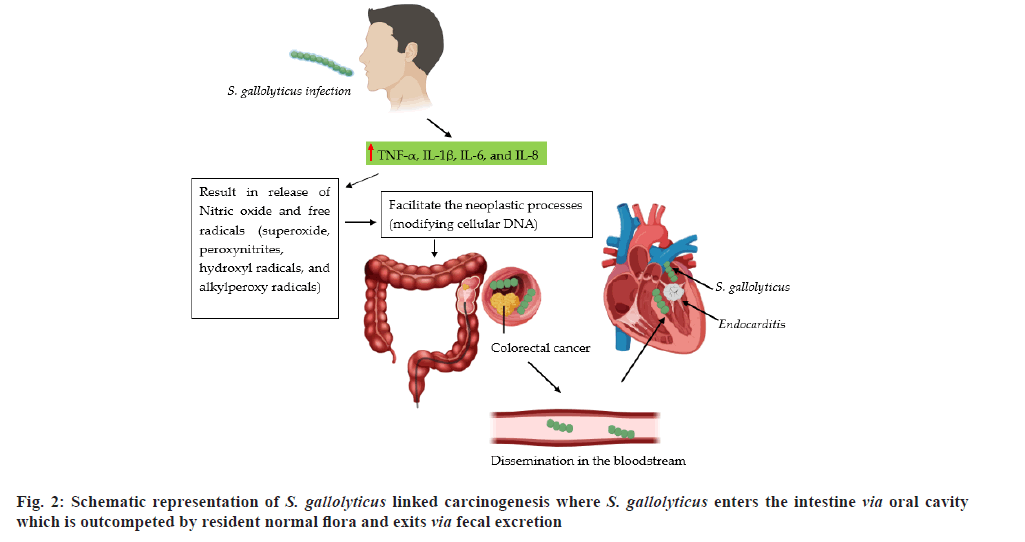
S. bovis is presently named Streptococcus gallolyticus (S. gallolyticus). According to the reports, about 25 %-80 % of the individuals with bacteremia due to S. gallolyticus, experience colorectal tumors[50]. It has been reported that S. bovis infected individuals have experienced CRC and incidence of colonic neoplasia[51]. It has been reported that 94 % and 18 % of individuals face the issue of bacteremia linked with CRC, was in fact specifically associated with S. bovis biotypes I and II, respectively[52].
The occurrence of CRC differs from country to country and it has been considered as one of the major public health concerns in the world. In the USA and United Kingdom (UK), this type of cancer is the 2nd most common cancer after lung or prostate cancer for men and breast cancer for women[53].
In a study conducted by Boleij et al.[54], indicated that S. gallolyticus, particularly their cell wall antigens, were found to increase the synthesis of inflammatory cytokines in rat’s colonic mucosa, suggesting direct association between colonic mucosal cells and S. bovis infection which is believed to be responsible for the development of CRC. Wide variety of interaction between CRC and S. gallolyticus might be attributed to different ethnic groups and geographical location. Currently, Lee et al.[55] reported that S. gallolyticus (S. bovis biotype II), rather than S. gallolyticus (subspecies gallolyticus (biotype I)), have showed superior associated with CRC while, USA and in Europe, S. gallolyticus (subspecies gallolyticus) was predominantly linked with colorectal tumors.
Various bacterial species have been associated with chronic colon infections and predispose patients for colon cancer including E. coli[56] and streptococci[57]. S. bovis is a normal resident of the human GIT which can cause bacteremia, urinary tract infection and endocarditis, etc. Though S. bovis is the 2nd utmost cause of streptococcal endocarditis[51], it is commonly linked with GIT lesions, particularly colon carcinoma. Remarkably, neoplasia of the colon may later develop into bacteremia or septic endocarditis.
A study indicated that there is direct association between CRC and bacteremia[58]. In a different study, the release of S. bovis/gallolyticus mediated inflammatory cytokines such as Tumor Necrosis Factor-Alpha (TNF-α), IL-1β, 6 and 8 is found to facilitate the normal host defense mechanisms leading to the production of free radicals, nitric oxide, peroxynitrites, superoxide, alkylperoxy and hydroxyl radicals[59]. The mutagenicity and neoplastic processes of these molecular species is mainly caused by modifying cellular DNA. On the contrary, formation of S. bovis/gallolyticus antigens triggered angiogenic modulators in colonic mucosa, such as IL-8 may also facilitate the process of colon carcinogenesis (fig. 2)[60].
C. pneumoniae:
C. pneumoniae, which is a gram-negative, obligate intracellular bacterium, has been identified in 1989, which frequently occurs as a respiratory pathogen resulting in chronic and persistent respiratory infections among humans[61]. C. pneumoniae infection is not limited to respiratory infections such as pharyngitis, pneumonia, sinusitis and bronchitis but it is also linked with chronic obstructive pulmonary disease, asthma and lung cancer[62]. C. pneumoniae is transmitted via aerosols[63]. Similar to all the other Chlamydia species, C. pneumoniae tends to persist in the tissue[64]. C. pneumoniae respiratory infections vary among countries and populations. It is hypothesized that C. pneumoniae is linked with other acute and chronic pulmonary diseases such as chronic obstructive respiratory disease, lung cancer and asthma[65]; additionally, it is also related with atherosclerotic cardiovascular diseases depicting several evidences[65].
Lung cancer is one of the main health problems which causes deadly illness in humans[66]. Research findings indicated that around 6 of 10 people with pulmonary cancer die within 1 y after diagnosis. Lung cancer is responsible for 2 093 876 (11.6 %) new cases and 18.4 % of deaths in 2018[10]. The dramatic increase in the prevalence of lung cancer is mainly facilitated by increased smoking among males and females, contributed to 90 % of lung cancer.
Laurila et al.[67] firstly discovered that C. pneumoniae infection could be an independent causative agent for lung carcinoma in 1997. Since then, the role of C. pneumoniae in the development of lung cancer has been intensely investigated[68], nevertheless the reports have been inconsistent. Presently, the mechanism of action by which the causal relationship between chronic C. pneumoniae infection and lung cancer is not clearly investigated. According to Chebak et al.[69], smoking may accelerate C. pneumoniae to colonize the lung whereby superoxide oxygen radicals, IL-1β, TNF-α and IL-8 play vital role, attributing to DNA and lung tissue damage ultimately causing carcinogenesis. Besides, the infection, C. pneumoniae may result in irregular apoptosis in lung tissues which leads to cancer.
Research into the interaction between lung cancer and C. pneumoniae risk is still in its initial stage. As per the previous studies, C. pneumoniae might be involved in the development of lung cancer. Carcinogenesis caused by this organism could be associated with chronic pulmonary diseases such as chronic bronchitis and asthma[67].
H. pylori:
H. pylori which is the inhabitant of the human stomach, was 1st identified from peptic ulcer diseased individuals by Marshall et al.[70]. The occurrence of H. pylori infection remains higher in most countries all over the globe. According to a study, there were 4.4 billion H. pylori infected individuals globally in 2015 where Africa continent had the highest rate (70.1%)[71].
H. pylori is a gram-negative helical-shaped bacterium and the ammonia production by H. pylori using urease enzyme counteracts the acidity of stomach, making it conducive niche for the bacterium. Moreover, the helical shape of H. pylori allows it to hideaway into the mucus layer[72].
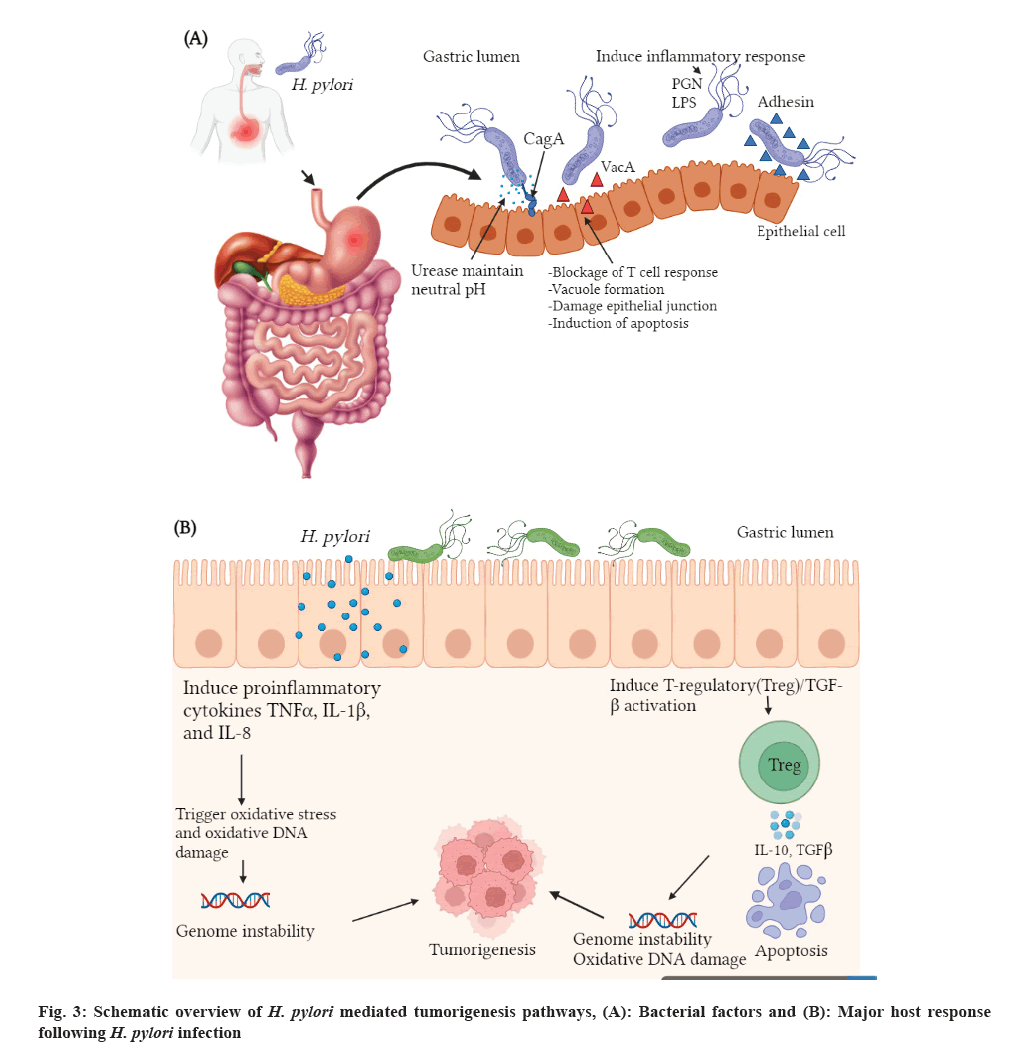
H. pylori is the most frequent cause of peptic ulcers in the GIT of humans, which has also been linked to several other malignant and benign GIT disorders, most notably gastric cancer[73]. H. pylori is now a major cause of duodenal and gastric ulcers, gastric Mucosa Assisted Lymphoid Tissue (MALT) lymphoma and Nocardia gastric adenocarcinoma[74]. Besides, epidemiologic investigations have explored the interaction between H. pylori and other GIT tumors, including CRC, pancreatic cancer[75,76] and esophageal tumors. A study indicated that CagA positive strains found to be of superior risk factor for cancer than CagA-negative strains[77]. A meta-analysis of 16 studies performed around the world revealed that CagA-positive H. pylori infected individuals experienced twice the risk of Nocardia gastric cancer than CagA-negative H. pylori infected individuals[78]. On the contrary, in Sweden, a study indicated that CagA-positive H. pylori infected patients experienced significantly lower risk of esophageal adenocarcinoma[79]. Likewise, in US, another study displayed that unlike CagA-negative strains, CagA-positive H. pylori infection was linked with lowered risk of gastric cardia cancer and/or esophageal adenocarcinoma[80].
Bacterial and host factors play an important role in the onset of gastric cancer in H. pylori infected individuals. Bacterial virulence factors such as CagA and Vacuolating cytotoxin A (VacA) of H. pylori play vital role in instability of cellular DNA and its damage. H. pylori tumorigenesis may be also driven by abnormal immune response (blockage of the normal immune response or overexpression of the immune cells) and dysregulation of apoptosis following infection[81] (fig. 3). According to Larussa et al.[82] arginase, CagA, VacA and other metabolic products of H. pylori alter T cell activation, proliferation and apoptosis. Recently, it has been reported that the semaphorin 5A-mediated pathway of H. pylori enhances the expression of matrix metalloproteinase 9 in gastric cancer cells and facilitate subsequent tumorigenesis process[83].
F. nucleatum:
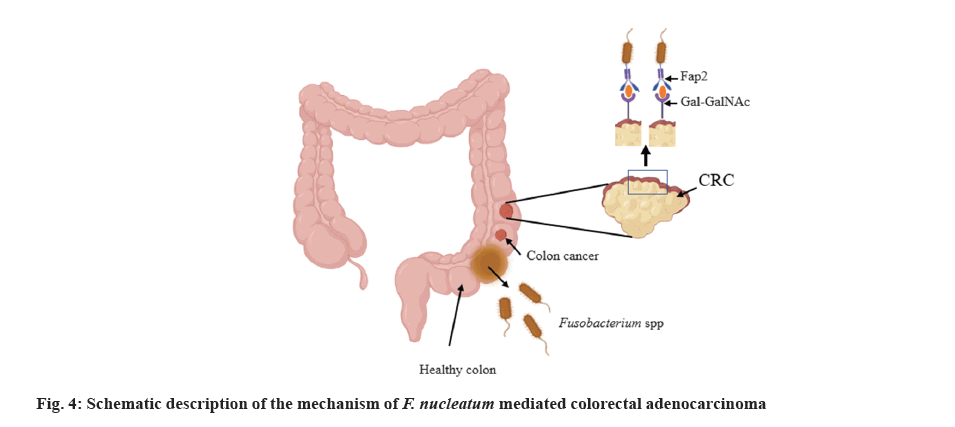
F. nucleatum is non-spore forming, gram-negative oral anaerobe. It is one of among the most prominent species localized in the oral environment[84]. F. nucleatum and the Fusobacterium adhesin gene A (FadA), are found to be abundant in stool samples of CRC diseased individuals[85]. CRC is the most prominent cause of mortality in humans worldwide[86]. Fusobacterial galactose adhesion hemagglutinin, Fap2, has been shown to intermediate F. nucleatum inhabitant of cells by attaching to the tumor induced host receptor D-Galactose and N-Acetyl-D-Galactosamine (Gal-GalNAc)[87].
Moreover, purified recombinant protein FadA has been identified to bind and colonize host cells, provoking the development of CRC cells. Interestingly, under disease and stress conditions, amyloid-like FadA might support acid resistance and further facilitate the internalization of the bacterial agent into the GIT, resulting in invasion of F. nucleatum. FadA has been recognized to bind to E-cadherin (Epithelial-cadherin) for attachment of the host epithelial cell[88]. This interaction led to the activation of the β-catenin/Wingless-related integration (Wnt) pathway, thus stimulating oncogenic and inflammatory responses[89]. Research findings exhibited that F. nucleatum might facilitate carcinogenesis by provoking, metabolism and proliferation of CRC cells[90]. F. nucleatum LPS might activate β-catenin via TLR4/Phosphor-p21-Activated Kinase 1 (PAK1) cascade in CRC cells[91]. LPS also facilitates TLR4 signalling to Myeloid Differentiation primary response 88 (MYD88), leading to the stimulation of Nuclear Factor Kappa B (NF-κB) and boosting miRNA-21 expression. Moreover, the oncogene miRNA-21 participated in colitis-linked CRC diminishes the expression of Rat Sarcoma p21 protein Activator 1 (RASA1) and stimulates the RAS-Mitogen-Activated Protein Kinase (MAPK) pathway, consequently leading to the accumulation of Synthesis phase (S phase) and enhances CRC cell proliferation[91,92].
Most cancer cells generate energy for the growth of tumor via aerobic glycolysis, which is also called as Warburg effect. It has been recognized that F. nucleatum infection triggers carcinogenesis and glycolysis by provoking H3K27ac-targeting of the genes Angiopoietin-Like Protein 4 (ANGPTL4) and Enolase 1 (ENO1) in CRC cells (fig. 4)[93].
Mycoplasma species:
The role of Mycoplasma and its oncogenic potential in the development of cancer has been explored since the 1950s. Mycoplasmas were 1st identified in leukemia diseased individuals, since then, several investigations displayed its presence either directly by PCR or indirectly by assessing the level of antibody in diseased individuals[94].
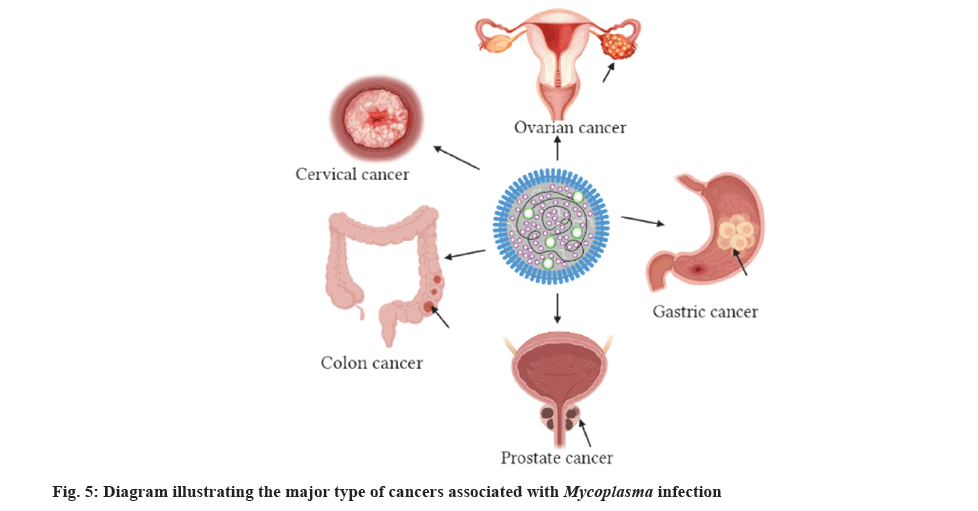
Mycoplasma species particularly Mycoplasma genitalium (M. genitalium), Mycoplasma hominis (M. hominis) and Ureaplasma urealyticum, have been identified in individuals from different countries such as Turkey, Australia, Russia, Japan and Iran[95]. Huang et al.[96], have documented exciting results depicting that Mycoplasma infection might be associated with the occurrence of cancer in various organs such as colon, prostate, breast, cervix, GIT, ovaries, esophagus and brain (fig. 5).
Recent research findings conducted by Klein et al.[97] indicated that Mycoplasmas have been identified in women who were positive for cervical cancer. Some of these species included M. genitalium, M. hominis and Mycoplasma penetrans (M. penetrans). The association between Mycoplasma fermentans (M. fermentans) infection, lymphatic system and renal cancer has also been studied[95]. Over 80 % of the DNA of Mycoplasmas have been found in tissues of cancer diseased individuals. Such fascinating results encouraged the investigators to explore the clinical importance of Mycoplasma infection in individuals who experienced kidney cancer[98]. Similarly, the occurrence of bladder cancer was formerly related with Mycoplasma infection whereby M. penetrans was mainly included in the progression of bladder cancer[99].
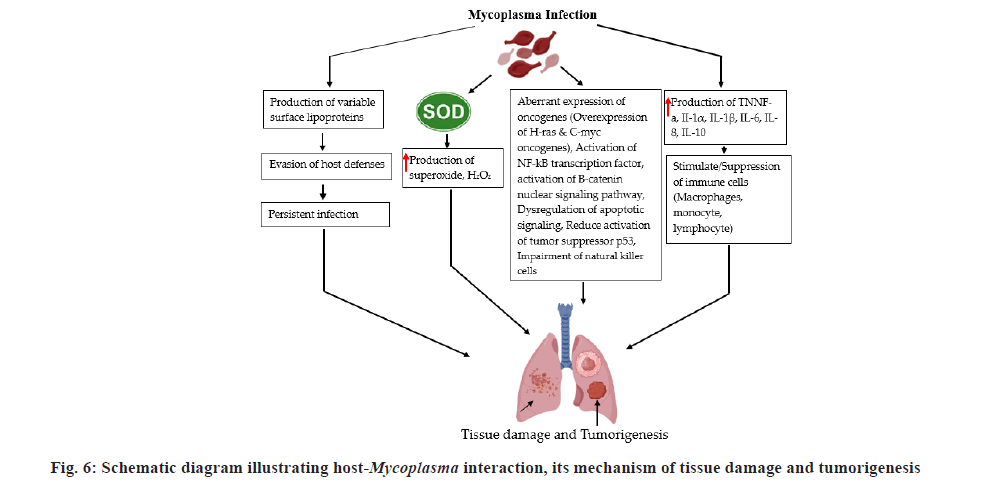
Several species of Mycoplasma have been shown to interfere with the coordination of cell cycle checkpoints in vivo and in vitro. Different cases of chromosomal anomalies, karyotypic and morphological alterations have been linked with Mycoplasma infections[100]. Mycoplasma is the best example to prove that infection-mediated inflammation helps in the progression of cancer via suppression of natural killer cell mediated macrophages[101]. Recent studies indicated that Mycoplasma hyorhinis infection enhances the accumulation of β-catenin in nuclear region, thereby facilitating gastric cancer cell motility via β-catenin signalling. In a study, Mycoplasma DnaK expression enhances the propagation of cancer via DNA damage in mouse model. Studies also indicated that Mycoplasma hyorhinis infection directly influences the level of Cluster of Differentiation (CD) 133+ cells in human CRC cell lines.
In an investigation, M. hominis, Mycoplasma arginini, M. fermentans and Mycoplasma arthritidis on the NF-κB pathways and tumor protein (p53) suppressor both engaged in the maintenance and stability of the cell cycle[102]. In vitro studies conducted on a panel of mice and human cell lines indicated that Mycoplasma infection suppressed the activity of p53 and induced NF-κB, which are the main traits of cancer cells in humans[103]. Bacteria encoded proteins such as M. hyorhinis-p37, facilitates the colonization of the agent into the human cells. This signifies its involvement in the carcinogenesis process[103]. Significant interaction between M. hyorhinis and carcinogenesis has been reported through in vitro and in vivo studies[104]. Role of M. hyorhinis in gastric cancer attained either by inducing the β?catenin signalling route which is vital for tissue homeostasis or the induction of the NLR family Pyrin domain containing 3 (NLRP3) inflammasome, which facilitates the process of pro-inflammatory cytokines maturation[105,106]. Mycoplasma species could disturb the anticancer activity of antibiotics either via the process of antibiotic resistance or by suppression of natural killer cells activity. Study conducted by Benedetti et al.[107], in the USA (Maryland University), chaperone and DnaK showed oncogenetic activities that intercalate with some of the proteins that regulate the critical cellular routes, resulting in reduction of effectiveness of anticancer drugs. Similarly, a group of German and Russian researchers have displayed that the cancer cells sensitivity to anticancer drugs decreases M. hyorhinis infection (fig. 6)[100].
Bartonella species:
Bartonella species are gram-negative facultative intracellular bacteria. Animals such as cats, rabbits, guinea pigs and dogs are the reservoirs of Bartonella henselae (B. henselae). Cat flea (Ctenocephalides felis) is the principal vector of B. henselae and infected flea faeces are the major source of transmission between cats and humans. Ticks (Ixodes ricinus) have been identified as potential vectors for transmitting Bartonellosis in humans. Ixodes ricinus is the most prevalent species in Western Europe, which commonly transmits the disease by biting[108].
Diseases caused by Bartonella species include asymptomatic skin lesions, unknown fever, local lymphadenopathy, encephalopathy, osteomyelitis, hepatomegaly and endocarditis. In immunosuppressed individuals, Bartonella species can cause opportunistic complications like peliosis hepatitis and bacillary angiomatosis[108].
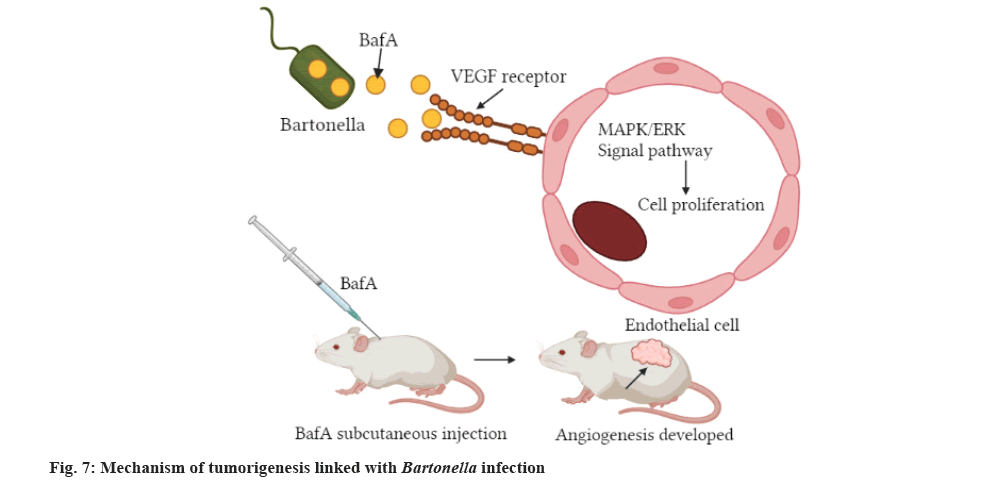
Bartonella species are neglected and emergent bacteria that cause disease worldwide[109]. In humans, Bartonella species cause neurological symptoms, endocarditis, trench fever, bacillary peliosis, bacillary angiomatosis and cat-scratch disease[110,111]. Bartonellosis is potentially fatal, particularly in immunodeficient people[112]. Uniquely many Bartonella species including B. henselae, stimulate Vascular Endothelial Growth Factor (VEGF) production causing the proliferation of blood vessels[113], which is also hallmark of malignant melanoma. It has been recognized that infection of melanoma cell cultures with B. henselae in vitro resulted in alteration of the cell morphology[114]. According to the study conducted by investigators, the infection cause by Bartonella species is linked with increased pro-angiogenic cytokine expression and inhibition of apoptosis in endothelial cells[110]. Published studies indicated that the expression of VEGF-C in melanoma cells co-cultured with B. henselae is upregulated like the case of formerly reported investigations of B. henselae co-cultures with HeLa or endothelial cells[115], whereby the organism upregulates the expression of IL-8 in endothelial cell lines[116]. Practically, Bartonella species rise VEGF-C expression in connection with cutaneous vasoproliferative metastasis growth, which is also crucial for the melanoma growth factor (fig. 7)[117].
B. fragilis is gram-negative, obligatory anaerobic bacterium that colonizes the colon of humans and is considered as one of the normal commensals of human colon. B. fragilis is categorized into two classes based on the toxin production, as Non-Toxigenic B. fragilis (NTBF) and Enterotoxigenic B. fragilis (ETBF); latter produced B. fragilis Toxin (BFT) is encoded by a chromosomal gene[118].
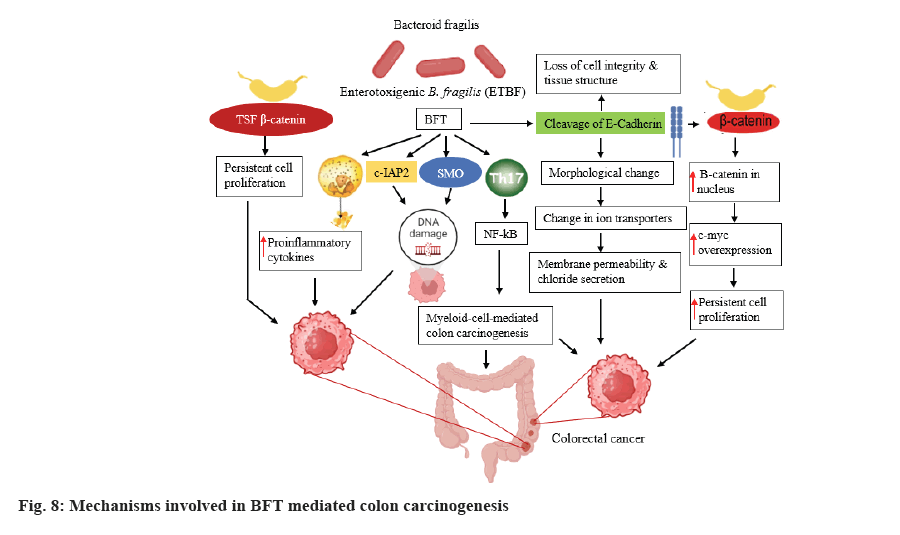
B. fragilis fragilysin, commonly called BFT, is a 20 kDa zinc dependent metalloproteinase toxin which participated in colon carcinogensis via formation of biofilm and enteritis that adversely affects the intestinal epithelium tight junction, resulting in increased permeability of the intestine. It has been recognized that tissue injury mediated by chronic inflammation accelerates the carcinogenesis process in B. fragilis infected individuals[118].
Several different mechanisms (fig. 8) have been involved in colon carcinogenesis mediated by the induction of the cleavage of E-cadherin, T cell factor-dependent β-catenin pathway T cell Factor (TCF) β-catenin, stimulation of IL-8 production leading to persistent proliferation of GIT epithelial cells (fig. 8). Further, BFT results in DNA damage, provokes the production of Reactive Oxygen Species (ROS) and proliferation of GIT epithelial cell via involvement of Spermine Oxidase (SMO) and cellular-Inhibitor of Apoptosis Protein 2 (c-IAP2)[119].
Conclusion
The interaction between infectious agents and development of cancer has been investigated previously. However, there are inadequate research findings on the causal relationship between mutagenic bacteria and proposed tumorigenesis pathways. Except for some findings, the mechanism of action of how bacterial infections predispose individuals for different types of cancer is not clearly elucidated. This review discussed the interaction between bacterial infection and development of different types of cancer in humans. As we discussed, several clues were presented by different researchers supporting the etiological role bacterial infection for the progression of cancer in humans. Therefore, it is impossible to neglect the contribution of bacterial infection in cancer development. This study also provides an idea that the limitations of the diagnostic assays are key factors which hindered such interactions. Hence, more detailed investigations are needed to explore the underlying mechanisms of action of tumor formation in humans. Advanced research is needed to identify the bacterial metabolites and/or bacterial specific proteins which facilite tumorigenesis process, for which highly sensitive as well as specific diagnostic assays would be needed.
To understand the actual interaction between different types of cancer and bacterial infections and/or their by-products, it would be crucial to follow-up patients with or without antibiotic interventions for a protracted period. Furthermore, in vivo and in vitro studies should be conducted to determine the mutagenic potential of bacterial pathogens and their associated precursors.
Acknowledgment:
This study was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah under grant number (GPIP: 928-305-2024). The authors, therefore, acknowledge with thanks to DSR for technical and financial support.
Conflicts of interests:
The authors declared no conflict of interests.
References
- Yin JJ, Duan FJ, Madhurapantula SV, Zhang YH, He G, Wang KY, et al. Helicobacter pylori and gastric cardia cancer: What do we know about their relationship? World J Meta-Anal 2020;8(2):89-97.
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: Cancer J Clin 2024;74(1):12-49.
[Crossref] [Google Scholar] [PubMed]
- Volesky-Avellaneda KD, Morais S, Walter SD, O’Brien TR, Hildesheim A, Engels EA, et al. Cancers attributable to infections in the US in 2017: A meta-analysis. JAMA Oncol 2023;9(12):1678-87.
[Crossref] [Google Scholar] [PubMed]
- Zella D, Gallo RC. Viruses and bacteria associated with cancer: An overview. Viruses 2021;13(6):1-10.
[Crossref] [Google Scholar] [PubMed]
- Schiller JT, Lowy DR. An introduction to virus infections and human cancer. Recent Results Cancer Res 2021;217:1-11.
[Crossref] [Google Scholar] [PubMed]
- Yusuf K, Sampath V, Umar S. Bacterial infections and cancer: Exploring this association and its implications for cancer patients. Int J Mol Sci 2023;24(4):3110.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Tian R, Sun C, Guo Y, Dong L, Li Y, et al. Microbial metabolites are involved in tumorigenesis and development by regulating immune responses. Front Immunol 2023;14:1-12.
[Crossref] [Google Scholar] [PubMed]
- Reyes VE. Helicobacter pylori and its role in gastric cancer. Microorganisms 2023;11(5):1-13.
[Crossref] [Google Scholar] [PubMed]
- Li Q. Bacterial infection and microbiota in carcinogenesis and tumor development. Front Cell Infect Microbiol 2023;13:1-12.
[Crossref] [Google Scholar] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394-424.
[Crossref] [Google Scholar] [PubMed]
- Bhagat TS, Gulati G, Bhagat R, Gupta S. Epidemiology of gallbladder cancer in India 2018-2023. Santosh Univ J Health Sci 2024;10(1):87-92.
- Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: Current status and future trends. Nature Rev Clin Oncol 2023;20(9):624-39.
[Crossref] [Google Scholar] [PubMed]
- Murthy SK, Kuenzig ME, Windsor JW, Matthews P, Tandon P, Benchimol EI, et al. The 2023 impact of inflammatory bowel disease in Canada: Cancer and IBD. J Can Assoc Gastroenterol 2023;6:83-96.
[Crossref] [Google Scholar] [PubMed]
- Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, et al. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol 2018;9:1-8.
[Crossref] [Google Scholar] [PubMed]
- Ghazaei C. Advances in the study of bacterial toxins, their roles and mechanisms in pathogenesis. The Malays J Med Sci 2022;29(1):4-17.
[Crossref] [Google Scholar] [PubMed]
- Shahid A, Ali S, Zahra T, Raza M, Shahid A, Saeed MU, et al. Influence of microbes in progression of cancer and DNA damaging effects. Haya Saudi J Life Sci 2020;5(11):246-52.
- Khatun S, Appidi T, Rengan AK. The role played by bacterial infections in the onset and metastasis of cancer. Curr Res Microb Sci 2021;2:1-10.
[Crossref] [Google Scholar] [PubMed]
- Luu TH, Michel C, Bard JM, Dravet F, Nazih H, Bobin-Dubigeon C. Intestinal proportion of Blautia sp. is associated with clinical stage and histoprognostic grade in patients with early-stage breast cancer. Nutr Cancer 2017;69(2):267-75.
[Crossref] [Google Scholar] [PubMed]
- Eyvazi S, Vostakolaei MA, Dilmaghani A, Borumandi O, Hejazi MS, Kahroba H, et al. The oncogenic roles of bacterial infections in development of cancer. Microb Pathog 2020;141:1-10.
[Crossref] [Google Scholar] [PubMed]
- Tolg C, Sabha N, Cortese R, Panchal T, Ahsan A, Soliman A, et al. Uropathogenic E. coli infection provokes epigenetic downregulation of CDKN2A (p16INK4A) in uroepithelial cells. Lab Invest 2011;91(6):825-36.
[Crossref] [Google Scholar] [PubMed]
- Zaidi AH, Kelly LA, Kreft RE, Barlek M, Omstead AN, Matsui D, et al. Associations of microbiota and toll-like receptor signaling pathway in esophageal adenocarcinoma. BMC Cancer 2016;16:1-10.
[Crossref] [Google Scholar] [PubMed]
- Nemunaitis JM, Brown-Glabeman U, Soares H, Belmonte J, Liem B, Nir I, et al. Gallbladder cancer: Review of a rare orphan gastrointestinal cancer with a focus on populations of new Mexico. BMC Cancer 2018;18:1-14.
[Crossref] [Google Scholar] [PubMed]
- Chandan K, Gupta M, Sarwat M. Role of host and pathogen-derived microRNAs in immune regulation during infectious and inflammatory diseases. Front Immunol 2020;10:1-13.
[Crossref] [Google Scholar] [PubMed]
- van Elsland D, Neefjes J. Bacterial infections and cancer. EMBO Rep 2018;19(11):1-10.
[Crossref] [Google Scholar] [PubMed]
- Palrasu M, Zaika E, El-Rifai W, Que J, Zaika AI. Role of bacterial and viral pathogens in gastric carcinogenesis. Cancers 2021;13(8):1-8.
[Crossref] [Google Scholar] [PubMed]
- Guo S, Zhu X, Huang Z, Wei C, Yu J, Zhang L, et al. Genomic instability drives tumorigenesis and metastasis and its implications for cancer therapy. Biomed Pharmacother 2023;157:114036.
[Crossref] [Google Scholar] [PubMed]
- Cheng WT, Kantilal HK, Davamani F. The mechanism of Bacteroides fragilis toxin contributes to colon cancer formation. Malays J Med Sci 2020;27(4):9-21.
[Crossref] [Google Scholar] [PubMed]
- Han S, Zhuang J, Wu Y, Wu W, Yang X. Progress in research on colorectal cancer-related microorganisms and metabolites. Cancer Manag Res 2020;12:8703-20.
[Crossref] [Google Scholar] [PubMed]
- Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis Primers 2022;8(1):69-75.
[Crossref] [Google Scholar] [PubMed]
- Gabrielli M, Hugo S, Dominguez A, Baez S, Venturelli A, Puga M, et al. Mortality due to gallbladder cancer: Retrospective analysis in three Chilean hospitals. Rev Med Chil 2010;138(11):1357-64.
[Google Scholar] [PubMed]
- Ferreccio C. Salmonella typhi and gallbladder cancer. Bacteria and Cancer; 2012. p. 117-37.
- Axelrod L, Munster AM, O'Brien TF. Typhoid cholecystitis and gallbladder carcinoma after interval of 67 y. JAMA 1971;217(1):83-7.
[Google Scholar] [PubMed]
- Mellemgaard AN, Gaarslev KN. Risk of hepatobiliary cancer in carriers of Salmonella typhi. J Natl Cancer Inst 1988;80(4):288-300.
[Crossref] [Google Scholar] [PubMed]
- Welton J, Marr J, Friedman S. Association between hepatobiliary cancer and typhoid carrier state. Lancet 1979;313(8120):791-4.
[Crossref] [Google Scholar] [PubMed]
- Caygill CP, Hill MJ, Braddick M, Sharp JC. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 1994;343(8889):83-4.
[Crossref] [Google Scholar] [PubMed]
- Saravanan S, Purushothaman V, Murthy TR, Sukumar K, Srinivasan P, Gowthaman V, et al. Molecular epidemiology of nontyphoidal Salmonella in poultry and poultry products in India: Implications for human health. Indian J Microbiol 2015;55(3):319-26.
[Crossref] [Google Scholar] [PubMed]
- Gunn JS, Marshall JM, Baker S, Dongol S, Charles RC, Ryan ET. Salmonella chronic carriage: Epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol 2014;22(11):648-55.
[Crossref] [Google Scholar] [PubMed]
- Hundal R, Shaffer EA. Gallbladder cancer: Epidemiology and outcome. Clin Epidemiol 2014;6:99-109.
[Crossref] [Google Scholar] [PubMed]
- Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol 2017;23(22):3978-98.
[Crossref] [Google Scholar] [PubMed]
- Nagaraja V, Eslick GD. Systematic review with meta?analysis: The relationship between chronic Salmonella typhi carrier status and gall?bladder cancer. Aliment Pharmacol Ther 2014;39(8):745-50.
[Crossref] [Google Scholar] [PubMed]
- Chouhan A. An epidemiological prospective study on the relation between gallbladder cancer and gall stones disease. Eur J Cardiovasc Med 2023;13(3):1-5.
- Koshiol J, Wozniak A, Cook P, Adaniel C, Acevedo J, Azócar L, et al. Salmonella enterica serovar Typhi and gallbladder cancer: A case-control study and meta?analysis. Cancer Med 2016;5(11):3310-235.
[Crossref] [Google Scholar] [PubMed]
- Strom BL, Soloway RD, Rios?Dalenz JL, Rodriguez?Martinez HA, West SL, Kinman JL, et al. Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer 1995;76(10):1747-56.
[Crossref] [Google Scholar] [PubMed]
- Shukla VK, Singh H, Pandey M, Upadhyay SK, Nath G. Carcinoma of the gallbladder-Is it a sequel of typhoid? Dig Dis Sci 2000;45:900-3.
[Crossref] [Google Scholar] [PubMed]
- Tewari M, Mishra RR, Shukla HS. Salmonella typhi and gallbladder cancer: Report from an endemic region. Hepatobiliary Pancreat Dis Int 2010;9(5):524-30.
[Google Scholar] [PubMed]
- Dwivedi M, Dwivedi M, Moitra M, Sanyal S. The implication of gall stones in gallbladder cancer and recent updates on its epidemiology. Gallstone Formation, Diagnosis, Treatment and Prevention; 2024. p. 207-222.
- Crawford RW, Rosales-Reyes R, Ramirez-Aguilar MD, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci USA 2010;107(9):4353-8.
[Crossref] [Google Scholar] [PubMed]
- Nayak S, Dasgupta IS. A comprehensive review on the development of Salmonella biofilm on gallbladder surface. Int J Innov Sci Res Technol 2022;7(6):295-304.
- Crump JA, Sjolund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev 2015;28(4):901-37.
[Crossref] [Google Scholar] [PubMed]
- Abdulamir AS, Hafidh RR, Bakar FA. The association of Streptococcus bovis/gallolyticus with colorectal tumors: The nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res 2011;30(1):1-13.
[Crossref] [Google Scholar] [PubMed]
- Corredoira J, Miguez E, Mateo LM, Fernández-Rodríguez R, García-Rodríguez JF, Pérez-González A, et al. The interaction between liver cirrhosis, infection by Streptococcus bovis, and colon cancer. Eur J Clin Microbiol Infect Dis 2023;42(7):907-12.
[Crossref] [Google Scholar] [PubMed]
- Tsai CE, Chiu CT, Rayner CK, Wu KL, Chiu YC, Hu ML, et al. Associated factors in Streptococcus bovis bacteremia and colorectal cancer. Kaohsiung J Med Sci 2016;32(4):196-200.
[Crossref] [Google Scholar] [PubMed]
- Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: A time?trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun 2021;41(10):1037-48.
[Crossref] [Google Scholar] [PubMed]
- Boleij A, Schaeps RM, Tjalsma H. Association between Streptococcus bovis and colon cancer. J Clin Microbiol 2009;47(2):516-27.
[Crossref] [Google Scholar] [PubMed]
- Lee H, Yoon Y. Etiological agents implicated in foodborne illness worldwide. Food Sci Anim Resour 2021;41(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Fu K. Genotoxins: The mechanistic links between Escherichia coli and colorectal cancer. Cancers 2023;15(4):1-11.
[Crossref] [Google Scholar] [PubMed]
- ?ahin T, Kiliç O, Acar AG, Severoglu Z. A review the role of Streptococcus bovis in colorectal cancer. Arts Humanit Open Access J 2023;5:165-73.
- Oberg J, Rasmussen M, Buchwald P, Nilson B, Inghammar M. Streptococcus bovis-bacteremia: Subspecies distribution and association with colorectal cancer: A retrospective cohort study. Epidemiol Infect 2022;150:1-8.
[Crossref] [Google Scholar] [PubMed]
- Nguyen IS, Biarc J, Pini A, Gosse F, Richert S, Thierse D, et al. Streptococcus infantarius and colonic cancer: Identification and purification of cell wall proteins putatively involved in colorectal inflammation and carcinogenesis in rats. International Congress Series 2006;1289:257-61.
- Abdulamir AS, Hafidh RR, Bakar FA. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: Inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer 2010;9:1-18.
[Crossref] [Google Scholar] [PubMed]
- Radouani F, El Yazouli L, Elyazghi Z, Hejaji H, Alami AA, Elmdaghri N. Chlamydia pneumoniae sero-prevalence in Moroccan patients with cardiovascular diseases. Infec Dis Health 2019;24(2):67-74.
[Crossref] [Google Scholar] [PubMed]
- Premachandra NM, Jayaweera JS. Chlamydia pneumoniae infections and development of lung cancer: Systematic review. Infect Agents Cancer 2022;17(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Cai F, Shou X, Ye Q. Epidemiological study on Mycoplasma pneumoniae and Chlamydia pneumoniae infection of hospitalized children in a single center during the COVID-19 pandemic. Front Cell Infect Microbiol 2022;12:1-8.
[Crossref] [Google Scholar] [PubMed]
- Scharbaai-Vázquez R, Font FJ, Rodríguez FA. Persistence in Chlamydia. Chlamydia-Secret Enemy from Past to Present; 2022.
- Porritt RA, Crother TR. Chlamydia pneumoniae infection and inflammatory diseases. For Immunopathol Dis Therap 2016;7:237-54.
[Crossref] [Google Scholar] [PubMed]
- Gu Z, Wu L, Li J, Zheng S, Huang M. A visual analysis of patient-reported outcomes in lung cancer from 2013 to 2023. Cancer Control 2024;31:1-10.
[Crossref] [Google Scholar] [PubMed]
- Laurila AL, von Hertzen L, Saikku P. Chlamydia pneumoniae and chronic lung diseases. Scand J Infect Dis Suppl 1997;104:34-6.
[Google Scholar] [PubMed]
- Smith JS, Kumlin U, Nyberg F, Fortes C, Zaridze D, Ahrens W, et al. Lack of association between serum antibodies of Chlamydia pneumoniae infection and the risk of lung cancer. Int J Cancer 2008;123(10):2469-71.
[Crossref] [Google Scholar] [PubMed]
- Chebak M, Azzouzi M, Chaibi H, Fakhkhari M, Benamri I, Mguil M, et al. Assessment of the association of Chlamydia pneumoniae infection with lung cancer in a Moroccan patients’ cohort. Asian Pac J Cancer Prev 2023;24(2):659-65.
[Crossref] [Google Scholar] [PubMed]
- Marshall B, Warren JR. Unidentified curved Bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;323(8390):1311-5.
[Crossref] [Google Scholar] [PubMed]
- Hooi JK, Lai WY, Ng WK, Suen MM, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017;153(2):420-9.
[Crossref] [Google Scholar] [PubMed]
- Mascellino MT, Pontone S, Vega AE, Malfertheiner P. Helicobacter pylori infection: Pathogenesis, antibiotic resistance, advances and therapy, new treatment strategies. Front Microbiol 2022;13:1-4.
[Crossref] [Google Scholar] [PubMed]
- Nintewoue GF, Mabeku LB. Helicobacter pylori infection promotes gastric premalignancies and malignancies lesions and demotes hyperplastic polyps: A 5 y multicentric study among Cameroonian dyspeptic patients. Asian Pac J Cancer Prev 2023;24(1):171-83.
[Crossref] [Google Scholar] [PubMed]
- Myrou A. Molecular mechanisms and treatment strategies for Helicobacter pylori-induced gastric carcinogenesis and Mucosa Associated Lymphoid Tissue (MALT) lymphoma. Cureus 2024;16(5).
[Crossref] [Google Scholar] [PubMed]
- Elgohary AM, Gomaa NM, Ibrahim MA, Ahmed HS, Ibraheem SM, Frag MH. Helicobacter pylori virulence factors, pathogenicity, and gastric cancer. InGastrointestinal Cancers: An interdisciplinary approach; 2023. p. 117-131. [Crossref]
- Raderer M, Wrba F, Kornek G, Maca T, Koller DY, Weinlaender G, et al. Association between Helicobacter pylori infection and pancreatic cancer. Oncology 1998;55(1):16-9.
[Crossref] [Google Scholar] [PubMed]
- Shmuely H, Passaro D, Figer A, Niv Y, Pitlik S, Samra Z, et al. Relationship between Helicobacter pylori cagA status and colorectal cancer. Am J Gastroenterol 2001;96(12):3406-10.
[Crossref] [Google Scholar] [PubMed]
- Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between CagA seropositivity and gastric cancer. Gastroenterology 2003;125(6):1636-44.
[Crossref] [Google Scholar] [PubMed]
- Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, et al. Helicobacter pylori infection and gastric atrophy: Risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J Natl Cancer Inst 2004;96(5):388-96.
[Crossref] [Google Scholar] [PubMed]
- Chow WH, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res 1998;58(4):588-90.
[Google Scholar] [PubMed]
- Dinc? AL, Meli? LE, M?rginean CO. Old and new aspects of H. pylori-associated inflammation and gastric cancer. Children 2022;9(7):1-16.
[Crossref] [Google Scholar] [PubMed]
- Larussa T, Leone I, Suraci E, Imeneo M, Luzza F. Helicobacter pylori and T helper cells: Mechanisms of immune escape and tolerance. J Immunol Res 2015;2015(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Pan G, Wang X, Wang Y, Li R, Li G, He Y, et al. Helicobacter pylori promotes gastric cancer progression by upregulating semaphorin 5A expression via ERK/MMP9 signalling. Mol Ther Oncolytics 2021;22:256-64.
[Crossref] [Google Scholar] [PubMed]
- Nozawa A, Oshima H, Togawa N, Nozaki T, Murakami S. Development of oral care chip, a novel device for quantitative detection of the oral microbiota associated with periodontal disease. PLoS One 2020;15(2):1-13.
[Crossref] [Google Scholar] [PubMed]
- Thomas AM, Manghi P, Asnicar F, Pasolli E, Armanini F, Zolfo M, et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med 2019;25(4):667-78.
[Crossref] [Google Scholar] [PubMed]
- Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015;148(6):1244-60.
[Crossref] [Google Scholar] [PubMed]
- Casasanta MA, Yoo CC, Udayasuryan B, Sanders BE, Umaña A, Zhang Y, et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci Signal 2020;13(641):1-12.
[Crossref] [Google Scholar] [PubMed]
- Meng Q, Gao Q, Mehrazarin S, Tangwanichgapong K, Wang Y, Huang Y, et al. Fusobacterium nucleatum secretes amyloid?like FadA to enhance pathogenicity. EMBO Rep 2021;22(7):1-19.
[Crossref] [Google Scholar] [PubMed]
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013;14(2):195-206.
[Crossref] [Google Scholar] [PubMed]
- Hong J, Guo F, Lu SY, Shen C, Ma D, Zhang X, et al. F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer. Gut 2021;70(11):2123-37.
[Crossref] [Google Scholar] [PubMed]
- Shi C, Yang Y, Xia Y, Okugawa Y, Yang J, Liang Y, et al. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut 2016;65(9):1470-81.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signalling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology 2017;152(4):851-66.
[Crossref] [Google Scholar] [PubMed]
- Zheng X, Liu R, Zhou C, Yu H, Luo W, Zhu J, et al. ANGPTL4-mediated promotion of glycolysis facilitates the colonization of Fusobacterium nucleatum in colorectal cancer. Cancer Res 2021;81(24):6157-70.
[Crossref] [Google Scholar] [PubMed]
- Yacoub E, Abdul-Wahab OMS, Al-Shyarba MH, Mardassi BBA. The relationship between mycoplasmas and cancer: Is it fact or fiction? Narrative review and update on the situation. J Oncol 2021(1):1-13.
[Crossref] [Google Scholar] [PubMed]
- Saadat S, Karami P, Jafari M, Kholoujini M, Rikhtegaran Tehrani Z, et al. The silent presence of Mycoplasma hominis in patients with prostate cancer. Pathog Dis 2020;78(7):1-5.
[Crossref] [Google Scholar] [PubMed]
- Huang S, Li JY, Wu J, Meng L, Shou CC. Mycoplasma infections and different human carcinomas. World J Gastroenterol 2001;7(2):266-9.
- Klein C, Samwel K, Kahesa C, Mwaiselage J, West JT, Wood C, et al. Mycoplasma co-infection is associated with cervical cancer risk. Cancers 2020;12(5):1-13.
[Crossref] [Google Scholar] [PubMed]
- Pehlivan M, Pehlivan S, Onay H, Koyuncuoglu M, Kirkali Z. Can Mycoplasma-mediated oncogenesis be responsible for formation of conventional renal cell carcinoma? Urology 2005;65(2):411-4.
[Crossref] [Google Scholar] [PubMed]
- Zhu YY, Yu ZX, Zhou LP. Detection of Mycoplasma penetrans in transitional cell carcinoma of bladder. Chin J Zoonoses. 2009;25:238-40.
- Boyarskikh UA, Shadrina AS, Smetanina MA, Tsepilov YA, Oscorbin IP, Kozlov VV, et al. Mycoplasma hyorhinis reduces sensitivity of human lung carcinoma cells to nutlin-3 and promotes their malignant phenotype. J Cancer Res Clin Oncol 2018;144:1289-300.
[Crossref] [Google Scholar] [PubMed]
- Choo QW, Koean RA, Chang SC, Chng WJ, Chan MC, Wang W, et al. Macrophages protect Mycoplasma?infected chronic myeloid leukemia cells from natural killer cell killing. Immunol Cell Biol 2020;98(2):138-51.
[Crossref] [Google Scholar] [PubMed]
- Hoesel B, Schmid JA. The complexity of NF-κB signalling in inflammation and cancer. Mol Cancer 2013;12:1-5.
[Crossref] [Google Scholar] [PubMed]
- Duan H, Chen L, Qu L, Yang H, Song SW, Han Y, et al. Mycoplasma hyorhinis infection promotes NF-κB-dependent migration of gastric cancer cells. Cancer Res 2014;74(20):5782-94.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signalling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology 2017;152(4):851-66.
[Crossref] [Google Scholar] [PubMed]
- Liu RD, Cui J, Liu XL, Jiang P, Sun GG, Zhang X, et al. Comparative proteomic analysis of surface proteins of Trichinella spiralis muscle larvae and intestinal infective larvae. Acta Trop 2015;150:79-86.
[Crossref] [Google Scholar] [PubMed]
- Xu Y, Li H, Chen W, Yao X, Xing Y, Wang X, et al. Mycoplasma hyorhinis activates the NLRP3 inflammasome and promotes migration and invasion of gastric cancer cells. PloS One 2013;8(11):1-14.
[Crossref] [Google Scholar] [PubMed]
- Benedetti F, Cocchi F, Latinovic OS, Curreli S, Krishnan S, Munawwar A, et al. Role of Mycoplasma chaperone DnaK in cellular transformation. Int J Mol Sci 2020;21(4):1-16.
[Crossref] [Google Scholar] [PubMed]
- Mazur?Melewska K, Macedulski T, Prusinowska J, Mania A, Figlerowicz M, S?u?ewski W, et al. Ró?norodno?? przebiegu klinicznego choroby kociego pazura. Pediatr Med Rodz 2012;2(8):176-9. [Crossref]
- Cheslock MA, Embers ME. Human bartonellosis: An underappreciated public health problem? Trop Med Infect Dis 2019;4(2):1-16.
[Crossref] [Google Scholar] [PubMed]
- Regier Y, O’Rourke F, Kempf VA. Bartonella spp.-A chance to establish one health concepts in veterinary and human medicine. Parasit Vectors 2016;9(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Álvarez-Fernández A, Breitschwerdt EB, Solano-Gallego L. Bartonella infections in cats and dogs including zoonotic aspects. Parasit Vectors 2018;11:1-21.
[Crossref] [Google Scholar] [PubMed]
- Chomel BB, Boulouis HJ, Maruyama S, Breitschwerdt EB. Bartonella spp. in pets and effect on human health. Emerg Infect Dis 2006;12(3):389.
[Crossref] [Google Scholar] [PubMed]
- Truttmann MC, Rhomberg TA, Dehio C. Combined action of the type IV secretion effector proteins BepC and BepF promotes invasome formation of Bartonella henselae on endothelial and epithelial cells. Cell Microbiol 2011;13(2):284-99.
[Crossref] [Google Scholar] [PubMed]
- Felcht M, Thomas M. Angiogenesis in malignant melanoma. J Dtsch Dermatol Ges 2015;13(2):125-35.
[Crossref] [Google Scholar] [PubMed]
- Zhu C, Bai Y, Liu Q, Li D, Hong J, Yang Z, et al. Depolymerization of cytokeratin intermediate filaments facilitates intracellular infection of HeLa cells by Bartonella henselae. J Infect Dis 2013;207(9):1397-405.
[Crossref] [Google Scholar] [PubMed]
- McCord AM, Resto-Ruiz SI, Anderson BE. Autocrine role for interleukin-8 in Bartonella henselae-induced angiogenesis. Infect Immun 2006;74(9):5185-90.
[Crossref] [Google Scholar] [PubMed]
- Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology 2017;153(6):1621-33.
[Crossref] [Google Scholar] [PubMed]
- Cheng WT, Kantilal HK, Davamani F. The mechanism of Bacteroides fragilis toxin contributes to colon cancer formation. Malays J Med Sci 2020;27(4):9.
[Crossref] [Google Scholar] [PubMed]
- Yekani M, Baghi HB, Naghili B, Vahed SZ, Sóki J, Memar MY. To resist and persist: Important factors in the pathogenesis of Bacteroides fragilis. Microb Pathog 2020;149:1-9.
[Crossref] [Google Scholar] [PubMed]