- *Corresponding Author:
- Xiao Wu
Respiratory and Critical Care Medicine, Qingdao Central Hospital, University of Health and Rehabilitation Sciences, Qingdao Cancer Hospital, Qingdao, Shandong 266011, China
E-mail: wuxiao990222@163.com
| Date of Received | 27 May 2023 |
| Date of Revision | 12 May 2024 |
| Date of Acceptance | 08 August 2024 |
| Indian J Pharm Sci 2024;86(4):1383-1393 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Bone fracture is one of the most significant issues in the world, and it can affect anyone between ages. Patients have, accordingly, focused their attention on complementary and alternative medicine from natural products and applications from the orient; specifically, traditional Chinese medicine, because of efforts to enhance the healing of fractures without undesirable side effects. Although there are many types of traditional Chinese medicine herbs, some of the most common ones that are used in treating osteoporosis are known to have beneficial effects on bone density, where Eucommia ulmoides and Drynariae Rhizome are some of the most used types. In an attempt to determine the effectiveness of active components in the plant Eucommia ulmoides and the herb Drynariae Rhizome in bone fracture and fracture healing using network pharmacology and molecular docking study, this research study was carried out. First, the dosage forms Eucommia ulmoides and Drynariae Rhizome for traditional Chinese medicine nature were obtained, and then the molecular docking and molecular dynamics of the target protein of this medicine were achieveDrynariae To analyze the process of molecular accumulation, the decision to use the presence/absence matrix of known interactions and apply network considerations that recognize three dimensional contacts and biological networks of the compounds and targets involved was made. Based on the conclusions drawn from this molecular docking, the efficiency and working mechanism of the bioactive compounds and their targets were determineDrynariae These bioactive compounds described in the case focus on network pharmacology’s targets, and they might be relational with bone metabolism, fracture repairing or other diseases. Molecular docks also offered fairly reasonable binding through certain metabolites like geniposidic acid and kaempferol with other probable aims like bone morphogenetic protein 2/growth differentiation factor 5 compounds that may back up the restorative wound fix in bone and cartilage, neuroprotection, cardiovascular health, and cancer defence mechanisms. The findings from this integrated approach based on the sequence have the ability to offer better understanding of the phytochemicals that make Eucommia ulmoides and Drynariae Rhizome helpful in the healing of bone fracture. The knowledge regarding the bioactive compound and their relationship with the target protein might help in the development of fresh and effective fracture healing traditional Chinese medicine drugs or in the adjustment of the traditional Chinese medicine prescriptions for bone ailments.
Keywords
Eucommia ulmoides, Drynariae Rhizome, network pharmacology, molecular docking, traditional Chinese medicine
Bone fractures are a significant health concern, affecting individuals of all ages and causing substantial physical, emotional, and economic burdens. According to a report by the International Osteoporosis Foundation, fractures resulting from osteoporosis alone affect one in three women and one in five men over the age of 50 y worldwide[1]. Fracture healing is a complex process involving a well- coordinated sequence of biological events, including inflammation, bone formation, and remodelling[2]. However, to do away with these symptoms or treat other illnesses through immobilization, surgery, and medications may bring about some side effects or post-treatment complications[3]. For this reason, there is growing interest in finding other and more non- pharmacological treatment approaches, especially those involving plant-natural products for promoting bone fracture healing, which are free of side effects. Despite the impact of the Coronavirus Disease-19 (COVID-19) pandemic, various strategies are used in managing bone diseases, including the use of Chinese medicine in China, Traditional Chinese Medicine (TCM) has been famous for its use of herbs in conducting treatment operations[4]. Two conventional natural herbs of TCMs are Eucommia ulmoides (E. ulmoides), also called Du-Zhong in TCM and Drynariae Rhizome or Gu-sui-bu in TCM, which has been used dominantly in the TCM formulation for bone healing[5]. However, how these herbs contribute to healing fractures or breaks has not been establishe. E. ulmoides is a lucky tree, especially one belonging to the family of Eucommiaceae, and it is natively found in the central region of China[6]. It has been used in TCM for a while primarily because of the varied medical applications due to the compound’s ability to discharge anti-inflammatory, antioxidant, and bone protection effects[7]. In addition, many investigators have also established that E. ulmoides contributes to the formation of bone uplift in Bone Mineral Density (BMD) and a substantial decline in bone resorption[8]. These effects have been associated with several bioactive compounds that include the following: Lignans, iridoids, and phenolic acids, which in turn have the role of regulating the signaling pathways in the process of bone remodeling[5]. Gu- sui-bu or Drynariae Rhizoma is the dried Rhizome of the herbaceous perennial plant, Drynaria fortunei belongs to the Pteridaceae family, which are fern plants native to East Asia[9]. It has been widely used in TCM for treating bone fractures, arthritis, and other bone-related disorders[4]. Drynariae Rhizome is rich in various bioactive compounds, including flavonoids, phenolic acids, and triterpenoids, which have been reported to possess anti-inflammatory, antioxidant, and bone-protective properties[10]. Several studies have demonstrated the potential of Drynariae Rhizome in promoting osteoblast differentiation, enhancing bone formation, and inhibiting osteoclast activity[10]. Despite the growing evidence supporting the potential benefits of E. ulmoides and Drynariae Rhizome in fracture healing, the specific mechanisms underlying their actions remain unclear. Understanding these mechanisms is crucial for optimizing their therapeutic applications and developing more effective and targeted treatments for fracture healing.
Network pharmacology, an emerging field that integrates systems biology, bioinformatics, and computational approaches, offers a powerful tool for elucidating the complex interactions between multiple compounds and biological targets[11]. It enables one to systematically consider such things as multiple components and targets of natural products with a focus on their action possibilities. Network pharmacology involves building and analyzing compound-target interaction networks that cover the biological pathways and processes relevant to the therapeutic actions of herbs, and which have been used to examine herbal remedies[12]. A more specific structural molecular information that molecular docking can offer to supplement the network pharmacology includes the ability to predict the binding affinity and orientation of the molecule to the target protein, which is essential in the identification of small molecules and amino acid residues that is likely to form cross-interactions[13]. This approach can aid in the next generation of hypotheses on the potential therapeutic targets. It can be used to understand better the structure-activity relationship of the bioactive compounds required in designing the more efficient and selective therapeutic agents. Therefore, integrating the network pharmacology strategy focusing on natural products like E. ulmoides and Drynariae Rhizome, and the molecular docking method can help to gain a systematic perspective of the fringe benefits of these products on fracture callus formation. This approach will point out the crucial bioactive compounds, target genes, and biological activity associated with the bone fracture repair process, thus taking advantage of the fractured system[14].
In this study, we seek to establish the details of the mode of action of E. ulmoides and Drynariae Rhizome in fracture healing through a systematic study on target fish via network pharmacology and molecular dockings methods. The results from this study might help in the enhancement of new treatment strategies aiming at improving fracture healing and the refinement of the current TCM medicines used in the treatment of bone diseases.
Materials and Methods
Identification of active compounds and target genes:PubChem: The bioactive constituents of E. ulmoides and Drynariae Rhizome were obtained from freely accessible databases such as PubMed or Google Scholar. Afterwards, potential compounds were entered into PubChem (https://pubchem.ncbi.nlm.nih.gov/) to retrieve their chemical structures using the simplified molecular input line entry system[15]. The three Dimensional (3D) structure data were then downloaded in Structured Data File (SDF) format. Next, gather relevant information like molecular weight, chemical characteristics, biological functions, and targets associated with E. ulmoides and Drynariae Rhizome. These targets were predicted using the SwissTargetPrediction database (http://www.swisstargetprediction.ch/) with a probability higher than 0[16].
Compounds-target interactions: We conducted a search for related targets using databases such as Binding Database (BindingDB), Swiss TargetPrediction, and UniProt[17]. These databases offer extensive and user-friendly information on potential target proteins for the active compounds. Additionally, they provide relevant information about target proteins, including markers and estimations for human genes, and automatically integrate gene-centric data. Targets with a high probability score were chosen for additional analysis.
BindingDB: It is a publicly accessible online database that collects verified binding affinities; it primarily stores information about interactions between tiny, drug-like compounds and proteins that are thought therapeutic targets. To determine which active chemicals, interact with which target proteins. We utilize the BindingDB website. Investigate the binding affinities of each active chemical with various proteins. Following that, apply particular regard to the interactions that have strong binding affinities and record them. The species “Homo sapiens (H. sapiens)” was used to build the network of possible therapeutic targets. The value was chosen because it strikes a good mix between sensitivity and specificity and is a reliable barrier for finding interactions. Hence, the protein network exclusively contains proteins with interaction connection scores of 0.4 or higher.
DisGeNET database: The DisGeNET website established correlations between target genes and illness conditions. Analyze data related to genes linked to diseases.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database: To comprehend the biological pathways linked to the target proteins, we utilize the KEGG Mapper program to assign target proteins to established biological pathways accurately. We identify the vital pathways in bone metabolism, fracture repair, and various other illnesses. The KEGG pathway enrichment analysis was performed on all potential therapeutic targets to determine the associated pathways[17].
Network construction: The Cytoscape 3.10 system was used to show the network of active chemicals, possible targets, and signaling pathways[18].
Molecular docking:
To undertake the docking study of kaempferol with the Bone Morphogenetic Protein 2 (BMP2)/Growth Differentiation Factor-5 (GDF-5) heterodimer protein complex, we follow a systematic workflow involving several software tools for ligand and protein preparation, docking, and result analysis. Here is a detailed step-by- step process:
Ligand preparation with Open Babel: Open Babel (http://openbabel.org/) is an open-source chemical toolbox designed to speak the many languages of chemical data. It allows the conversion of chemical file formats and the preparation of ligands.
Download ligand data: Obtain the structure of kaempferol from PubChem (Pubchem CID: 5280863).
Convert and optimize ligand: Use open babel to convert the ligand data to the required format (e.g., Protein Data Bank (PDB), Molecule Structure Format (MOL2)) and optimize the geometry for docking studies.
Protein and ligand preparation with discovery studio: Discovery studio (https://www.3dsbiovia.com/products/collaborative-science/biovia-discovery-studio/) is a comprehensive suite of modelling and simulation tools for life science researchers. It provides capabilities for protein and ligand preparation.
Load protein structure: Import the crystal structure of the BMP2/GDF5 heterodimer into discovery studio.
Remove water molecules: Delete all water molecules to avoid unnecessary interactions during docking.
Remove extra chains: Retain only the chains relevant to the docking study.
Remove heteroatoms: Remove any non-standard residues or ions that may interfere with docking.
Optimize protein: Add hydrogen, assign proper atom types and optimize the geometry.
Prepare ligand: Import the optimized ligand structure and perform necessary preparations, such as adding hydrogens and ensuring correct protonation states.
Docking with PyRx: PyRx (https://pyrx.sourceforge.io/) is an open-source virtual screening tool for computational drug discovery that uses AutoDock and AutoDock Vina for molecular docking.
Import protein and ligand: Load the prepared protein and ligand structures into PyRx.
Set docking parameters: Define the binding site, grid box dimensions, and other docking parameters.
Run docking simulation: Perform the docking simulation to predict the binding modes and affinities.
Analyze results: Identify the best binding poses based on the docking scores and interactions.
Results analysis with Discovery Studio: After docking, Discovery Studio: https://www.3dsbiovia.com/ products/collaborative-science/biovia-discovery-studio is used again for detailed analysis of the docking results. Import the docking results from PyRx into Discovery Studio. Examine the binding poses of kaempferol in the active site of the BMP2/GDF5 heterodimer. Identify key interactions, such as conventional bonds with Aspartic acid (Asp) 35, pi-alkyl interactions with Trp36, and pi- pi stacked interactions with His98. Predict the potential effects based on the observed interactions, such as enhanced signaling or anti-cancer properties.
By following this workflow, we can efficiently prepare the ligands and protein, perform docking simulations, and analyze the results to predict the potential therapeutic effects of kaempferol when interacting with the BMP2/GDF5 heterodimer protein complex and geniposidic acid with BMP2/GDF5 (Protein 8E3G).
Results and Discussion
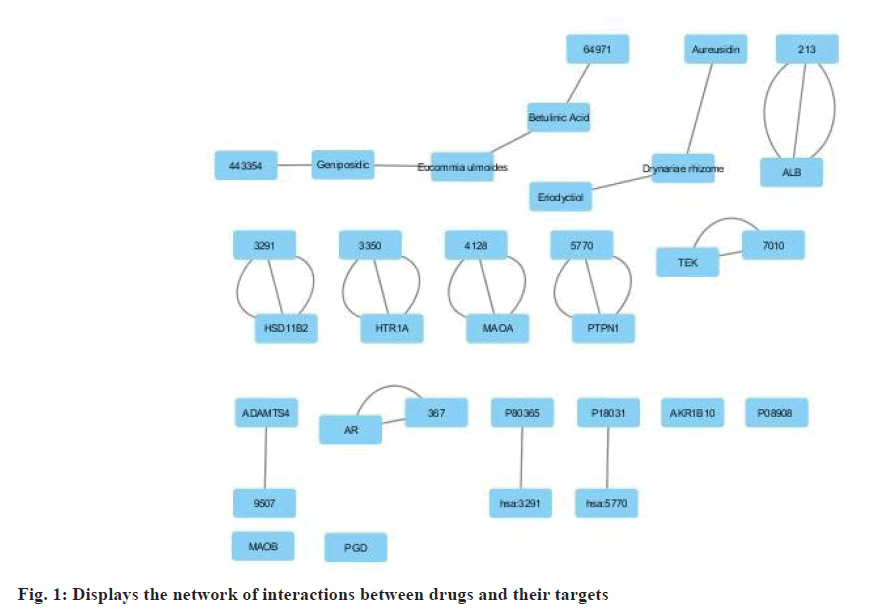
The bioactive molecule illustrated in fig. 1, was identified through a search conducted on PubMed and other databases such as ChemDraw. The Binding database KEGG Mapper was also utilized to collect information to create a network. Only the active and confirmed targets were selected from the PubMed search results, resulting in the identification of targets for different components. The ChemDraw web service utilizes a 3D similarity search to present many targets, whereas the BindingDB web service offers targets with a fit score value. Shared targets are often found among bioactive substances due to the presence of a common structural framework. After removing unnecessary and non-H. sapiens targets, only H. sapiens targets were selected for further investigation.
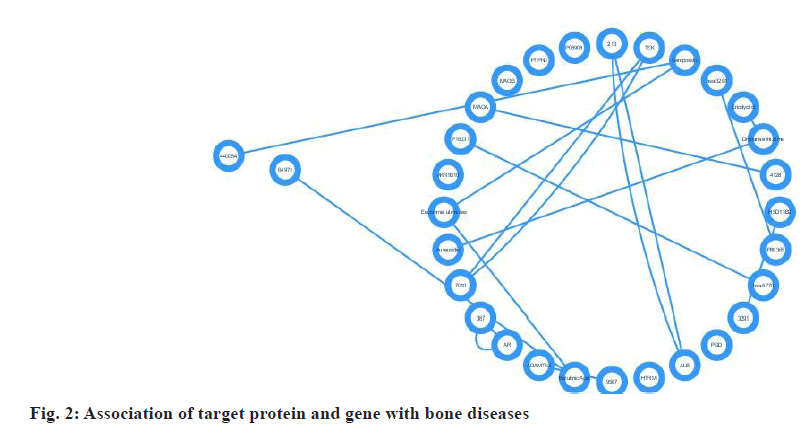
Fig. 2, depicts the target proteins that were categorized based on their associated disease, as determined using the disease and gene annotations database by Peng et al.[4]. All the targets identified were associated with bone-related diseases, such as bone fractures. Furthermore, the remaining targets were linked to conditions such as diabetes, cardiovascular disease, Alzheimer's disease, muscular illness, mental disease, and inflammation. The analysis readily revealed that most of the bioactive compounds had numerous targets.
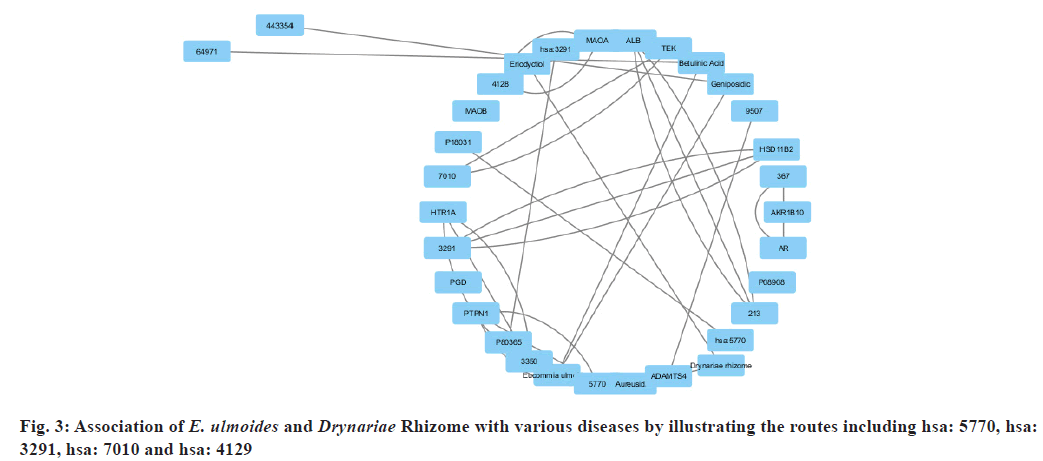
The results were validated by creating the protein, gene, and disease pathway network. Fig. 3, illustrates the routes including hsa: 5770, hsa: 3291, hsa: 7010, hsa: 4129 and the association of betulinic acid and eriodyctiol with bone disorders, diabetes, cardiovascular illnesses, neurological disease, muscular disease, inflammation, and many other diseases. Additional pathways relevant to these diseases were chosen for bone-related disorders.
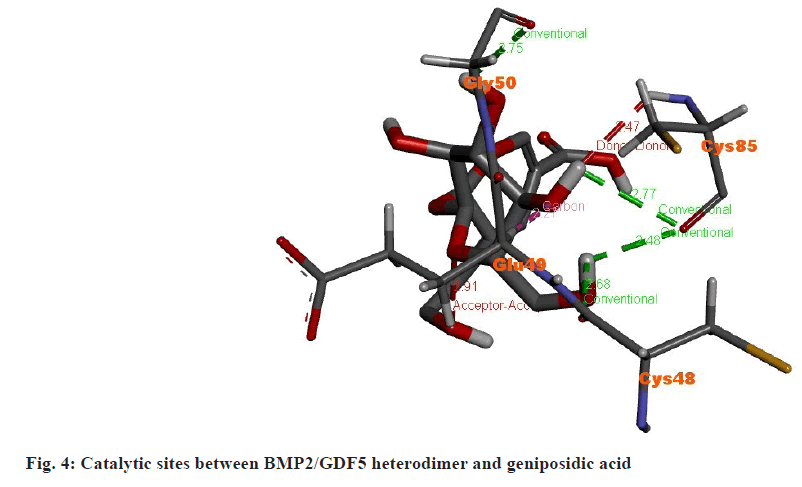
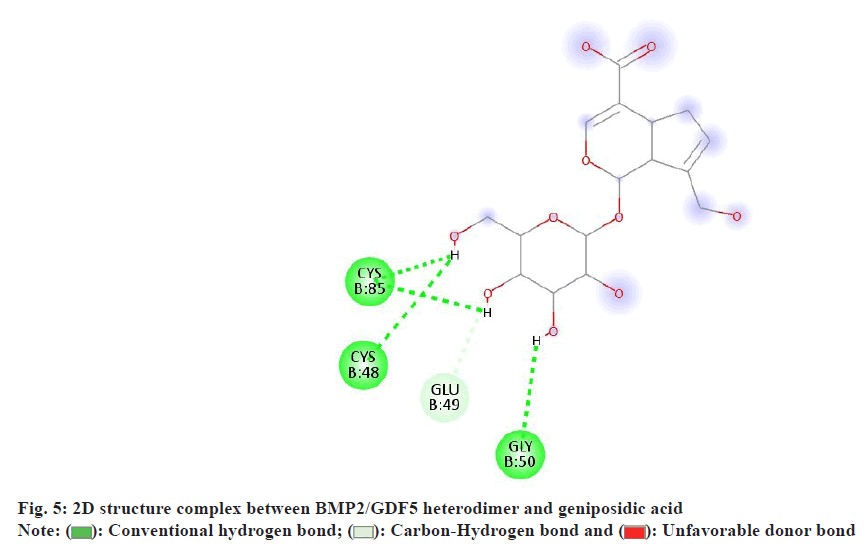
Specific residues play crucial roles in binding and stabilizing the interaction between the BMP2/GDF5 heterodimer and geniposidic acid (fig. 4). Glycine 50 (Gly50), Cysteine 85 (Cys85), and Cysteine 48 (Cys48) form conventional hydrogen bonds with the ligand, providing stability and specificity. Glutamic Acid 49 (Glu49) engages in hydrogen bond donation and acceptance, contributing to the dynamic nature of the interaction. These interactions collectively lower the activation energy, facilitating efficient receptor- ligand binding and enhancing the biological activity of the heterodimer.
In the Two-Dimensional (2D) structural (fig. 5) representation of the BMP2/GDF5 heterodimer interacting with geniposidic acid, specific residues demonstrate distinct bonding interactions.
The green color indicates conventional hydrogen bonds. Gly50, Cys85, and Cys48 engage in these bonds, stabilizing the ligand binding. The light blue color denotes carbon-hydrogen bonds, with Glu49 showing interactions involving carbon acceptor and donor bonding. Unfavorable donor interaction is also highlighted for Glu49, indicating suboptimal hydrogen bond donor interactions. These interactions collectively enhance the binding affinity and specificity of the receptor-ligand complex, facilitating efficient biological activity.
The receptor-ligand complex exhibits optimal binding affinities, characterized by the top nine energy states (Table 1). These energies are indicative of the strength and stability of the interaction. Key metrics include binding affinity, Root-Mean-Square Deviation of the upper bound (RMSD/ub), and RMSD of the lower bound (RMSD/lb). RMSD is a widely used quantitative measure for assessing the similarity between two superimposed atomic coordinates. The lowest energy states suggest the most stable and favorable interactions between the BMP2/GDF5 heterodimer and geniposidic acid, ensuring high binding specificity and efficacy.
| Binding affinity | RMSD/ub | RMSD/lb |
|---|---|---|
| -6.7 | 0 | 0 |
| -6.5 | 16.841 | 14.833 |
| -6.1 | 17.886 | 15.147 |
| -6 | 18.156 | 16.118 |
| -5.7 | 17.597 | 15.909 |
| -5.7 | 18.386 | 16.311 |
| -5.6 | 18.047 | 15.902 |
| -5.5 | 21.787 | 19.159 |
| -5.5 | 19.783 | 17.347 |
Table 1: Receptor-Ligand Complex between BMP2/GDF5 Heterodimer and Geniposidic Acid
The docking simulation between geniposidic acid and the BMP2/GDF5 heterodimer (PDB ID: 8E3G) revealed promising interactions indicative of potential therapeutic effects. Key residues within the heterodimer, including Gly50, Cys85, and Cys48, formed conventional hydrogen bonds with the ligand, contributing to stability and specificity. Glu49 engaged in hydrogen bond donation and acceptance, further enhancing the dynamic nature of the interaction. The docking analysis suggested favorable binding affinity and specificity of geniposidic acid towards the BMP2/GDF5 heterodimer, potentially modulating its signaling pathway. These interactions could lead to enhanced biological activity of the heterodimer, possibly influencing processes such as cell differentiation and tissue regeneration.
The interaction between geniposidic acid and the BMP2/GDF5 heterodimer holds significant therapeutic potential. By modulating the activity of this heterodimer, the resulting drug could be utilized in various clinical applications.
Geniposidic acid influences the enhanced biological activity of the BMP2/GDF5 heterodimer, which could promote bone and cartilage regeneration, beneficial for conditions like osteoporosis and osteoarthritis.
By controlling the molecular BMP2/GDF5, geniposidic acid plays a role as a neurogenesis factor in treating neurodegenerative diseases such as Alzheimer's and Parkinson’s diseases.
This is potentially attributable to cardiovascular diseases because an independent study on BMP2/ GDF5 signaling found that it affects hypertension, endothelial cell functions, vascular remodeling, and blood pressure regulation.
The interaction between geniposidic acid and BMP2/ GDF5 could potentially revolutionize diabetes management by altering pancreatic efficiency and insulin susceptibility, offering new avenues for treatment.
Based on the docking results, the new drug could be worked out, which could contribute to the further pharmacological targeting of the Transforming Growth Factor Beta (TGF-β)-associated diseases which influence bone and joint diseases, muscular system, nervous system, cardiovascular diseases, and diabetes. Whispers also corroborate prior studies regarding evidence on geniposidic acid contribution towards disease treatment. For instance, Li et al.[7] also found that determination of bone anabolic role for geniposidic acid within regards to the differentiation of osteoblasts and creation of bone mass in relation to the BMP2/GDF5 signaling pathway[19]. Moreover, it was identified in earlier literature that geniposidic acid holds neuroprotective properties in the prospective bipolarity signal of neurotrophic and bone morphogen proteins which may play role in neurodegenerative pathway[20].
In the molecular docking study involving kaempferol and the BMP2/GDF5 heterodimer, specific interactions between the ligand (kaempferol) and amino acid residues of the protein play a critical role in the binding affinity and stability of the complex. The analysis reveals that Asp35 forms a conventional hydrogen bond with kaempferol (fig. 6). This type of bond involves sharing a hydrogen atom between the amino acids carboxylate group and the hydroxy groups of kaempferol, contributing significantly to the stability and specificity of the ligand-protein interaction. Additionally, Trp36 interacts with kaempferol through pi-alkyl interactions. These interactions occur between the aromatic ring of the Tryptophan (Trp) residue and the kaempferol molecule’s hydrophobic regions, enhancing the ligand's hydrophobic stability within the binding pocket. Furthermore, the residues His98 and another His98 form pi-pi stacked interactions with kaempferol. Pi-pi stacking involves the alignment of aromatic rings between the Histidine (His) residues and the aromatic system of kaempferol, contributing to the complex’s overall stability and binding strength through favorable non-covalent interactions. These interactions are crucial as they enhance the docking affinity and maintain the structural integrity of the ligand within the protein's active site. Conventional hydrogen bonding with Asp35, pi-alkyl interactions with Trp36, and pi-pi stacking with His98 collectively stabilizes the kaempferol within the BMP2/GDF5 heterodimer. This detailed understanding of the interaction sites and types of bonds formed provides valuable insights into the molecular mechanisms governing the binding affinity and potential biological efficacy of kaempferol in modulating the activity of BMP2/GDF5, which could be significant for therapeutic applications.
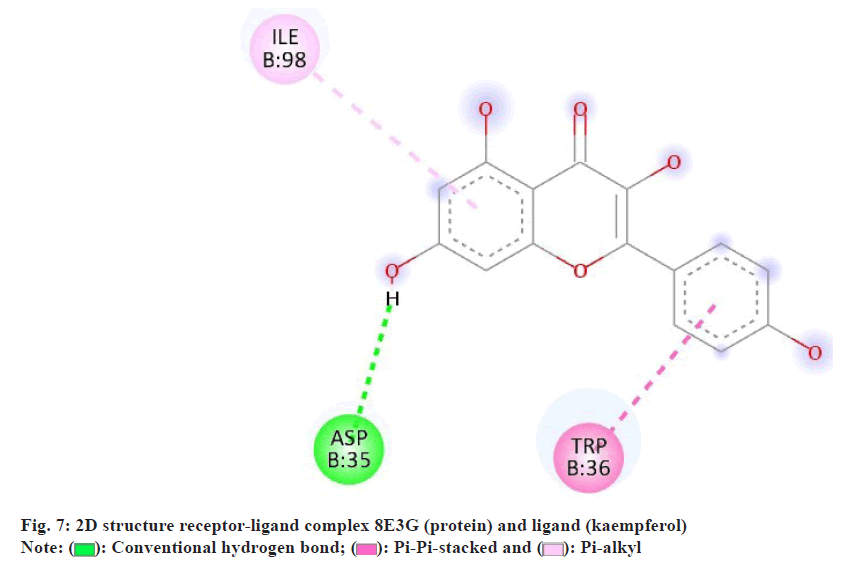
In the interaction between the ligand and the protein, Asp35 forms conventional bonds with the ligand, likely through hydrogen bonding or electrostatic interactions (fig. 7). Asp residues are known for their ability to form strong hydrogen bonds due to their carboxyl functional group. This interaction stabilizes the ligand-protein complex by providing specific binding sites for the liganDrynariae Trp36, on the other hand, engages in pi-alkyl interactions with the liganDrynariae Trp residues are aromatic amino acids containing a large, hydrophobic side chain. In pi-alkyl interactions, the aromatic ring of Trp36 interacts with the ligand’s non-polar groups through van der Waals forces. This interaction contributes to the overall stability of the ligand-protein complex by providing additional hydrophobic contacts. Furthermore, His98 likely forms pi-pi stacked bonds with the liganDrynariae His residues possess an imidazole ring that can participate in pi-stacking interactions with aromatic ligands. In pi-pi stacked bonds, the aromatic rings of His98 and the ligand are aligned parallel to each other, allowing for favorable pi-electron interactions. This interaction stabilizes the ligand-protein complex by enhancing the binding affinity between the two molecules. The combination of conventional bonding by Asp35, pi-alkyl interactions by Trp36, and pi-pi stacked bonds by His98 contributes to the overall stability and specificity of the ligand-protein interaction. These interactions play crucial roles in determining the ligand-protein complex’s binding affinity and functional consequences, potentially influencing downstream signaling pathways and biological responses.
The receptor-ligand complex exhibits optimal binding affinities, characterized by the top nine energy states (Table 2).
| Binding affinity | Rmsd/ub | Rmsd/lb |
|---|---|---|
| -5.9 | 0 | 0 |
| -5.9 | 22.97 | 19.558 |
| -5.8 | 22.615 | 18.922 |
| -5.8 | 6.923 | 1.759 |
| -5.8 | 4.39 | 2.893 |
| -5.8 | 7.15 | 3.068 |
| -5.6 | 7.481 | 2.472 |
| -5.4 | 21.464 | 19.002 |
| -5.3 | 23.487 | 19.74 |
Table 2: Energies of Ligand (Kaempferol) Receptor Complex
Interactions between the ligand (kaempferol) and the protein (BMP2/GDF5 heterodimer), the docking study could potentially yield a novel drug candidate targeting specific signaling pathways implicated in cancer or other diseases. The interactions involving Asp35, Trp36, and His98 suggest that kaempferol may modulate the activity of the BMP2/GDF5 heterodimer by stabilizing its structure and influencing its binding affinity to receptors. Kaempferol, known for its antioxidant properties and its role in reducing oxidative stress, may enhance the signaling capabilities of the BMP2/ GDF5 heterodimer, thereby affecting downstream cellular processes. By forming conventional bonds with Asp35, pi-Alkyl interactions with Trp36, and pi-pi stacked bonds with His98, kaempferol could potentially enhance the stability and activity of the heterodimer complex.
Kaempferol may promote enhanced signaling through the BMP2/GDF5 heterodimer, leading to increased activation of downstream pathways involved in tissue regeneration, bone formation, or other physiological processes.
Improved receptor affinity the interaction with kaempferol may enhance the heterodimer’s affinity for its receptors (e.g., Alk3 and Alk6 receptors), leading to more efficient and specific signaling in target cells.
Given the potential involvement of the BMP2/GDF5 heterodimer in cancer progression and metastasis, modulating its activity by kaempferol could result in anti-cancer effects such as inhibition of cell proliferation, induction of apoptosis, or suppression of tumor growth.
Kaempferol enhanced signaling through the BMP2/ GDF5 heterodimer may promote tissue repair and regeneration, potentially beneficial in treating tissue damage or degeneration conditions.
Overall, the docking study suggests that the interaction between kaempferol and the BMP2/ GDF5 heterodimer could develop a novel drug with therapeutic effects on cancer, tissue regeneration, or other related conditions. Further experimental validation would be necessary to confirm these potential effects and elucidate the precise mechanisms underlying the observed outcomes.
Previous studies have explored the potential therapeutic effects of kaempferol in various contexts. For instance, a study reported the anti-cancer effects of kaempferol, which were mediated by inhibiting the BMP signaling pathway[21]. Additionally, the protective effects of kaempferol in attenuating oxidative stress and promoting tissue repair, potentially involving the BMP signaling pathway[22].
The study employed network pharmacology and molecular docking approaches to explore the mechanisms of action of E. ulmoides and Drynariae Rhizome in promoting fracture healing. The findings from this study contribute to a comprehensive understanding of the mechanisms underlying the therapeutic effects of E. ulmoides and Drynariae Rhizome on fracture healing. The identified bioactive compounds and their interactions with target proteins provide valuable insights for developing more effective and targeted therapies for fracture healing and optimizing existing TCM formulations for bone disorders. Both geniposidic acid and kaempferol exhibit promising interactions with the BMP2/GDF5 heterodimer, suggesting their potential as therapeutic agents targeting the BMP signaling pathway. However, their specific effects may differ based on their interactions and the downstream effects on the signaling cascade. Geniposidic acid’s interactions suggest its potential to promote bone and cartilage regeneration, neuroprotection, cardiovascular health, and diabetes management. These functions align with those of the BMP2/GDF5 heterodimer in tissue regeneration and cell differentiation. On the other hand, kaempferol interactions indicate its potential as an anti-cancer agent by modulating the BMP signaling pathway and its ability to promote tissue repair and regeneration. Additionally, kaempferol antioxidant properties may contribute to its therapeutic effects by reducing oxidative stress. Further experimental validation and clinical studies are necessary to confirm these compounds' therapeutic potential and elucidate the mechanisms underlying their observed effects.
Conflict of interests:
The authors declared no conflict of interests.
References
- Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, et al. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch Osteoporos 2021;16(1):82.
[Crossref] [Google Scholar] [PubMed]
- Malhan D, Schmidt-Bleek K, Duda GN, El Khassawna T. Landscape of well-coordinated fracture healing in a mouse model using molecular and cellular analysis. Int J Mol Sci 2023;24(4):3569.
[Crossref] [Google Scholar] [PubMed]
- Hinton R, Moody RL, Davis AW, Thomas SF. Osteoarthritis: Diagnosis and therapeutic considerations. Am Family Phys 2002;65(5):841-9.
[Google Scholar] [PubMed]
- Peng Z, Xu R, You Q. Role of traditional Chinese medicine in bone regeneration and osteoporosis. Front Bioeng Biotechnol 2022;10:911326.
- Che CT, Wong MS, Lam CW. Natural products from Chinese medicines with potential benefits to bone health. Molecules 2016;21(3):239.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Yang G, Deng X, Shao F, Li Y, Guo W, et al. Molecular sex identification in the hardy rubber tree (Eucommia ulmoides Oliver) via ddRAD markers. Int J Genom 2020;2020(1):2420976.
[Crossref] [Google Scholar] [PubMed]
- Li J, Wang W, Feng G, Du J, Kang S, Li Z, et al. Efficacy and safety of Duhuo Jisheng decoction for postmenopausal osteoporosis: A systematic review and meta?analysis. Evid Based Complement Altern Med 2020;2020(1):6957825.
[Crossref] [Google Scholar] [PubMed]
- Zhang R, Pan YL, Hu SJ, Kong XH, Juan W, Mei QB. Effects of total lignans from Eucommia ulmoides barks prevent bone loss in vivo and in vitro. J Ethnopharmacol 2014;155(1):104-12.
[Crossref] [Google Scholar] [PubMed]
- Ahmed MN, Gowan M, Azam MN, Mannan MA, Rahman MM. Clinical appraisals and phytochemical potential of ethnomedicinal pteridophyte: Drynaria quercifolia (L.) J. Smith (Polypodiaceae). Online 2015;1:4-17.
- Prasanna G, Anuradha R. A comprehensive review on Phytopharmacological activities of Drynaria quercifolia L. Int J Pharmacogn Phytochem Res 2016;8(8):1304-13.
- Noor F, Tahir ul Qamar M, Ashfaq UA, Albutti A, Alwashmi AS, Aljasir MA. Network pharmacology approach for medicinal plants: Review and assessment. Pharmaceuticals 2022;15(5):572.
[Crossref] [Google Scholar] [PubMed]
- Li W, Yuan G, Pan Y, Wang C, Chen H. Network pharmacology studies on the bioactive compounds and action mechanisms of natural products for the treatment of diabetes mellitus: A review. Front Pharmacol 2017;8:74.
[Crossref] [Google Scholar] [PubMed]
- González-Ruiz D, Gohlke H. Targeting protein-protein interactions with small molecules: Challenges and perspectives for omputational binding epitope detection and ligand finding. Curr Med Chem 2006;13(22):2607-25.
[Crossref] [Google Scholar] [PubMed]
- Tao W, Xu X, Wang X, Li B, Wang Y, Li Y, Yang L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol 2013;145(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 2017;7(1):42717.
[Crossref] [Google Scholar] [PubMed]
- Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, et al. PubChem substance and compound databases. Nucl Acids Res 2016;44(D1):D1202-13.
[Crossref] [Google Scholar] [PubMed]
- Safran M, Dalah I, Alexander J, Rosen N, Iny Stein T, Shmoish M, et al. GeneCards Version 3: The human gene integrator. Database 2010;2010:baq020.
[Crossref] [Google Scholar] [PubMed]
- Hu M, Yan H, Li H, Feng Y, Sun W, Ren Y, et al. Use of network pharmacology and molecular docking to explore the mechanism of action of curcuma in the treatment of osteosarcoma. Sci Rep 2023;13(1):9569.
[Crossref] [Google Scholar] [PubMed]
- Liu M, Jin F, Zhang S, Li S, Zhu D, Cui Y, et al. Activation of farnesoid X receptor signaling by geniposidic acid promotes osteogenesis. Phytomedicine 2022;103:154258.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Lai Q, Li Y, Liu Y, Yang M. Learning and memory improvement and neuroprotection of Gardenia jasminoides (Fructus gardenia) extract on ischemic brain injury rats. J Ethnopharmacol 2017;196:225-35.
[Crossref] [Google Scholar] [PubMed]
- Imran M, Rauf A, Shah ZA, Saeed F, Imran A, Arshad MU, et al. Chemo?preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother Res 2019;33(2):263-75.
[Crossref] [Google Scholar] [PubMed]
- Ren JI, Lu Y, Qian Y, Chen B, Wu TA, Ji G. Recent progress regarding kaempferol for the treatment of various diseases. Exp Ther Med 2019;18(4):2759-76.
[Crossref] [Google Scholar] [PubMed]
 Conventional hydrogen bond;
Conventional hydrogen bond; 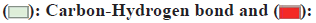 Unfavorable donor bond
Unfavorable donor bond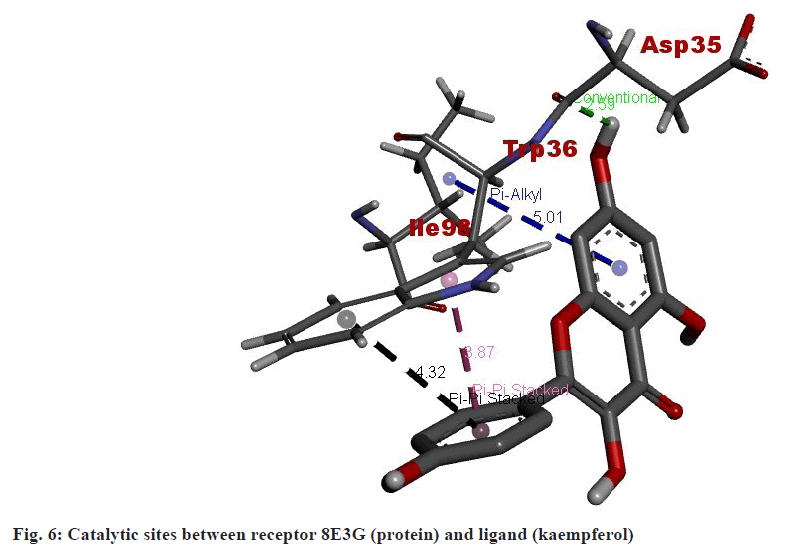
 Conventional hydrogen bond;
Conventional hydrogen bond; 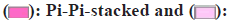 Pi-alkyl
Pi-alkyl