- *Corresponding Author:
- S. R. Kanala
Raghavendra Institute of Pharmaceutical Education and Research, Anantapur, Andhrapradesh 515721, India
E-mail: somu.reddyvaru@gmail.com
| Date of Received | 19 December 2023 |
| Date of Revision | 18 March 2024 |
| Date of Acceptance | 24 June 2024 |
| Indian J Pharm Sci 2024;86(3):1042-1050 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The study aimed to identify the impact of oral supplementation of ethanolic extract of Boswellia ovalifoliolata in treating diabetes. Oral administration of ethanolic extract of Boswellia ovalifoliolata at doses of 200 mg/kg and 400 mg/kg was carried out for 21 d in rats that had been induced to diabetes by alloxan. To explore the effect of ethanolic extract of Boswellia ovalifoliolata on stress-induced pancreatic inflammation in alloxan-induced diabetic rats, the following parameters were assessed viz., blood glucose, hemoglobin A1c, insulin, lipid content, liver biochemical parameters, enzymatic and non-enzymatic antioxidants and inflammatory mediators. There was a marked improvement in blood glucose and hemoglobin A1c levels after 21 d of treatment as well as insulin and serum biochemical parameter restoration. This suggests that they may have the ability to boost endogenous insulin synthesis. Research on ethanolic extract of Boswellia ovalifoliolata's effects revealed that it possesses strong anti-inflammatory and antioxidant capabilities. The fact is that it could regulate the contents of malondialdehyde, protein carbonyl content, tumour necrosis factor-α and interleukin-6 in pancreatic tissue, as well as raise levels of superoxide dismutase, catalase and glutathione, proved this. In addition, ethanolic extract of Boswellia ovalifoliolata considerably reduced the cellular damage caused by alloxan to the islet and acinar cells of the pancreas. One possible explanation is that ethanolic extract of Boswellia ovalifoliolata contains boswellic acid. The results of this study provide promising evidence that ethanolic extract of Boswellia ovalifoliolata can improve diabetic indices, which could pave the way for a new approach to diabetes care. Finding new, all-natural ways to manage diabetes is a major goal of this study.
Keywords
Boswellia ovalifoliolata, diabetes, alloxan, oxidative stress, inflammation
Addressing the prevalence and difficulties of diabetes mellitus, one of the most significant non-communicable diseases, demands immediate action from all relevant areas globally[1]. Claiming the lives of almost 1.6 million individuals worldwide, it is among the top 10 causes of mortality globally[2]. Inflammation, oxidative stress due to high blood sugar and the onset and advancement of type 2 diabetes are strongly related[3]. Numerous studies have established a correlation between chronic low-level inflammation and an elevated risk of acquiring type 2 diabetes. Insulin resistance and metabolic syndrome features, such as elevated blood sugar levels are accompanied by sub-clinical inflammation. Various oral antidiabetic medication classes target diabetes in different ways. These classes include biguanides, sulfonylureas, meglitinide, thiazolidinedione, dipeptidyl peptidase 4, sodium-glucose cotransporter-2 inhibitors and α-glucosidase inhibitors. The most common adverse reaction to sulfonylureas is hypoglycemia, which means low blood sugar. Weight gain, vertigo, nausea, headache and hypersensitivity reactions are among the other less serious side effects. The possible risk of extended hypoglycemia effects on pregnancy and people with liver or kidney problems should not use sulfonylureas[4]. Many treatments incorporate medicinal plant components, such as carotenoids, flavonoids, terpenoids, alkaloids and glycosides, which can enhance pancreatic function by increasing insulin secretions or decreasing intestinal glucose absorption and thus have anti-diabetic effects[5,6]. Phytomedicines are an alternative to sulfonylureas. Increased insulin secretion, enhanced glucose absorption by adipose and muscle tissues, inhibition of intestinal glucose absorption and inhibition of glucose synthesis by liver cells are the methods by which hypoglycemic plants work. Diabetes problems can be reduced or eliminated by focusing on these factors[7]. Because of its potential therapeutic benefits on conditions like diabetes mellitus, the resin of the Boswellia tree has been the subject of extensive research[8]. The antioxidant and anti-inflammatory actions of boswellic acids on conditions like ulcerative colitis, chronic colitis and asthma are among the many well-documented biological activities of these acids. The following molecules are found in Boswellia ovalifoliolata (B. ovalifoliolata) includes oleo-gum-resin, terpenoids, α-amyrin, ovalifoliolatins A and B, acetogenin C, and sitost-4-en-3-one[9]. Researchers have discovered that oleo-gum resin can increase blood insulin levels, regenerate β-cells of Langerhans islets, improve glycogenesis and decrease glycogenolysis in rats that have developed diabetes due to alloxan[10].
Henceforth, the study aimed to identify the potential antihyperglycemic activity of one of the Boswellia species i.e. stem bark extract of B. ovalifoliolata in alloxan-induced diabetes in rats based on the presence of boswellic acid as a principal active constituent in Boswellia gums.
Materials and Methods
Collection of plant material:
In Tirupati, Andhra Pradesh, India, the stem barks of B. ovalifoliolata were gathered from nearby locations. Dr. Madhava Chetty from Sri Venkateswara University's Department of Botany, verified the plant's legitimacy at Tirupati, Andhra Pradesh. The stem barks were collected, washed, let to dry at room temperature, mechanically powdered, sieved (using a #10/#44 sieve) and then sealed in containers to prevent airtightness.
Preparation of extract:
Isolated stem bark powder weighing around 100 g was subjected to a 72 h extraction using 70 % ethanol. The next step was to use a low-temperature rotary flash evaporator to distill the solvent under reduced pressure. The resulting mixture was dried in a 40° oven, ground into a powder, labeled as Ethanolic Extract of Boswellia ovalifoliolata (EEBO) and then placed in desiccators for storage.
Experimental animals:
Adult health Wistar albino rats that weighed 150-200 g were selected. The rats were housed in optimal conditions for 7 d before the experiment, including a temperature of 26±2°, a relative humidity of 45 %-55 %, and a 12 h light-dark cycle, following conventional husbandry practices. The rats were provided with commercial pellet rat food (Mysore Enterprises Bangalore) and were allowed unlimited access to water. The protocols used in this investigation were approved by CPCSEA no: 1423/PO/A/11/CPCSEA/113/2012.
Chemicals and instruments:
All other chemicals and reagents used were of analytical grade and were purchased from Coral Diagnostics Ltd in India. Glibenclamide and Alloxan were procured from Sigma-Aldrich, Mumbai. Mini centrifuge (Remi, Model No: KKLO-9013), tissue homogenizer (Ever Shine, Model No: 607), ultraviolet-visible spectrophotometer (Systronics, Model No: 2203), electronic balance (Maxlyser, Avecon Model No: NB-201), and semi autoanalyzer (Avecon, Model No: NB-201) are the equipment used in this research.
Experimental design:
Study on alloxan-induced diabetic rats: The normal rats that had fasted overnight were injected with a 0.9 % w/v NaCl solution that included 150 mg/kg of alloxan monohydrate. Blood glucose levels were measured after 72 h to confirm the diagnosis of diabetes. Rats with Fasting Blood Glucose (FBG) levels above 250 mg/dl were included in the study. The first group received a vehicle control; second with alloxan; third glibenclamide (5 mg/kg p.o.); and fourth and fifth groups served with EEBO (200 mg/kg and 400 mg/kg, respectively) orally once daily for 21 d. Every group's rats had starved the night prior. The animals were given EEBO suspended in distilled waterby an oral feeding needle.
Collection of blood samples:
Before dosing on 1st d and plasma glucose levels were measured by drawing blood samples from the retro-orbital plexus at regular intervals on 7th, 14th, and 21st d for all animal groups. Rats were sacrificed by cervical dislocation after being euthanized with pentobarbitone sodium (60 mg/kg) on the 21st d of the experiment. Post-cardiac puncture blood samples were centrifuged at 2500 rpm for 15 min to separate serum and plasma. Serum samples were stored at -20° until biochemical parameter estimations. The treatment period is 21 d. The pancreas was extracted and cleansed with ice-cold saline. It was then combined with 2 ml of ice-cold phosphate buffer (20 mM, pH 7.4) in a glass homogenizer with a Teflon pestle. After centrifugation, the supernatant was used for further studies.
Serum biochemical parameters:
Rats fasted for a night after the 21 d study, then blood samples were taken and serum glucose levels were measured using Trinder et al.[11] approach. Following Coral Diagnostics Ltd's. instructions, serum glycosylated hemoglobin, insulin, Serum Glutamic-Oxaloacetic Transaminase (SGOT), Serum Glutamic-Pyruvic Transaminase (SGPT), Alkaline Phosphatase (ALP), bilirubin, urea, uric acid and creatinine were measured.
Biochemical assays:
The rats were anesthetized after 21 d of injecting EEBO. The pancreas was cleaned with cold saline after dissection. On a Teflon pestle in a glass homogenizer, 2 ml of ice-cold phosphate buffer (20 mM, pH 7.4) were stirred in. The centrifuged supernatant was taken for examination. The lens homogenate total and soluble protein levels were estimated using the Lowry technique[12]. The superoxide dismutase was measured using Nitroblue Tetrazolium (NBT) photoreduction. 1.2 ml of sodium pyrophosphate buffer (0.052 M, pH 8.3) was added to 0.1 ml of pancreatic homogenate supernatant. Next, 0.1 M 186 µm phenazoniummethosulphate, 0.3 ml 300 µm NBT and 0.2 ml of 780 µm nicotinamide adenine dinucleotide hydrogen were added. The process stopped after 90 s of incubation at 30° with 1 cc glacial acetic acid. After shaking the reaction mixture vigorously and adding 4 ml of n-butanol, it was centrifuged at 4000 RPM for 10 min. The organic layer's absorbance was measured at 560 nm[13]. The sample's H2O2 degradation capacity determined Catalase (CAT) estimation. To incubate pancreatic homogenate, mix 20 µl with 1 M Tris-HCl, 5 mM Ethylenediamine Tetraacetic Acid (EDTA) at pH 8.0, 900 µl of 10 mM H2O2 enzyme substrate and up to 30 µl of distilled water. H2O2 degradation was measured at 230 nm using a spectrophotometer[14]. To test reduced Glutathione (GSH) activity, 0.2 ml of pancreas homogenate and 0.2 ml of 0.8 mM EDTA, 0.1 ml of sodium azide, 0.1 ml of 4 mM GSH, 0.1 ml of H2O2 solution and 0.4 ml of 0.4M phosphate buffer (pH 7) were added. Following 10 min incubation at 37° and subsequent cooling to room temperature, 0.5 ml of 10 % trichloroacetic acid was added to the mixture. A 10 min centrifugation run at 2000 RPM was applied to the mixture. A 5,5′-dithiobis-(2-nitrobenzoic acid (DTNB) solution with a concentration of 0.04 % was added to 0.1 ml of the supernatant. At 420 nm, the optical density of the resulting mixture was measured[15]. Ohkawa et al.[16] method was used to test Malondialdehyde (MDA) levels in pancreas homogenates. In this assay, 0.1 ml of pancreas homogenate was mixed with 2 ml of distilled water, then 0.1 ml of thiobarbituric acid and 1 ml of trichloroacetic acid were added. Once the mixture had cooled, butanol was added to it after it had been cooked in a water bath. The optical density was measured at 532 nm after centrifugation separated the organic phase[16]. To determine the pancreatic homogenates' protein carbonyl content, 1 ml of a solution containing 10 mM 2,4-dinitrophenylhydrazine in 2 mM hydrochloric acids was added to the reaction mixture. For 30 min, the samples were left at room temperature to incubate. After that, 1 ml of cold trichloroacetic acid (10 % w/v) was added to the concoction and then spins at 3000 RPM for 10 min. The protein pellet was dissolved in 1 ml of guanidine hydrochloride (6 M, pH 2.3), after being rinsed three times with 2 ml of ethanol/ethyl acetate [1:1, v/v]. A wavelength of 370 nm was used to measure the sample's absorbance[17]. Interleukin-6 (IL-6) and Tumour Necrotic Factor (TNF-α) in the Pancreas Commercially available TNF-α and IL-6 ELISA kits (eBioscience Inc., San Diego, CA, USA) were used to assess the amounts of biochemical parameters in the liver homogenate, according to the manufacturer's instructions.
Histological studies:
Following the collection of blood samples for biochemical analysis, the experimental animals were euthanized and swiftly dissected and pancreas tissues were carefully procured. These tissue specimens were then fixed in a 10 % formalin solution. Sequential dehydration of the specimens was performed using ascending concentrations of butanol. Subsequently, clearing was achieved using xylene, followed by embedding the tissues in paraffin wax. Thin sections of approximately 6 μm thickness were meticulously prepared. These sections were subjected to staining with hematoxylin and eosin, facilitating the visualization of cellular structures. Microscopic analysis was then conducted to gain insights into the histological features and alterations within the pancreatic tissues.
Results and Discussion
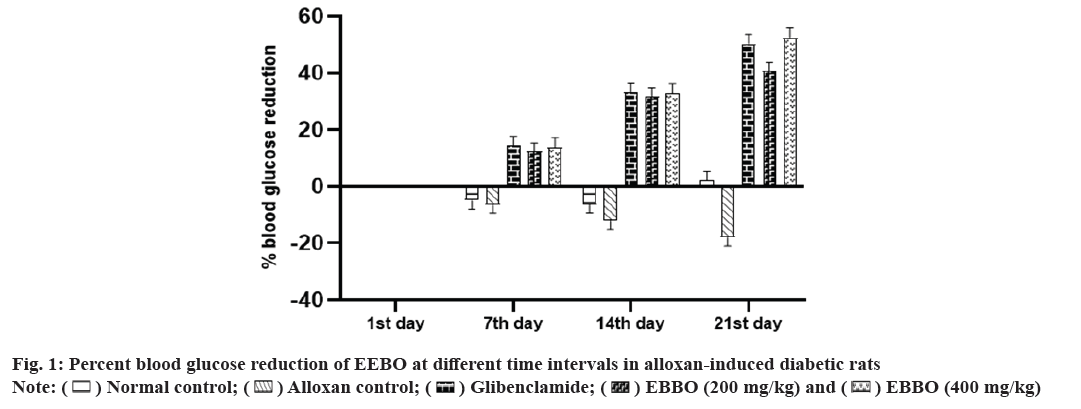
The long-term complications of diabetes mellitus might be due to uncontrolled or persistent diabetes mellitus[18]. In recent years, dietary supplements and phytochemicals have gained popularity as potential treatments for this illness. Reasons for this include the widespread interest in all-natural remedies and the relative safety of medicines derived from plant extracts. Given the potential of Boswellia species to treat metabolic dysfunction, we set out to assess the efficacy of B. ovalifoliolata in treating diabetes-induced preclinical models.In research concerning diabetes, alloxan is one of the common diabetogenic agents used to evaluate the antidiabetic potential of both pure chemicals and plant extracts. The model utilizes two separate pathogenic effects: first, it selectively inhibits insulin secretion in response to glucose, and second, it induces the creation of Reactive Oxygen Species (ROS), which promotes the selective necrosis of pancreatic beta cells[19]. The chosen animals were given 150 mg/kg of alloxanintraperitoneally (i.p.) to induce them to undergo treatment for duration of 21 d. On the 1st, 7th, 14th and 21st d of the treatment period, blood glucose levels were assessed at various intervals. Table 1 shows that on the 21st d of EEBO treatment, there is a substantial decrease (p<0.05) in blood glucose levels, with a reduction of up to 50 % as illustrated in fig. 1. Research on other Boswellia species reported that administering B to those with type 2 diabetes led to a marked reduction in FBG and an increase in insulin level[20]. In a similar way, an increase in pancreatic enzyme and a decrease in blood sugar were both brought about by Boswellia glabra aqueous extract, which enhanced beta-cell secretory granule synthesis[21].
| Group | 1st d | 7th d | 14th d | 21st d |
|---|---|---|---|---|
| Normal control |
120.483 | 120.483 | 120.483 | 120.483 |
| 91.17±0.47 | 95.67±1.20ns | 97.00±1.52ns | 89.2±0.60* | |
| Alloxan control |
120.483 | 120.483 | 120.483 | 120.483 |
| 210.3±0.61 | 224.0±2.01** | 235.8±3.11*** | 248.3±1.25*** | |
| Glibenclamide (5 mg/kg) | 206.0±1.63 | 176.3±1.58** | 137.5±2.34*** | 102.3±3.87*** |
| EEBO (200 mg/kg) | 209.2±1.44 | 183.3±1.74*** | 142.8±2.88*** | 123.81±4.44*** |
| EEBO (400 mg/kg) | 207.5±1.50 | 179.0±1.75*** | 139.5±1.87*** | 98.84±1.42*** |
Note: Ns: Not significant, nsp>0.05 and *p<0.05 compared with 1st d, all values are expressed as mean±Standard Error of Mean (SEM) and the statistical comparisons were made by using Analysis of Variance (ANOVA) followed by Dunnett? s multiple comparison test
Table 1: Effect of EEBO on serum glucose levels at different time intervals in alloxan-induced diabetic rats.
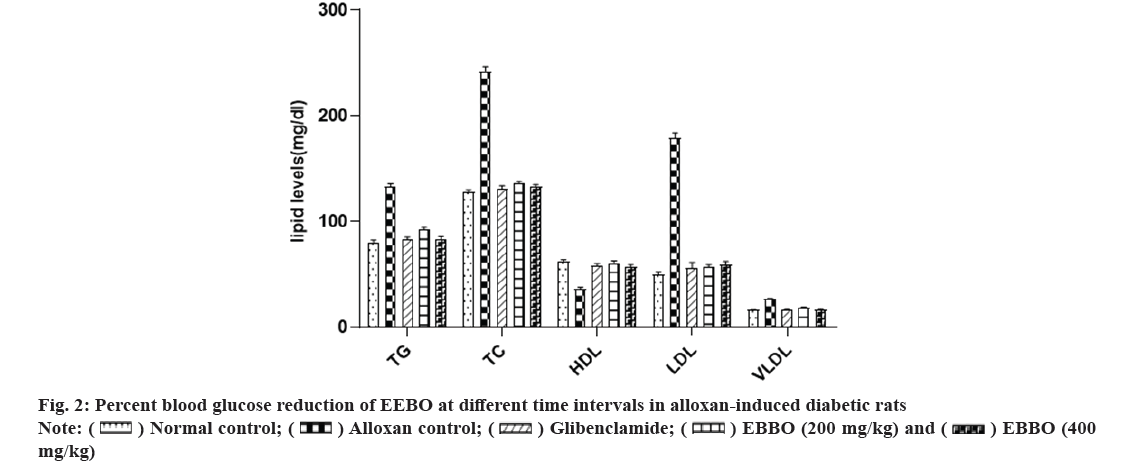
As an alternative to FBG, the American Diabetes Association suggests utilizing hemoglobin A1c as a diagnostic tool for diabetes. There is a strong correlation between Hemoglobin A1C (HbA1c) and the risk of long-term diabetic complications and it also provides a valid indicator of chronic hyperglycaemia[22]. Following exposure to alloxan, which blocks the selectivity of insulin secretion, the binding of the glucose sensor glucokinase allows for insulin secretion[23]. Table 2 shows that the study found that alloxan intoxication led to higher levels of blood glucose and HbA1c and lower levels of insulin in the blood. The β-cells may have been protected from the ROS produced by alloxan, as these parameters were considerably improved to normal levels after treatment with EEBO. Consistent with earlier research, this study found that 15 mg/kg body weight of Boswellia serrata significantly reduced blood glucose and HbA1c in STZ-induced diabetic rats[24] and it also improved insulin sensitivity or perhaps increased endogenous insulin secretion[25]. Fig. 2 and Table 2 show that the EEBO significantly reduced bilirubin, urea, uric acid and creatinine in a dose-dependent manner; decreased elevated liver enzymes (SGOT, SGPT, and ALP) and normalized elevated triglycerides, cholesterol, Low Density Lipoprotein (LDL), Very Low Density Lipoprotein (VLDL) while increasing High Density Lipoprotein (HDL). The EEBO also demonstrated significant hypolipidemic and hepatoprotective activity. The oleo gum resin and Boswellic acid found in B. ovalifoliolata may be responsible for this action. According to research by Mahesh et al.[26], B. ovalifoliolata therapy reduced liver damage, protected against lipid peroxidation, SGOT, SGPT, ALP, and LDH, and preserved glutathione status in the face of paracetamol poisoning[26]. Another study found that diabetic individuals who took 900 mg of Boswellia serrata gum resin orally for six weeks had significantly lower levels of cholesterol (LDL), fructosamine SGPT and SGOT and significantly higher HDL levels[27]. Over the course of 28 d, Boswellia glabraroot reduced enzyme activity, serum glucose, urea, triglycerides, cholesterol, creatinine and had significant hypoglycemic effects[20]. According to another source, the anti-inflammatory properties of Boswellia serrata are responsible for the observed effects. It was found that animals given Boswellia as a supplement significantly reduced levels of serum glucose, TC, TG, LDL-C, FFA, IL-1β, TNF-α, insulin, and leptin as well as HDL-C and adiponectin. Additionally, creatinine bilirubin, SGOT, SGPT, and ALP were also significantly reduced. In a separate trial using rats that were diabetic due to alloxan, the researchers found that administering Boswellia glabra significantly reduced levels of serum glucose, cholesterol, triglycerides, urea, and creatinine, as well as enzyme activities such as alkaline phosphatase and glucose-6-phosphatase[20]. Not only that, but Boswellia serrate was found to significantly lower blood glucose, HbA1c, cholesterol, LDL, and fructosamine levels after dosing[30].
| Parameters | Normal control |
Alloxan control |
Glibenclamide (5 mg/kg) | EEBO (200 mg/kg) | EEBO (400 mg/kg) |
|---|---|---|---|---|---|
| HbA1c | 4.45±0.32* | 12.67±0.76# | 4.89±0.24* | 6.23±0.32* | 5.12±0.23* |
| Insulin | 8.23±0.34* | 5.12±0.11# | 7.89±0.17* | 6.78±0.34* | 8.09±0.23* |
| SGOT | 73.6±1.56* | 131.00±2.23# | 75.23±4.41* | 98.09±4.11* | 81.21±3.34* |
| SGPT | 39.89±1.65* | 78.09±1.23# | 43.44±2.23* | 54.43±1.64* | 40.50±1.71* |
| ALP | 3.59±0.14* | 7.32±0.20# | 4.04±0.20* | 4.46±0.12* | 3.97±0.13* |
| Bilirubin | 0.70±0.04* | 1.61±0.03# | 0.75±0.04* | 1.12±0.01* | 0.76±0.02* |
| Urea | 22.32±0.55* | 34.50±0.61# | 24.09±0.15* | 27.45±0.34* | 23.70±0.78* |
| Uric acid | 3.43±0.11* | 8.12±0.23# | 3.89±0.11* | 5.39±0.23* | 3.90±0.23* |
| Creatinine | 0.67±0.03* | 1.78±0.07# | 0.81±0.01* | 0.93±0.03* | 0.65±0.04* |
Note: *p<0.05 and #p<0.001 respectively when disease control compared with the control group, all values are expressed as Mean±Standard Error of Mean (SEM) and the statistical comparisons were made by using Analysis of Variance (ANOVA) followed by Dunnett? s multiple comparison test.
Table 2: Effect of EEBO on serum biochemical parameters in alloxan-induced diabetic rats.
One of the most important factors in the onset and development of diabetes is oxidative stress, which can be triggered by hyperglycemia. The body's antioxidant defense mechanism is unable to degrade ROS quickly enough, leading to oxidative stress[31]. Catalase-reduced glutathione, and superoxide dismutase are the three enzymes that make up the antioxidant defense system in the body. An overabundance of ROS in hyperglycemic states raises levels of MDA, a biomarker for lipid peroxidation and cellular oxidative stress. To avoid additional issues with insulin action and secretion, it is essential to keep a good antioxidant balance[32]. Due to the damage it causes to DNA, proteins, cell membranes, and plasma lipids, endothelial dysfunction results from this oxidative stress. The activation of inflammatory mediators like Nuclear Factor Kappa-light-chain-enhancer of activated B cells (NF-κB) occurs as a result of this damage. According to reference 33, pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and other similar proteins are produced when NFκB is activated. Developing antioxidant and anti-inflammatory therapies is of utmost importance, considering the detrimental impact of oxidative stress and inflammation on diabetes.
Research into B. ovalifoliolatawa's effects on pancreatic tissue inflammation and alloxan-induced oxidative stress began at that time. Table 3 shows that EEBO dose-dependently reduced oxidative stress markers including MDA and protein carbonyls in pancreatic homogenate and increased SOD, CAT and GSH, thereby balancing the free radicals and antioxidant system. More importantly, it returns the alloxan-induced rats' increased inflammatory mediator levels to normal levels, suggesting a possible antioxidant mechanism (fig. 3). Prabhakar YK's study, which found a marked reduction in hepatic and renal TBARs, provided support for the substantial results; treatment with B. ovalifoliolata resulted in a marked improvement in the activities of SOD, GPx, and GST in the liver and kidney of diabetic rats induced with STZ[33,34]. Arthroscopic elastase, MPO, LPO, GSH, catalase, SOD, and NO were among the metrics that were found to undergo substantial alterations when exposed to Boswellia serrata gum resin, according to another study. The levels of inflammatory mediators (IL-1β, IL-6, TNF-α, IFN-γ, and PGE2) were markedly decreased and levels of IL-10 were markedly elevated after oral administration of Boswellia[35]. Boswelliadalzielii increased levels of glutathione (GSH), superoxide dismutase (SOD), and catalase, while simultaneously decreasing MDA. In rats that were diabetic due to alloxan, these extracts also reduced levels of inflammatory markers such as TNF-α, IL-6 and IL-1β.
| Parameter | Normal control | Alloxan control | Glibenclamide (5 mg/kg) | EEBO (200 mg/kg) | EEBO (400 mg/kg) |
|---|---|---|---|---|---|
| Protein | 47.90±0.45 | 33.67±1.10# | 46.50±1.42* | 43.34±2.09* | 50.23±1.23* |
| SOD | 2.89±0.04 | 0.89±0.03# | 2.58±0.07* | 2.27±0.07* | 2.45±0.09* |
| CAT | 1.26±0.06 | 0.39±0.08# | 1.01±0.06* | 0.77±0.05* | 0.99±0.09* |
| GSH | 20.87±0.23 | 13.56±0.23# | 19.98±0.23* | 17.17±0.39* | 18.70±0.43* |
| MDA | 77.34±1.45 | 125.22±2.23# | 81.27±2.34* | 101.78±2.34* | 83.23±4.46* |
| PCO | 4.82±0.27 | 12.12±0.32# | 5.23±0.45* | 8.76±0.56* | 6.54±0.34* |
Note: All values are expressed as mean±S.E.M. Statistical comparisons were made by using One way ANOVA followed by Dunnett? s multiple comparison test and foundsignificantly different at *p<0.05when compared to Disease Control and #p<0.001 when disease control is compared with the control group
Table 3: Effect of EEBO on oxidative stress parameters in pancreatic homogenate in alloxan-induced diabetic rats.
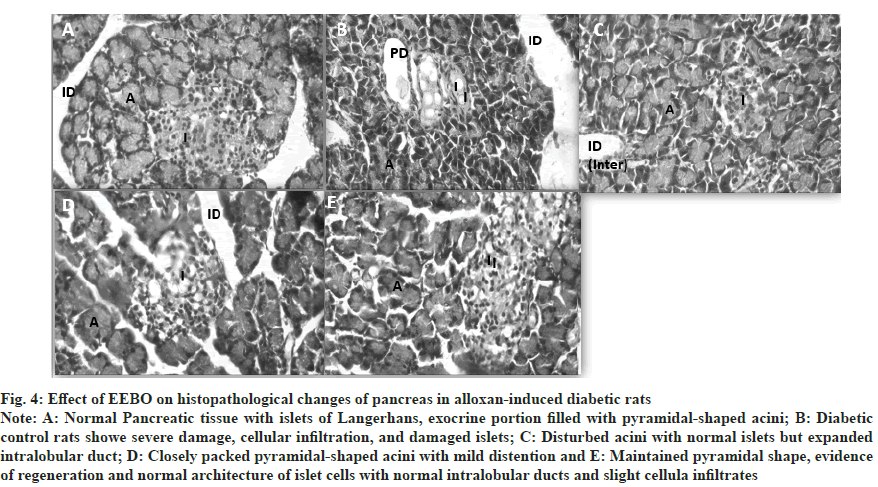
In conclusion, the histological investigations demonstrated, as shown in fig. 4, that the administration of EEBO 400 mg/kg significantly reduced damage to pancreatic acinar cells and β cells. This might be because the antidiabetic effects of boswellic acid and oleo-gum-resin are felt in the liver through changes in phosphoenolpyruvate carboxykinase, hepatic gluconeogenesis, and pyruvate carboxylase[36]. There is a lack of effective treatments for diabetes because it is a complex condition. B. ovalifoliolata is a plant that has a long history of usage in diabetes management; we investigated its potential as a safer alternative. The plant's capacity to decrease oxidative stress and pancreatic inflammation may be responsible for the protective effects on the liver observed at dosages of 200 mg/kg and 400 mg/kg of its EEBO, which we found to considerably lower hyperglycemia and hyperlipidaemia. The results show that B. ovalifoliolata may work as an oral antidiabetic medication. The processes and long-term impacts, however, need additional research.
Fig. 4: Effect of EEBO on histopathological changes of pancreas in alloxan-induced diabetic rats.
Note: A: Normal Pancreatic tissue with islets of Langerhans, exocrine portion filled with pyramidal-shaped acini; B: Diabetic control rats showe severe damage, cellular infiltration, and damaged islets; C: Disturbed acini with normal islets but expanded intralobular duct; D: Closely packed pyramidal-shaped acini with mild distention and E: Maintained pyramidal shape, evidence of regeneration and normal architecture of islet cells with normal intralobular ducts and slight cellula infiltrates.
Acknowledgments:
The authors expressed their sincere thanks to the Srikrishna Devaraya University College of Pharmaceutical Sciences and Raghavendra Institute of Pharmaceutical Education and Research (Autonomous) for providing facilities to carry out the research work.
Conflict of interest:
The authors declare no conflict of interest.
References
- Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol 2019;11(3):45.
[Google Scholar] [PubMed]
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76(25):2982-3021.
[Crossref] [Google Scholar] [PubMed]
- Sharma K, Akre S, Chakole S, Wanjari MB. Stress-induced diabetes: A review. Cureus 2022;14(9).
[Crossref] [Google Scholar] [PubMed]
- Chaudhury A, Duvoor C, Reddy DVS, Kraleti S, Chada A, Ravilla R, et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front Endocrinol (Lausanne) 2017;8:6.
[Crossref] [Google Scholar] [PubMed]
- Kooti W, Moradi M, Ali-Akbari S, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Therapeutic and pharmacological potential of Foeniculum vulgare Mill: A review. J. Herbmed Pharmacol 2015;4(1):1-9.
- Afrisham R, Aberomand M, Ghaffari MA, Siahpoosh A, Jamalan M. Inhibitory effect of Heracleum persicum and Ziziphus jujuba on activity of alpha-amylase. J Bot 2015;2015.
- Hegazy GA, Alnoury AM, Gad HG. The role of Acacia Arabica extract as an antidiabetic, antihyperlipidemic, and antioxidant in streptozotocin-induced diabetic rats. Saudi Med J 2013;34(7):727-33.
[Google Scholar] [PubMed]
- Devi PS, Adilaxmamma K, Rao GS, Srilatha C, Raj MA. Safety evaluation of alcoholic extract of Boswellia ovalifoliolata stem-bark in rats. Toxicol Int 2012;19(2):115.
[Crossref] [Google Scholar] [PubMed]
- Rao AR, Veeresham C, Asres K. In vitro and in vivo inhibitory activities of four Indian medicinal plant extracts and their major components on rat aldose reductase and generation of advanced glycation endproducts. Phytother Res 2013;27(5):753-60.
[Crossref] [Google Scholar] [PubMed]
- Al-Yasiry RM, Jawad SA, Menati KJ, Naji SA, Lokman IH. Effects of Boswellia carterii and Boswellia serrata in drinking water on the growth performance, hematology traits and immune response of broiler chicken. Int J Dairy Technol 2016;4:27-37.
- Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 1969;6(1):24-7.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J biol Chem 1951;193(1):265-75. [Crossref]
[Google Scholar] [PubMed]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170-5.
[Google Scholar] [PubMed]
- Bergmeyer HU, editor. Methods of enzymatic analysis. Elsevier 2012:673-84.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 1968;25:192-205.
[Crossref] [Google Scholar] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
[Crossref] [Google Scholar] [PubMed]
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 1994:357-63.
[Crossref] [Google Scholar] [PubMed]
- Abbas M, Ali M. Protective effects of beetroot on streptozotocin induced diabetes in adult male albino rats. Bulletin of ESPS 2021;41(2):270-82.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological research. 2001;50(6):537-46.
[Google Scholar] [PubMed]
- Kavitha JV, Rosario JF, Chandran J, Anbu P. Hypoglycemic and other related effects of Boswellia glabra in alloxan-induced diabetic rats. Indian J Physiol Pharmacol. 2007;51(1):29-39. [Crossref]
[Google Scholar] [PubMed]
- Helal EG, Mostafa AM, Ashour FA, Kahwash AA. Effect of Boswellia carterii Birdw on carbohydrate metabolism in diabetic male albino rats. Egypt J Hosp Med. 2005;20(1):38-45.
- Shehata AM, Quintanilla-Fend L, Bettio S, Singh CB, Ammon HP. Prevention of Multiple Low-Dose Streptozotocin (MLD-STZ) diabetes in mice by an extract from gum resin of Boswellia serrata (BE). Phytomedicine 2011;18(12):1037-44.
[Crossref] [Google Scholar] [PubMed]
- Kronenberg-Versteeg D, Eichmann M, Russell MA, de Ru A, Hehn B, Yusuf N, et al. Molecular pathways for immune recognition of preproinsulin signal peptide in type 1 diabetes. Diabetes 2018;67(4):687-96.
[Crossref] [Google Scholar] [PubMed]
- Andreani G, Ferlizza E, Macrì E, Beghelli D, Isani G. Effect of Boswellia serrata supplementation in addition to insulin on glycemic control in a diabetic dog. Slov Vet Res 2017;54(4):173-9.
- Mehrzadi S, Tavakolifar B, Huseini HF, Mosavat SH, Heydari M. The effects of Boswellia serrata gum resin on the blood glucose and lipid profile of diabetic patients: A double-blind randomized placebo-controlled clinical trial. J Evid Based Integr Med 2018;75(3):169-90.
[Crossref] [Google Scholar] [PubMed]
- Mahesh BU, Shrivastava S, Pragada RR, Naidu VG, Sistla R. Antioxidant and hepatoprotective effects of Boswellia ovalifoliolata bark extracts. Chin J Nat Med 2014;12(9):663-71.
[Crossref] [Google Scholar] [PubMed]
- Ahangarpour A, Heidari H, Fatemeh RA, Pakmehr M, Shahbazian H, Ahmadi I, et al. Effect of Boswellia serrata supplementation on blood lipid, hepatic enzymes and fructosamine levels in type 2 diabetic patients. J Diabetes Metab Disord 2014;13:1-5.
[Crossref] [Google Scholar] [PubMed]
- Gomaa AA, Farghaly HS, El-Sers DA, Farrag MM, Al-Zokeim NI. Inhibition of adiposity and related metabolic disturbances by polyphenol-rich extract of Boswellia serrata gum through alteration of adipo/cytokine profiles. Inflammopharmacology 2019;27:549-59.
[Crossref] [Google Scholar] [PubMed]
- Alotaibi SH, Nasir O, Elsayed S, Ahmed O, Baty RS, Abushal SA, et al. Biomedical and histological evidence of Boswellia sp. Burseraceae on kidney and liver function in mice. J King Saud Univ Sci. 2022;34(1):101691.
- Shehata AM, Quintanilla-Fend L, Bettio S, Jauch J, Scior T, Scherbaum WA, et al. 11-keto-β-boswellic acids prevent development of autoimmune reactions, insulitis and reduce hyperglycemia during induction of Multiple Low-Dose Streptozotocin (MLD-STZ) diabetes in mice. Horm Metab Res 2015;47(06):463-9.
[Crossref] [Google Scholar] [PubMed]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107(9):1058-70.
[Crossref] [Google Scholar] [PubMed]
- Derosa G, D'Angelo A, Bonaventura A, Bianchi L, Romano D, Maffioli P. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin Biol Ther 2013;13(4):475-82.
[Crossref] [Google Scholar] [PubMed]
- Hamadjida A, Mbomo RE, Minko SE, Ntchapda F, Mingoas JP, Nnanga N. Antioxidant and anti-inflammatory effects of Boswellia dalzielii and Hibiscus sabdariffa extracts in alloxan-induced diabetic rats. Metabolism Open 2024:498-5.
[Crossref] [Google Scholar] [PubMed]
- Prabhakar Y, Ali MS, Kumar MJ, Tilak TK, Rao CA. Evaluation of antioxidant activities of aqueous extract of stem bark of Boswellia ovalifoliolata in streptozotocin induced diabetic rats. J Pharm Chem 2013;7(4):19-24.
- Umar S, Umar K, Sarwar AH, Khan A, Ahmad N, Ahmad S, et al. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine 2014;21(6):847-56.
[Crossref] [Google Scholar] [PubMed]
- Mahdian D, Abbaszadeh-Goudarzi K, Raoofi A, Dadashizadeh G, Abroudi M, Zarepour E, et al. Effect of Boswellia species on the metabolic syndrome: A review. Iran J Basic Med Sci 2020;23(11):1374.
[Crossref] [Google Scholar] [PubMed]
 .
.
 mg/kg).
mg/kg).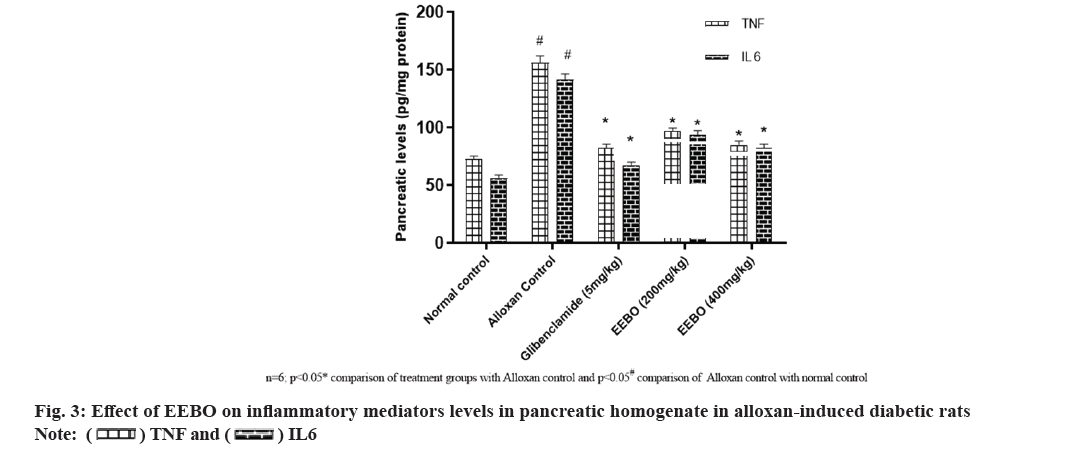
 .
.