- *Corresponding Author:
- I. Wei
Department of Pediatrics, Beijing University of Traditional Chinese Medicine Subsidiary Dongfang Hospital, Beijing, Fengtai 065001, China
E-mail: lunwen202403@163.com
| Date of Received | 19 September 2022 |
| Date of Revision | 24 March 2023 |
| Date of Acceptance | 04 June 2024 |
| Indian J Pharm Sci 2024;86(3):968-979 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aims to explore the mechanism of action of spleen-tonifying herbs in the spleen-tonifying and spasm-alleviating decoction for treating tic disorders based on network pharmacology and mitochondrial metabolism. Active ingredients and targets of spleen-tonifying herbs were collected from the Traditional Chinese Medicine Systems Pharmacology database. Tic disorder related targets were obtained from Online Mendelian Inheritance in Man, GeneCards, Therapeutic Target database and DisGeNet databases and the intersection targets of drugs and diseases were obtained using Venn diagram. Key targets were obtained through Search Tool for the Retrieval of Interacting Genes/Proteins database to construct a protein-protein interaction network, followed by gene ontology and Kyoto Encyclopedia of Genes and Genomes analysis using the MetaScape online platform. 25 healthy specific pathogen free-grade Sprague-Dawley male rats of 3 w old, were randomly divided into five groups, model group, Western medicine group, spleen-tonifying and spasm-alleviating decoction group, spleen-tonifying herb group and blank control group, with 5 rats in each group. Rats in each group were orally administered interventions at a dose of 1 ml/100g body weight/day for 4 w. Rats in the blank control and model groups were given physiological saline; rats in the Western medicine group were given metoclopramide hydrochloride suspension; rats in the spleen-tonifying herb group were given the spleen-tonifying herb formula and rats in the spleen-tonifying and spasm-alleviating decoction group were given the decoction. The content of mitochondrial respiratory chain enzyme complex IV and sodium-potassium-adenosine triphophatase enzyme in rats were detected. Spleen-tonifying herb group may exert its effects on neurotransmitter regulation, anti-inflammation, regulation of apoptosis, reduction of mitochondrial damage, and protection of neurons through core active ingredients such as baicalein, beta-sitosterol and stigmasterol. Core targets regulate signalling pathways, thereby exerting the spleen-tonifying effects against spasms. Iminodipropionitrile induced tic disorder rats showed a tendency of growth retardation while metoclopramide hydrochloride, spleen-tonifying herb group, and spleen-tonifying and spasm-alleviating decoction group showed acceleration of the growth and development of model rats. However, short-term treatment of 1 mo did not significantly improve the growth and development of tic disorder rats. Spleen-tonifying therapy may provide a new direction for the treatment of tic disorder.
Keywords
Tic disorders, spleen-tonifying and spasm-alleviating decoction, mitochondrial function, network pharmacology
Tic Disorder (TD) is a neurodevelopmental disorder that typically emerges in childhood. Meta-analyses of domestic TD literature from the mid-1960s to 2015 showed a prevalence rate of 6.1 %, while epidemiological studies abroad suggest rates ranging from approximately 0.3 %-0.9 %[1,2]. The incidence of gender parity is approximately 3 to 9:1. Urban areas tend to have a slightly higher prevalence at 2.6 % compared to rural areas at 2.2 %[3]. TD typically manifests between the age of 2 and 21, with peak onset between 5 and 10 y old and maximum severity around (10-12) y old. 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) categorizes TD into Transient Tic Disorder (TTD), Chronic Motor or Vocal Tic (CMVT) disorder, and Tourette Syndrome (TS)[4]. Beyond the tics themselves, most TD patients also experience comorbidities such as Attention Deficit Hyperactivity Disorder (ADHD) or Obsessive- Compulsive Disorder (OCD), as well as conditions like autism spectrum disorders, depression, anxiety, and behavioral disorders, leading to social and psychological distress and impaired quality of life[5,6]. The precise etiology of TD remains unclear, though current research suggests it arises from the interaction of various factors during different stages of childhood development, including genetics, immune infections, central neurotransmitters, and psychological environment[7]. Western medicine primarily treats TD with dopamine receptor blockers, often supplemented with psychological and behavioral therapies. However, there lacks a unified standard regarding dosage and combination of medications in Western pharmacotherapy and clinical practice often focuses on short-term symptom control, leading to adverse reactions, high relapse rates upon discontinuation and low longterm adherence[8]. Given that TD predominantly affects children and adolescents, and it significantly impacts their learning and daily life, thereby imposing heavy burden on families and society[9]. With its increasing prevalence and characteristic of chronicity and relapse, there is a growing societal demand for understanding the pathogenesis of TD, achieving early and accurate diagnosis, avoiding misdiagnosis and implementing effective treatments. Seeking treatments with confirmed efficacy and minimal adverse effects is a primary focus of the current research.
Traditional Chinese Medicine (TCM) and clinical practice suggests that tonifying spleen is an effective approach for treating TD. Herbal formulations primarily tonifying the spleen demonstrate significant clinical efficacy, particularly in treating TD children with syndromes of spleen deficiency and liver hyperactivity[10]. Thus, the spleen is considered as a crucial organ in the pathogenesis of TD and spleen tonification constitutes as an important aspect of TD treatment[11]. However, current research on TCM treatment for pediatric TD relies on empirical summaries and clinical observations, with limited mechanistic studies. Therefore, improving the pharmacological mechanism research of spleen-tonifying drugs against tics will provide an objective evidence for their clinical application[12].
Current research suggests that mitochondrial damage is not only a pathological consequence of TD but is also a factor exacerbating tic symptoms. Mitochondrial energy metabolism may play a role in the occurrence and development of neurological and psychiatric disorders, particularly abnormalities in mitochondrial structure and the Electron Transport Chain (ETC), affecting oxidative phosphorylation function and causing energy supply disruptions[13]. Variations in mitochondrial genes, alterations in striatal mitochondrial structure and function may be associated with the pathogenesis of TD[14]. Shi et al.[15], using transmission electron microscopy, found alterations in mitochondrial structure in rats with TD induced by intraperitoneal injection of Iminodipropionitrile (IDPN) combined with uncertain empty water bottle stimulation, accompanied by anxiety disorders. Irregular mitochondria were observed in the prefrontal cortex with partial mitochondrial membrane loss and swelling, indicating functional impairment[16]. The number of abnormal mitochondria in the striatum significantly increased with double membrane rupture and disruption of inner membranes. Spleen-tonifying and spasm-alleviating decoction was able to reverse the mitochondrial functional impairment in affected animals. Therefore, this study aims to elucidate the mechanism of tics based on network pharmacology and mitochondrial energy metabolism, aiming to provide data support for the clinical treatment.
Materials and Methods
Screening of active ingredients and corresponding targets of TCM:
The effective chemical constituents and target genes of Radix Codonopsis, Rhizoma Atractylodes macrocephala, Poria cocos, Pericarpium Citri Reticulatae and Pinellia ternata were obtained through the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP). Chemical constituents with an Oral Bioavailability (OB) ≥30 % and Drug-Likeness (DL) ≥0.18 were considered as effective ingredients and their corresponding targets were retrieved.Protein target standardization was performed using the Universal Protein Resource (UniProt) database (https://www.uniprot.org/), with the species set to "Homo sapiens". The network of herbal medicines, active ingredients and key targets was constructed using Cytoscape 3.9.0 software.
Acquisition of disease targets:
All potential targets for treating TS were collected from databases such as Online Mendelian Inheritance in Man (OMIM), GeneCards, Therapeutic Target Database (TTD), and DisGeNet. DrugBank database (https://www.drugbank.ca) was used to search supplement disease-related genes with clinical 1st-line drug targets for TD treatment. After merging the obtained target databases, duplicate values were removed to obtain TD targets.
Construction of Protein-Protein Interaction (PPI) network:
Using bioinformatics software, the intersection of disease and drug targets was obtained and visualized using a Venn diagram. The intersection targets were uploaded to the online PPI network analysis platform Search Tool for the Retrieval of Interacting Genes/Proteins (STRING), selecting "Multiple proteins" and "Homo sapiens" as species. The PPI network and core genes of Radix Codonopsis, Rhizoma Atractylodis macrocephala, Poria cocos, Pericarpium Citri Reticulatae and Pinellia ternata with TD were obtained.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis:
The key targets of Radix Codonopsis, Rhizoma Atractylodis macrocephala, Poria cocos, Pericarpium Citri Reticulatae, and Pinellia ternata in treating TD were entered into the MetaScape platform. GO annotation analysis and KEGG pathway enrichment analysis were performed for Homo sapiens species. Top-ranked biological processes and pathways were screened.
Experimental animals and drug preparation:
25 healthy, male Specific-Pathogen Free (SPF) grade Sprague Dawley (SD) rats of 3 w old weighing (50±10) g which were provided by the Beijing Institute for Food and Drug Control (Daxing) (License no: 2022-0002). The rats were conventionally raised in the SPF-grade experimental animal room of the Institute of Basic Theory of Chinese Medicine and China Academy of Chinese Medical Sciences, with a Light (L)- Dark (D) cycle of 12L/12D (6:00-18:00) at room temperature (20°±2°) and having relative humidity of 50 %-60 %. The environment was quiet, with free access to water, and the rats were acclimatized for 1 w.
Chinese herbal granules which were provided by Sichuan New Green Pharmaceutical Technology Development Co., Ltd., and Jiawei Zhidong decoction were used as test drug. Jiawei Zhidong decoction comprised of 3 g of Gentian species, 6 g of Saposhnikovia divaricata, 6 g of Ligusticum chuanxiong, 10 g of Angelica sinensis, 10 g of Radix Codonopsis, 10 g of Rhizoma Atractylodis macrocephala, 10 g of Poria cocos, 6 g of Pinellia ternata, 6 g of Pericarpium Citri Reticulatae and 10 g of Uncaria. Similarly, spleen-tonifying group comprised of 10 g of Radix Codonopsis, 10 g of Rhizoma Atractylodis macrocephala, 10 g of Poria cocos, 6 g of Pinellia ternata and 6 g of Pericarpium Citri Reticulatae.
Further, loxapine hydrochloride which is a 1stline clinical drug for TD treatment was used as a positive control drug. It was provided by the Jiangsu Enhua Pharmaceutical Co., Ltd., with specification of 100 mg/tablet.
Experimental reagents and equipment:
Rat mitochondrial respiratory chain complex IV Enzyme-Linked Immunosorbent Assay (ELISA) kit/96T (Provided by Jiangsu Crystal Biotech Co., Ltd.), rat Sodium/Potassium (Na+/K+) Adenosine Triphosphatase (ATPase) ELISA kit/96T (Provided by Jiangsu Crystal Biotech Co., Ltd.). Bicinchoninic Acid (BCA) protein quantification kit was provided by Shanghai Biyun Tian company. Acetonitrile was provided by Sigma, United States of America (USA) (100 ml/bottle, 317306). The enzyme labeling instrument model used was Rayto RT-6100.
Animal model preparation and drug treatment:
After acclimatization feeding for 1 w, the animals were numbered according to their weight, and were randomly divided into 5 groups, model group, Western medicine group, spleen-tonifying and spasm-alleviating decoction group, spleentonifying herb group and blank control group, each with 5. Except for the blank control group, other 4 groups of rats were intraperitoneally injected with 250 mg/kg of IDPN which was dissolved in normal saline according to 1 ml/kg/d volume and the blank control group was injected with an equivalent volume of normal saline, once consecutively for 7 d. On the 7th d of modeling, rats with choreiform movements scoring ≥1 were considered successfully modelled and included in the groups. From the 8th d after modeling, the rats in all the 5 groups were orally administered with interventions, with a volume of 1 ml/100 g, administered daily from 5 AM to 7 PM. Blank control group and model group were given normal saline; Western medicine group was given loxapine hydrochloride suspension; spleen-tonifying herb group was given the spleen-tonifying herb decoction, and the spleen-tonifying and spasmalleviating decoction group was given the spleentonifying and spasm-alleviating decoction.
Evaluation indicators:
Choreiform movement scoring was conducted after 7 d of modeling and after 4 w of oral administration; each rat was observed for 5 min. The scoring was performed in a double-blind manner, and the average of two observers' scores was used for final calculation. Choreiform movement scoring criterion was referenced from the Diamond test scoring method. 0 points denoted no choreiform movement; 1 point means rotational behavior (clockwise or counterclockwise rotation); 2 points comprehends to excessive vertical movement of the head and neck; 3 points denotes excessive vertical movement of the head and neck combined with rotational behavior and 4 points comprehend to head tilting, combined with excessive vertical movement of the head and neck.
Specimen collection and indicator detection:
After 4 w of administration, rats were euthanized with a 4 % sodium pentobarbital solution (0.1 ml/100 g) administered intraperitoneally 24 h after the last administration. Then, the rats' brains were rapidly removed on ice after decapitation, and the striatum was dissected and weighed. The striatum was then placed in 1.5 ml Eppendorf (EP) tubes, weighed, and stored in a -80° freezer for later use. The time from decapitation to entry into the -80° freezer was within 5 min. The striatum was rinsed with pre-cooled Phosphate-Buffered Saline (PBS) (0.01 M, pH=7.4) to remove residual blood (lysing red blood cells in the homogenate could affect measurement results). After weighing, an equivalent volume of PBS (100 μl PBS per 10 mg sample) was added to the striatum tissue at a ratio of 1:10 (10 mg sample in 100 μl PBS), followed by thorough grinding on ice using a glass homogenizer. Finally, the homogenate was centrifuged at 5000 ×g for 5-10 min, and the supernatant was collected for testing. The precipitate was washed with washing solution, centrifuged, and stored in 0.5 ml aliquots for later use.
BCA protein quantification assay:
According to the instructions of the BCA kit, standard protein was prepared at a concentration of 0.5 mg/μl. Standard protein (0 μl, 1 μl, 2 μl, 4 μl, 8 μl, 12 μl, 16 μl and 20 μl) was added to the wells of a 96-well plate, and each well was supplemented with distilled water to a total volume of 20 μl. Then, 1 μl of striatal tissue homogenate was added to each corresponding well, and distilled water was added to a total volume of 20 μl. Subsequently, 200 μl of BCA working solution was added to each well. After incubating at 37° for 30 min, the absorbance at 562 nm was measured using ELISA reader, and a standard curve of protein concentration was plotted. The protein concentration of the samples was calculated based on their Optical Density (OD) values.
Measurement of rat mitochondrial respiratory chain complex IV and Na+-K+ATPase enzyme levels:
20× buffer was diluted with distilled water at a ratio of 1:19 and then washed. After balancing at room temperature for 20 min, the required strips were taken out from the aluminium foil bag, and the remaining strips were sealed in a self-sealing bag and the temperature was returned to 4°. Standard and sample wells were set up, with 50 μl of different concentrations of standard protein added to each standard well and 50 μl of the test sample added to each sample well. No solution was added to the blank wells. In addition to the blank wells, 100 μl of Horseradish Peroxidase (HRP)-labeled detection antibody was added to each standard and sample well and the reaction wells were sealed with sealing film and incubated at 37° for 60 min. After discarding the liquid, the plate was tapped dry on absorbent paper, filled with wash solution (350 μl/well) left to stand for 1 min, and the solution was removed by flicking the plate. This washing process was repeated 5 times. Subsequently, 50 μl of substrates A and B were added to each well and was incubated at 37° in the dark for 15 min. Finally, 50 μl of stop solution was added to each well and the OD values of each well were measured at a wavelength of 450 nm within 15 min.
Statistical analysis:
Each sample was assayed in duplicate and the average of three measurements was calculated. Using the OD values of the standard protein as the X-axis and the concentration values of the standard protein as the Y-axis, a standard curve was plotted either on graph paper or using relevant software to obtain a linear regression equation. OD values of the samples were substituted into the equation to calculate the sample concentration. The protein concentration of the samples was divided by the protein concentration quantified by the protein quantification assay to obtain the protein equivalent concentration, for data analysis. Statistical analysis was performed using the R language software (4.1.1 version). Normally distributed data was expressed as mean±standard deviation (x±s). For data with homogeneous variance, One-Way Analysis of Variance (ANOVA) was used, followed by pairwise comparisons using Tukey's Honestly Significant Difference (HSD) test. For data with inhomogeneous variance, Welch's ANOVA was used, and multiple comparisons between groups were performed using Tamhane’s T2 test. Nonnormally distributed data were analysed using non-parametric tests with a statistically significant value, p<0.05.
Results and Discussion
Active ingredients and targets of the drugs were studied. By searching the TCMSP database and reviewing relevant literature, a total of 33 effective ingredients were obtained in the spleen-invigorating drug group by limiting the parameters of OB and DL value (12 for Pinellia effective ingredients, 5 for tangerine peel effective ingredients, 6 for Poria effective ingredients, 6 for Astragalus effective ingredients and 4 for Atractylodes effective ingredients) (Table 1). Among them, Beta (β)- sitosterol exists in both Astragalus and Pinellia. After removing duplicate targets, a total of 172 related targets were involved. A total of 1753 targets related to TD were extracted from the databases GeneCards, OMIM, TTD, DisGeNet and DrugBank database. There were 69 intersection targets between the disease and the drugs.
| Number | Molecular (MOL) ID | Component |
|---|---|---|
| TZS1 | MOL001790 | Linarin |
| TSZ2 | MOL006756 | Schottenol |
| TZS3 | MOL002464 | 1-monolinolein |
| A1 | MOL000358 | β-sitosterol |
| TZS4 | MOL000006 | Luteolin |
| TZS5 | MOL001689 | Acacetin |
| BZ1 | MOL000022 | 14-acetyl-12-senecioyl-2E,8Z,10E-atractylentriol |
| BZ2 | MOL000033 | (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R,5S)-5-propan-2-yloctan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| BZ3 | MOL000049 | 3β-acetoxyatractylone |
| BZ4 | MOL000072 | 8β-ethoxy atractylenolide Ⅲ8 |
| FL1 | MOL000273 | (2R)-2-[(3S,5R,10S,13R,14R,16R,17R)-3,16-dihydroxy-4,4,10,13,14-pentamethyl-2,3,5,6,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-methylhept-5-enoic acid |
| FL2 | MOL000275 | Trametenolic acid |
| FL3 | MOL000279 | Cerevisterol |
| FL4 | MOL000282 | Ergosta-7,22E-dien-3β-ol |
| FL5 | MOL000283 | Ergosterol peroxide |
| FL6 | MOL000296 | Hederagenin |
| CP1 | MOL000359 | Sitosterol |
| CP2 | MOL004328 | Naringenin |
| CP3 | MOL005100 | 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one |
| CP4 | MOL005815 | Citromitin |
| CP5 | MOL005828 | Nob |
| BX1 | MOL001755 | 24-Ethylcholest-4-en-3-one |
| BX2 | MOL002670 | Cavidine |
| BX3 | MOL002714 | Baicalein |
| A1 | MOL000358 | β-sitosterol |
| BX4 | MOL000449 | Stigmasterol |
| BX5 | MOL005030 | Gondoic acid |
| BX6 | MOL000519 | Coniferin |
| BX7 | MOL006936 | 10,13-eicosadienoic |
| BX8 | MOL006957 | (3S,6S)-3-(benzyl)-6-(4-hydroxybenzyl)piperazine-2,5-quinone |
| BX9 | MOL003578 | Cycloartenol |
| BX10 | MOL006967 | beta-D-Ribofuranoside, xanthine-9 |
| BX11 | MOL002776 | Baicalin |
Table 1: Effective ingredients in the spleen-invigorating drug group
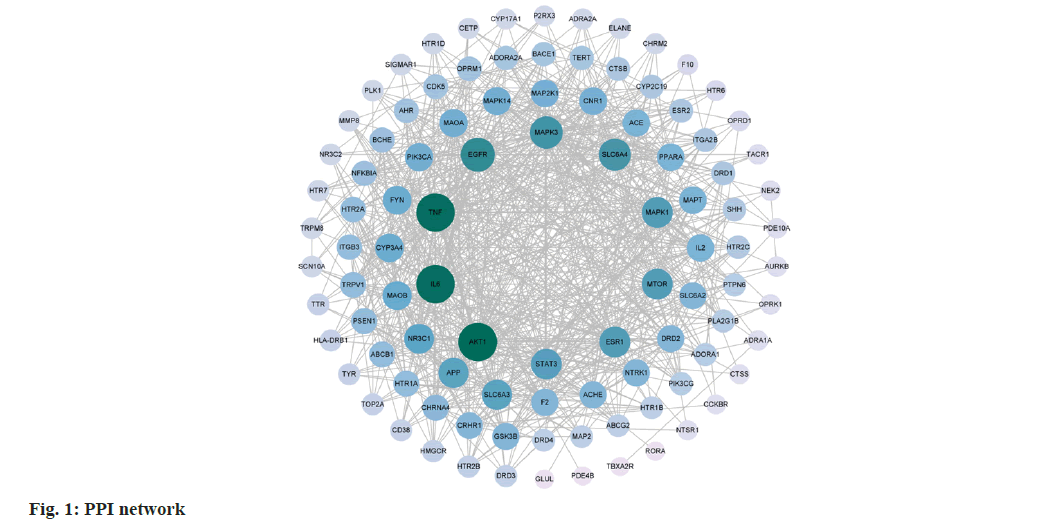
Then PPI network was constructed. 69 target genes were imported into the String database to obtain a "tsv" format file, which was then imported into Cytoscape 3.9.0 software for visualization analysis. A confidence level >0.4 was chosen, resulting in a network with 100 nodes and 762 edges (fig. 1). Network analyser was used to analyse the topological data and core targets for the treatment of TD with spleen-invigorating drug group were selected based on degree centrality. The top 10 ranked targets are Threonine Kinase 1 (AKT1), Interleukin 6 (IL6), Tumor Necrosis Factor-Alpha (TNF-α), Epidermal Growth Factor Receptor (EGFR), Mitogen-Activated Protein Kinase 3 (MAPK3), Solute Carrier Family 6 Member 4 (SLC6A4), mammalian Target of Rapamycin (mTOR), Estrogen Receptor 1 (ESR1), MAPK1 and Signal Transducer and Activator of Transcription 3 (STAT3).
Establishment and analysis of the network of herbal medicine-effective components-key targets was studied. The network of herbal medicineeffective components-key targets was constructed and analysed by importing the main target genes of Radix Ginseng, Rhizoma Atractylodis macrocephala, Poria cocos, Pericarpium Citri Reticulatae and Rhizoma Pinellia, as well as the intersection targets related to TD into Cytoscape 3.7.2 software (fig. 2). There are a total of 69 common targets between the medicine and the disease, with 95 nodes in total. The interactions between nodes, represented by edges, amount to 240. Effective components selected were those with a degree value >10, including cavidine (degree=14), baicalein (degree=10), β-sitosterol (degree=36), stigmasterol (degree=15), naringenin (degree=12), Nobiletin (NOB) (degree=13) and luteolin (degree=21).
Analysis of target function and pathway enrichment was studied. Using Metascape database, GO and KEGG pathway analysis were performed on the 69 key gene targets. There were 87 related pathways of Molecular Function (MF), 74 related pathways of Cellular Component (CC), 449 related pathways of Biological Processes (BP) (fig. 3a). There were 128 modified pathways in KEGG analysis (fig. 3b). GO enrichment analysis revealed significantly enriched pathways in biological processes such as G protein-coupled receptor signalling pathway, cyclic nucleotide 2nd messenger, response to external stimulus, response to drugs, chemical synaptic transmission, regulation of synaptic vesicle exocytosis, response to ethanol, response to cocaine, excitatory postsynaptic potential, phospholipase C-activating G protein-coupled receptor signalling pathway and positive regulation of nitric oxide biosynthetic process, etc. Significant pathways enriched in CC included synaptic membrane, integral component of plasma membrane, dendrites, cytoplasmic membrane, synaptic membrane integral component, membrane raft, neuron cell body, synapse, glutamatergic synapse and axon, etc. Enriched pathways in MFs included G protein-coupled serotonin receptor activity, identical protein binding, serotonin binding, neurotransmitter receptor activity, enzyme binding, AKT1, Ribonucleic Acid (RNA) polymerase II transcription factor activity ligandactivated sequence-specific Deoxyribonucleic Acid (DNA) binding protein, AKT1 activity, opioid receptor activity and protein binding, etc. KEGG pathway enrichment analysis indicated that these target genes were associated with serotonin synapse, cyclic Adenosine Monophosphate (cAMP), calcium, prolactin, T-cell receptor, cholinergic synapse, neurotrophin and sphingolipid signalling pathways. Programmed Cell Death Ligand 1 (PD-L1) expression and Programmed cell Death protein 1 (PD-1) checkpoint pathway in cancer, Fc Epsilon RI (FcεRI) signalling pathway, endocrine resistance, chemical carcinogenesisreceptor activation, C-type lectin receptor signalling pathway, toll-like receptor signalling pathway, TNF signalling pathway, gap junction, platelet activation, protein glycosylation in cancer and osteoclast differentiation, etc.
Rate of weight gain in rats was observed. On 1st d after modelling, there was no statistically significant difference (p=0.703 and p>0.05) in the comparison of body weight among the groups except in the blank group (Table 2). The body weight of rats in the blank group was significantly higher than that of the other four groups (p=0.000 and p<0.05). After following group gavage administration, there were differences in body weight growth rate between groups except for the 2nd w (p<0.05), further twoby- two comparison of body weight growth rate between groups, except for the blank group, there was no difference between groups (p<0.05). The growth rate of body weight in the blank group was faster than that of the model group in the 1st w, but there was no statistically significant difference (p>0.05), and in the 2nd w, the body weight growth rate of the Western medicine group reached the peak, at which its growth rate was faster than that of the model group (p>0.05). Peak value refers to higher growth rate which was faster than the model group, the spleen-strengthening and stopping the movement of the soup group and the spleenstrengthening medicine group, but there was no statistical significance (p=0.180 and p>0.05), the blank group in the 3rd w body weight growth rate was slower than that of the modelled model group (p=0.001 and <0.05), the spleen-strengthening medicine group (p=0.000 and p<0.05), the spleenstrengthening and stopping the movement of the soup group (p=0.000 and p<0.05), and the model group, The model group, the spleen-strengthening and stopping movement soup group, and the spleen-strengthening medicine group showed the peak of weight gain rate, and the weight gain rate of each group became a decreasing trend in the 4th w.
| Group | Body weight on 1st d after modelling (g) | 1st w | 2nd w | 3rd w | 4th w |
|---|---|---|---|---|---|
| Control | 0.342±0.052 | 0.276±0.016 | 0.192±0.015 | 0.089±0.042 | |
| Model | 104.46±13.223 | 0.273±0.128 | 0.304±0.047 | 0.302±0.050* | 0.144±0.007 |
| Western medicine | 104.82±7.568 | 0.160±0.033* | 0.351±0.045 | 0.261±0.036 | 0.165±0.022* |
| Spleen-tonifying and spasm-alleviating decoction | 106.68±4.926 | 0.207±0.072 | 0.300±0.040 | 0.315±0.024* | 0.103±0.030 |
| Spleen-tonifying herb | 110.8±5.271 | 0.130±0.033* | 0.293±0.057 | 0.318±0.024* | 0.121±0.049 |
| F | 0.476 | 5.577 | 1.743 | 10.699 | 3.561 |
| p | 0.703 | 0.003 | 0.180 | 0.000 | 0.024 |
Note: Compared with the control group, *p<0.05
Table 2: Comparison of body weight and growth rate of rats in each group on 1st D after modelling
Rat stereotyped movement score was studied. After successful modeling, there was no statistical difference in stereotyped movement scores among the groups compared to the control group (*p=0.673 and p>0.05). After 4 w of gastric lavage administration, there were differences in stereotyped movement scores among the groups (*p<0.05). Further pairwise comparisons revealed differences in stereotyped movement scores among the groups, with the stereotyped movement score in the herbal spleen-tonifying group lower than that in the model group (*p=0.022 and <0.05). The stereotyped movement score in the herbal spleentonifying group was also lower than that in the herbal spleen-stopping decoction group (*p=0.016 and p<0.05). There were no statistical differences in stereotyped movement scores among the other groups (p>0.05) (Table 3).
| Group | Before drug administration | After 4 w of drug administration |
|---|---|---|
| Model | 2.9±1.02 | 2.7±0.98 |
| Western medicine | 3.4±0.58 | 3.0±0.71 |
| Spleen-tonifying and spasm-alleviating decoction | 3.1±0.86 | 3.6±0.49 |
| Spleen-tonifying herb | 3.5±0.45 | 1.4±1.46*# |
| F | 0.523 | 5.053 |
| p | 0.673 | 0.012 |
Note: *p<0.05 and #p<0.05, respectively
Table 3: Stereotyped motor scores of rats before and after drug administration in each group
Rat striatum mitochondrial respiratory chain complex IV content and Na+-K+ATPase enzyme content was measured. Based on one-way ANOVA and Tukey's HSD test, there was no statistically significant difference in the content of rat striatum mitochondrial respiratory chain complex IV among the groups (p=0.306 and p>0.05). Similarly, the analysis showed no significant difference in the content of rat striatum mitochondrial Na+- K+ATPase enzyme among the groups (p=0.133 and p>0.05) (Table 4).
| Group | Rat striatum mitochondrial respiratory chain complex IV | Na+/K+-ATPase |
|---|---|---|
| Blank | 657.21±176.388 | 1.786±0.448 |
| Model | 567.11±82.561 | 1.78±0.199 |
| Western medicine | 637.614±135.075 | 1.796±0.401 |
| Spleen-tonifying and spasm-alleviating decoction | 544.738±111.379 | 1.444±0.311 |
| Spleen-tonifying herb | 503.526±106.089 | 1.294±0.440 |
| F | 1.293 | 2.002 |
| p | 0.306 | 0.133 |
Table 4: Conversion of enzyme content in rat striatum mitochondria
The main active components of the drug, such as baicalein and β-sitosterol have complex mechanisms that can function in multiple aspects such as cell apoptosis, mitochondrial damage and neuronal injury to improve neurological and psychiatric disorders. Baicalein can regulate B cell lymphoma-2 (Bcl-2) family members, reduce DNA fragmentation and prevent mitochondrial apoptosis. β-sitosterol possesses various biological activities such as cholesterol-lowering, hypoglycemic, antioxidant, anti-inflammatory, antimicrobial and anticancer effects. Stigmasterol can increase the levels of Gamma (γ)-Aminobutyric Acid (GABA) and Glutathione (GSH), reduce the levels of dopamine, acetaldehyde and TNF-α and decreases acetylcholinesterase activity, indicating its potential in ameliorating neurological and psychiatric symptoms, which suggests its involvement in TD by modulating various neurotransmitter levels.
The key targets, AKT1 and mTOR belong to the Phosphoinositide 3 Kinase (PI3K)/Akt/mTOR signalling pathway, which is not only crucial for mitochondrial quality control and cellular homeostasis but also participates in neuronal growth, differentiation, and development, while inhibiting the activity of the PI3K/Akt/mTOR pathway may lead to neuronal damage[17]. Among them, mTOR can promote the synthesis of many nuclear-encoded mitochondrial regulatory factors, providing a basis for mitochondrial biogenesis[18]. MAPK1 and MAPK3 belong to the MAPK family, which is a cascade phosphorylation process composed mainly of three kinase modules namely, MAPK kinase kinase (MAPKKK), MAPK kinase (MAPKK) and MAPK[19]. This pathway can be activated by exogenous stimuli such as cytokines, growth factors, neurotransmitters, and hormones. The pathway MAPK1/3, in which MAPK1 and MAPK3 are involved, participates in various cellular physiological functions such as cell growth, proliferation, migration, differentiation, homeostasis regulation, and apoptosis by sensing and regulating mitochondrial energy metabolism levels[20]. Therefore, the core targets AKT1, mTOR, MAPK1, MAPK3, EGFR, etc., by regulating the PI3K/Akt/mTOR signalling pathway and MAPK signalling pathway, modulate mitochondrial homeostasis, energy metabolism, thereby affecting cell apoptosis, neuronal differentiation, and growth, influencing the occurrence of TD. The key targets, IL-6 and TNF mainly participate in neuroinflammatory responses[21]. They may impair intracellular neuronal signalling through inflammatory reactions and immune mechanisms, thereby contributing to the occurrence and development of TD[22].
Network pharmacology predicts mechanisms of TD suggest that in addition to neurotransmitter hypotheses and immune responses, mitochondria may also influence the occurrence and development of TD[23]. Animal experiments have also validated this conclusion. Currently, the widely used stereotypic movement scoring (using the Diamond rating method as reference) evaluates abnormal behaviors in IDPN experimental rats[24]. The cortico-striato-thalamo-cortical loop plays a key role in generating stereotypic movements[25]. Repetitive administration of dopamine agonists or selective dopamine reuptake inhibitors, either directly or indirectly, has been shown to induce stereotypic behavior or self-injury. Studies in mice with reduced inhibitory synaptic function due to Neuroligin 2 (NLGN2) gene deletion have found a negative correlation between NLGN2 expression and stereotypic movement[26]. Therefore, the stereotypic movement behavior of rats can indicate the excitability of the central nervous system. The higher the score of stereotypic movement evaluation, the closer the performance is to that of TD patients[27]. The current mainstream hypothesis suggests that TD may be related to abnormal cortico-striato-thalamo-cortical loop, among many factors affecting this loop, the excessive dopamine activity or dopamine receptor hypersensitivity in the striatum is considered as a key factor in the pathogenesis of TD[28]. Mitochondria provide 93 % of the ATP required by the brain, which is involved in various stages of cortico-striatothalamo- cortical loop, including neurotransmitter transmission. ATP is essential for driving ion pumps, maintaining ion gradients, and supporting vesicle cycling and mitochondrial movement at synapses[29]. Additionally, they have other functions at synapses, such as regulating Calcium ion (Ca2+) concentration. Ca2+ released into the cytosol through mitochondrial Permeability Transition Pore (mPTP) or Sodium-Calcium exchangers (NCX) triggers synaptic vesicle Exocytosis, which in turn triggers neurotransmitter release. Mitochondria adjust their morphology and distribution in neurons to meet specific needs. Morphological adjustments include mitochondrial fusion and fission, where mitochondrial fusion networks can repair damage and share genetic information[30]. Mitochondrial fusion and fission need to be balanced to ensure neuronal health and help maintain normal synaptic function[31]. Synaptic activity is directly related to mitochondrial transport and distribution[32]. Large amounts of energy are required during synaptic activity, which requires mitochondria to aggregate at presynaptic terminals and dendritic spines for energy supply. This suggests that we should pay attention to the role of mitochondrial energy metabolism in the pathogenesis of TD.
This study has certain limitations. Previous literature has shown that changes in mitochondrial structure and function in spleen deficiency model animals are more commonly observed in gastric mucosa, intestinal epithelial mucosa, and liver. However, in this experiment, the site was limited to the striatum of model rats, and it is possible that neuronal mitochondrial damage expression may be lower than other sites such as the gastrointestinal mucosa, liver, and cerebral cortex. Secondly, there are various methods available for detecting mitochondrial damage, including immunohistochemistry, ELISA, Real- Time quantitative Polymerase Chain Reaction (qRT-PCR), Western blot for detecting proteins and DNA, fluorescence probes for detecting mitochondrial membrane potential, and morphological observations such as transmission electron microscopy for observing mitochondrial morphology and staining techniques for observing mitochondrial quantity. Single ELISA test may not fully reflect the state of mitochondrial structural and functional damage. We will address the current limitations in further research.
The spleen medicine group may exert its anti- TD effects through core active ingredients such as baicalein, β-sitosterol, and stigmasterol by regulating neurotransmitters, anti-inflammatory responses, apoptosis, reducing mitochondrial damage, and protecting neurons. Through core targets such as AKT1, mTOR, EGFR, MAPK1, and MAPK3, the spleen medicine group regulates signalling pathways such as PI3K/Akt/mTOR, cAMP signalling, Ca+2 signaling, neurotrophic signalling and sphingolipid signalling to modulate apoptosis and protect neurons, thereby exerting anti-TD effects. TD rats induced by IDPN showed a tendency of growth retardation. Hydrochloride metoclopramide, the spleen medicine group, and the spleen-calming decoction all showed a tendency to accelerate the growth and development of model rats, but short-term treatment (1 mo) did not significantly improve the growth and development of TD rats.
Conflict of interests:
The authors declared no conflict of interests.
References
- Fu Q, Zhang X, Yan H, Xu J, Liu H, Yang L, et al. Acupuncture for treating tic disorders in children: A protocol for systematic review and meta-analysis. Medicine 2021;100(12):1-12.
[Crossref] [Google Scholar] [PubMed]
- Chang YC, Daza R, Hevner R, Costa LG, Cole TB. Prenatal and early life diesel exhaust exposure disrupts cortical lamina organization: Evidence for a reelin-related pathogenic pathway induced by interleukin-6. Brain Behav Immun 2019;78:105-15.
[Crossref] [Google Scholar] [PubMed]
- Liu H, Tang L, Li Y, Xie W, Zhang L, Tang H, et al. Nasopharyngeal carcinoma: Current views on the tumor microenvironment's impact on drug resistance and clinical outcomes. Mol Cancer 2024;23(1):1-20.
[Crossref] [Google Scholar] [PubMed]
- Andrén P, de la Cruz LF, Isomura K, Lenhard F, Hall CL, Davies EB, et al. Efficacy and cost-effectiveness of therapist-guided internet-delivered behaviour therapy for children and adolescents with Tourette syndrome: study protocol for a single-blind randomised controlled trial. Trials 2021;22(1):669-75.
[Crossref] [Google Scholar] [PubMed]
- Taylor MJ, Martin J, Lu Y, Brikell I, Lundstrom S, Larsson H, et al. Association of genetic risk factors for psychiatric disorders and traits of these disorders in a Swedish population twin sample. JAMA Psychiatry 2019;76(3):280-9.
[Crossref] [Google Scholar] [PubMed]
- Ramsey KA, de Nadai AS, Espil FM, Ricketts E, Stiede JT, Schild J, et al. Urge intolerance predicts tic severity and impairment among adults with Tourette syndrome and chronic tic disorders. Front Psychiatry 2022;13:1-13.
[Crossref] [Google Scholar] [PubMed]
- Zhou F, Zhu Q, Zheng PF, Feng YL. Association of fucosyltransferase 2 gene variant with inflammatory bowel diseases: A meta-analysis. Med Sci Monit 2019;25:184-92.
[Crossref] [Google Scholar] [PubMed]
- Chen Z, Zhuang X. Meta-analysis of the efficacy and safety of integrated Chinese and Western medicine in the treatment of acute myeloid leukaemia in elderly people. Heliyon 2024;10(1):1-13.
[Crossref] [Google Scholar] [PubMed]
- Deng S, Feng X, Yang M, Yu W, Wu Z, Zhu X, et al. LAMP1 as a novel molecular biomarker to predict the prognosis of the children with autism spectrum disorder using bioinformatics approaches. Sci Rep 2023;13(1):1-14.
[Crossref] [Google Scholar] [PubMed]
- Guo X, Yan Z, Wang J, Fan X, Kang J, Niu R, et al. Effect of Traditional Chinese Medicine (TCM) and its fermentation using Lactobacillus plantarum on ceftriaxone sodium-induced dysbacteriotic diarrhea in mice. Chin Med 2022;17(1):1-20.
[Crossref] [Google Scholar] [PubMed]
- Chaudhari R, Tandel N, Sahu K, Negi S, Bashir H, Rupareliya A, et al. Transdermal immunization of elastic liposome-laden recombinant chimeric fusion protein of P. falciparum (PfMSP-Fu24) mounts protective immune response. Nanomaterials 2021;11(2):1-11.
[Crossref] [Google Scholar] [PubMed]
- Guo DH, Hao JP, Li XJ, Miao Q, Zhang Q. Exploration in the mechanism of Zhisou San for the treatment of cough variant asthma based on network pharmacology. Evid Based Complement Alternat Med 2022:1-15.
[Crossref] [Google Scholar] [PubMed]
- He Y, Han Y, Liao X, Zou M, Wang Y. Biology of cyclooxygenase-2: An application in depression therapeutics. Front Psychiatry 2022;13:1-13.
[Crossref] [Google Scholar] [PubMed]
- Gao AW, El Alam G, Lalou A, Li TY, Molenaars M, Zhu Y, et al. Multi-omics analysis identifies essential regulators of mitochondrial stress response in two wild-type C. elegans strains. iScience 2022;25(2):1-25.
[Crossref] [Google Scholar] [PubMed]
- Shi J, Hou J, Sun Y, Jia Z, Zhou Y, Wang C, et al. Chaihujialonggumulitang shows psycho-cardiology therapeutic effect on acute myocardial infarction with comorbid anxiety by the activation of Nrf2/HO-1 pathway and suppression of oxidative stress and apoptosis. Biomed Pharmacother 2022;153:1-13.
[Crossref] [Google Scholar] [PubMed]
- Gao S, Wang C, Qi L, Liang S, Qu X, Liu W, et al. Bushen huoxue formula inhibits IL-1β-induced apoptosis and extracellular matrix degradation in the nucleus pulposus cells and improves intervertebral disc degeneration in rats. J Inflamm Res 2024;17:121-36.
[Crossref] [Google Scholar] [PubMed]
- Qi H, Tian D, Luan F, Yang R, Zeng N. Pathophysiological changes of muscle after ischemic stroke: A secondary consequence of stroke injury. Neural Regen Res 2024;19(4):737-46.
[Crossref] [Google Scholar] [PubMed]
- Rinaldi L, Sepe M, Donne DR, Conte K, Arcella A, Borzacchiello D, et al. Mitochondrial AKAP1 supports mTOR pathway and tumor growth. Cell Death Dis 2017;8(6):1-12.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Zhang L, Xue C, Fang H, Zhao J, Liu M. Genome-wide identification and analysis of MAPK and MAPKK gene family in Chinese jujube (Ziziphus jujuba Mill.). BMC Genomics 2017;18(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Cherkasova V, Ilnytskyy Y, Kovalchuk O, Kovalchuk I. Transcriptome analysis of cisplatin, cannabidiol, and intermittent serum starvation alone and in various combinations on colorectal cancer cells. Int J Mol Sci 2023;24(19):1-10.
[Crossref] [Google Scholar] [PubMed]
- Zhao J, Liang G, Huang H, Yang W, Pan J, Luo M, et al. Potential mechanisms underlying the therapeutic roles of Gancao Fuzi decoction in cold-dampness obstruction syndrome-type knee osteoarthritis. Curr Comput Aided Drug Des 2024;20(4):384-95.
[Crossref] [Google Scholar] [PubMed]
- Jiang H, Wang HY, Wang JW, Lou DY, Niu N, Li GH, et al. NF-κB inhibitor on toll-like receptor 4 signal-induced expression of angiotensinogen and AT1a receptor in neonatal rat left ventricular myocytes. Exp Ther Med 2018;16(5):3875-82.
[Crossref] [Google Scholar] [PubMed]
- Nived P, Jönsson G, Settergren B, Einarsson J, Olofsson T, Jørgensen CS, et al. Prime-boost vaccination strategy enhances immunogenicity compared to single pneumococcal conjugate vaccination in patients receiving conventional DMARDs, to some extent in abatacept but not in rituximab-treated patients. Arthritis Res Ther 2020;22(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Weston CSE. Four social brain regions, their dysfunctions, and sequelae, extensively explain autism spectrum disorder symptomatology. Brain Sci 2019;9(6):1-10.
[Crossref] [Google Scholar] [PubMed]
- Newhouse A, Kritzer MD, Eryilmaz H, Praschan N, Camprodon JA, Fricchione G, et al. Neurocircuitry hypothesis and clinical experience in treating neuropsychiatric symptoms of postacute sequelae of severe acute respiratory syndrome Coronavirus 2. J Acad Consult Liaison Psychiatry 2022;63(6):619-27.
[Crossref] [Google Scholar] [PubMed]
- Alexander J, Potamianou H, Xing J, Deng L, Karagiannidis I, Tsetsos F, et al. Targeted re-sequencing approach of candidate genes implicates rare potentially functional variants in Tourette syndrome etiology. Front Neurosci 2016;10:1-7.
[Crossref] [Google Scholar] [PubMed]
- de Calbiac H, Dabacan A, Marsan E, Tostivint H, Devienne G, Ishida S, et al. Depdc5 knockdown causes mTOR-dependent motor hyperactivity in zebrafish. Ann Clin Transl Neurol 2018;5(5):510-23.
[Crossref] [Google Scholar] [PubMed]
- Widomska J, de Witte W, Buitelaar JK, Glennon JC, Poelmans G. Molecular landscape of Tourette's disorder. Int J Mol Sci 2023;24(2):1-10.
[Crossref] [Google Scholar] [PubMed]
- Santos JM, Mendonça VA, Ribeiro VG, Tossige-Gomes R, Fonseca SF, Prates AC, et al. Does whole body vibration exercise improve oxidative stress markers in women with fibromyalgia?. Braz J Med Biol Res 2019;52(8):1-9.
[Crossref] [Google Scholar] [PubMed]
- van Huynh T, Rethi L, Rethi L, Chen CH, Chen YJ, Kao YH. The complex interplay between imbalanced mitochondrial dynamics and metabolic disorders in type 2 diabetes. Cells 2023;12(9):1-7.
[Crossref] [Google Scholar] [PubMed]
- Meshrkey F, Ayuso AC, Rao RR, Iyer S. Quantitative analysis of mitochondrial morphologies in human induced pluripotent stem cells for Leigh syndrome. Stem Cell Res 2021;57:1-20.
[Crossref] [Google Scholar] [PubMed]
- Sheng ZH. The interplay of axonal energy homeostasis and mitochondrial trafficking and anchoring. Trends Cell Biol 2017;27(6):403-16.
[Crossref] [Google Scholar] [PubMed]
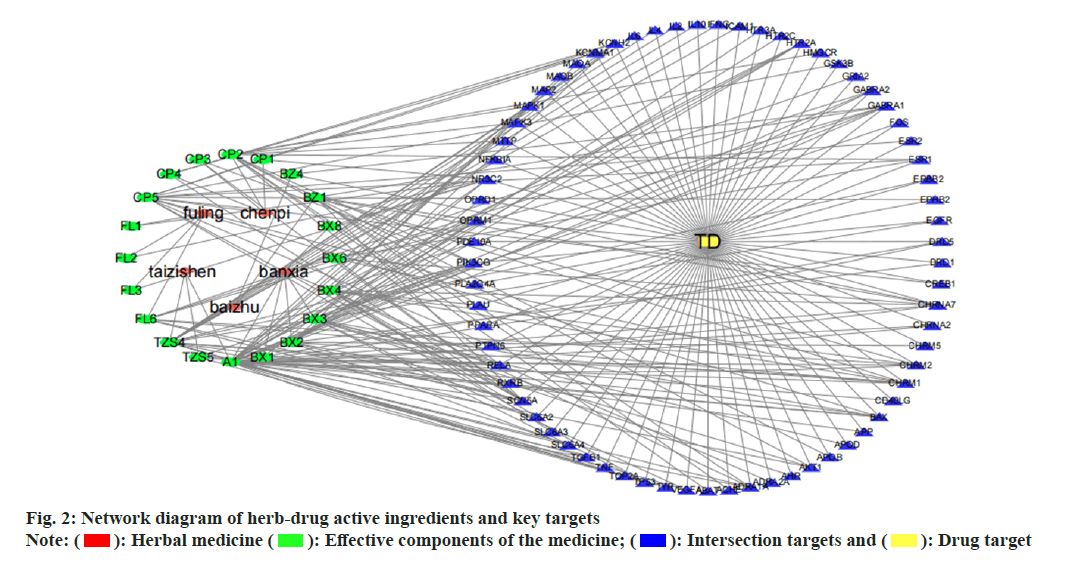
 ): Herbal medicine (
): Herbal medicine ( ): Effective components of the medicine; (
): Effective components of the medicine; (  ): Intersection targets and (
): Intersection targets and ( ): Drug target
): Drug target 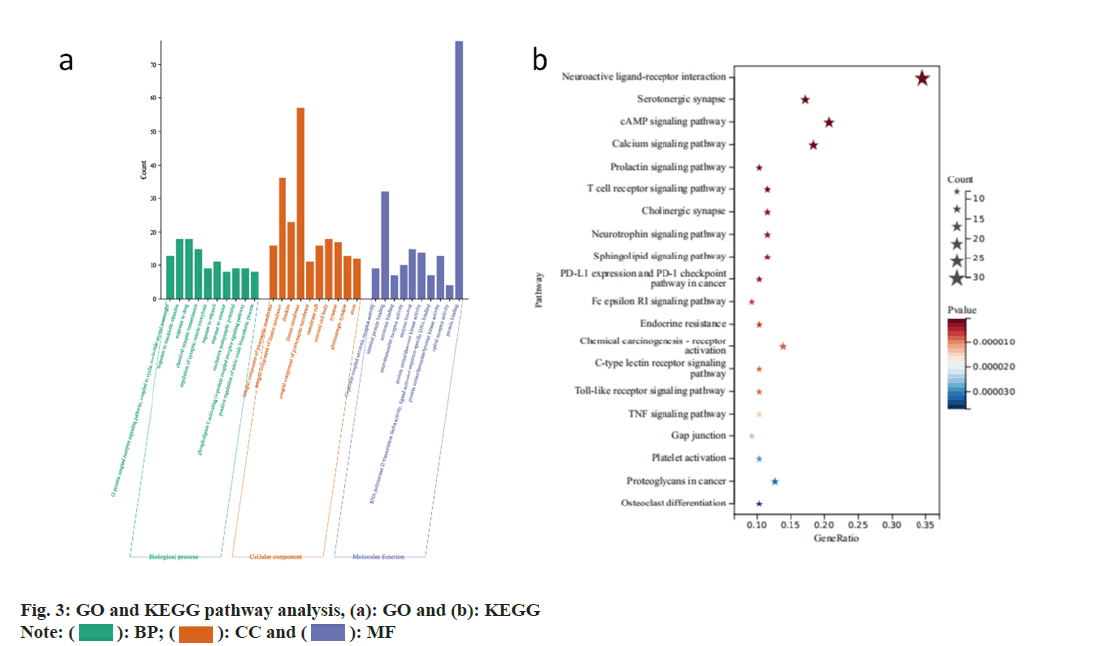
 ): BP; (
): BP; ( ): CC and (
): CC and ( ): MF
): MF