- *Corresponding Author:
- Yang Xing
Department of Emergency Medicine, Shengjing Hospital of China Medical University, Shenyang, Liaoning Province 110000, China
E-mail: chenny1030@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “122-127” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study focused to study the efficacy and safety of magnesium sulfate with nifedipine for pregnancy-induced hypertension receiving evidence-based care. 100 pregnancy-induced hypertension individuals who were treated between January 2022 and December 2022 were selected. On the basis of evidence-based care, 54 patients were given magnesium sulfate with nifedipine and were assigned as research group while 46 individuals were treated with magnesium sulfate alone and were regarded as control group. Data on clinical efficacy, systolic and diastolic blood pressure, heart rate and other complications rate like dyspnea, accelerated heart rate, and nausea and vomiting were comparatively analyzed. Similarly, information regarding umbilical artery resistance index, blood viscosity and adverse pregnancy outcomes such as postpartum hemorrhage, placental abruption and cesarean section were collected among the patients for comparison. The data showed an obviously higher overall response rate of treatment in the research group compared with the control group. Besides, the research group exhibited markedly reduced systolic and diastolic blood pressure, heart rate, umbilical artery resistance index and blood viscosity after treatment were low compared with the control group. Moreover, statistically lower incidence rates of complications and adverse pregnancy outcomes were determined in the research group. The above shows that magnesium sulfate with nifedipine has a beneficial effect on improving the curative effect of pregnancy-induced hypertension patients receiving evidence-based care while ensuring the treatment safety, contributing to better blood pressure and heart rate control, inhibited umbilical artery resistance index and blood viscosity, and a reduced risk of adverse pregnancy outcomes, which is worth of clinical promotion.
Keywords
Magnesium sulfate, nifedipine, hypertension, clinical efficacy, pre-eclampsia, blood pressure
Pregnancy Induced Hypertension (PIH) is a pregnancy-associated disease mainly occurring in the 3rd trimester of pregnancy, which may adversely affect maternal and neonatal outcomes[1,2]. This disease is mainly manifested as chronic hypertension, gestational hypertension, preeclampsia and eclampsia, and chronic hypertension complicated with pre-eclampsia, etc., which may be accompanied by clinical symptoms such as edema, dizziness, headache, convulsion, and elevated blood pressure[3-5]. Its etiology is related to placental ischemia, decreased immunity and heredity[6]. According to statistics, the global prevalence of PIH can be as high as 13.1 %, contributing to a risk of neonatal death up to nearly 10.0 %[7]. Currently, the treatment of PIH includes non-pharmacological methods such as weight loss and salt intake reduction, as well as drug interventions including Alpha (α) adrenergic agonists, Beta (β) blockers and calcium channel blockers[8]. However, non-pharmacologic approaches benefited a narrower slice of people, while pharmacotherapy leads to uneven efficacy and is associated with unavoidable risks[9,10]. Therefore, the treatment of PIH may still need to be explored to optimize management, which is of great value in reducing the morbidity and mortality associated with this disease.
Magnesium Sulfate (MS) is a choice of drug intervention for the basic treatment of PIH, as well as an anticonvulsant. It can regulate blood vessels and neuromuscular function in these patients through central inhibition and its hypotensive mechanism is related to its effect on vasodilation[11,12]. In addition, the drug has the effects of relieving spasm, diuresis and pain, and can relax and regulate vascular smooth muscle, which can be used for the treatment of moderate and severe PIH[13,14]. In the study of Ma et al.[15], MS can achieve therapeutic effects by improving the 24 h urine protein, Mean Arterial Pressure (MAP), homocysteine and C-reactive protein in pregnant women with PIH. Nifedipine (NIF), a dihydropyridine calcium channel blocker, can effectively inhibit the transmembrane transport of calcium ions to myocardium and smooth muscle without affecting plasma calcium ion concentrations[16], which exerts a relaxing effect on intravascular smooth muscle and thus helps to lower blood pressure and systolic pressure[17]. In the study of Easterling et al.[18], NIF, as an oral antihypertensive drug, could be used to treat severe hypertension during pregnancy and was more effective as a monotherapy than with methyldopa.
Considering that there are few analysis of the effect of MS with NIF on clinical outcomes in PIH patients receiving Evidence-Based Care (EBC), this study attempts to conduct this analysis in order to contribute optimization of the management of such patients.
Materials and Methods
General information:
This study selected 100 PIH individuals admitted between January 2022 and December 2022 in our hospital and were divided into research (n=54) and control groups (n=46). On the basis of EBC, the research group received MS+NIF, while the control group received MS monotherapy. The patients in the two groups were not statistically different in baseline data and were clinically comparable (p>0.05). This research has obtained approval from the hospital’s ethics committee.
Inclusion criteria:
All patients who met the diagnostic criteria for PIH; patients who underwent regular obstetric examination and scheduled deliveries in our hospital; patients with complete history of data; patients with good compliance; patients who are willing to cooperate with the research and patients having no history of hypertension before pregnancy were included in the study.
Exclusion criteria:
Patients having other pregnancy complications or comorbidities; patients with abnormal coagulation function or immune dysfunction; patients having heart, lung, kidney dysfunction and other diseases and patients having the history of allergy to MS or NIF were excluded from the study.
Treatment method:
Patients of both the groups received EBC, following some measures like all the patients were placed in quiet, soft lit and comfortable wards to avoid the stimulation of adverse factors, and were asked to rest in bed more to ensure adequate sleep. Similarly, patients’ vital signs were closely observed by monitoring the changes in blood pressure, and the emergencies of hypertension were dealt with in a timely manner. In addition, the risk of cerebral hemorrhage, hypertensive encephalopathy and heart failure was prevented, and the blood pressure was controlled to gain time to promote fetal maturity and prevent the occurrence of maternal and infant complications. Further, medication guidance was followed. Before medication, the medical staff guided the patients with patience on drug use and explained the possible adverse reactions, so that the patients could be psychologically prepared.
At the same time, the health assistants rationally regulated the infusion speed and input amount of MS during medication, closely observed the potential adverse reactions after medication, and provided timely intervention. The medical staff also monitored the fetal condition closely, strengthened education for pregnant women after admission, guided and cooperated with the monitoring of fetal movements, and timely found abnormalities for prompt intervention. The patient was taken complete care and comfort, receiving psychological support and explanation work in a timely manner, so as to keep the patient in a good psychological state and mood as much as possible and cooperate with the treatment. Similarly, dietary care was observed as the rise of blood pressure is closely associated with the patient’s diet. Therefore, patients were advised to follow nutrient-rich, digestible, high-protein dietary principles in their daily diet, while appropriately controlling salt intake according to the severity of the disease.
The control group received 4 g of MS monotherapy where the drug was dissolved in 100 ml of 5 % glucose water and was intravenously dripped for 30 min, followed by intravenous infusion of 7.5 g of MS mixed with 500 ml of 5 % glucose water with the infusion time controlled within (6-8) h. This treatment was continued until 1st d after delivery.
Similarly, the research group received combination of MS+NIF. MS was administered in the same way as the control group. In addition, NIF was administered orally at a dose of 10 mg per time, three times a day, for continuous treatment until 1st d of postpartum.
Endpoints:
Clinical efficacy: Marked response is that the clinical symptoms and signs disappear completely and the MAP returns to normal. Response refers to significantly improved clinical symptoms and signs and decreased MAP by ≥20 mmHg compared with the baseline (before treatment) while improvement corresponds to improved clinical symptoms and signs with a reduction in MAP by ≥10 mmHg compared with the baseline; non-response means no change or worsening of clinical symptoms and signs, and a decrease in MAP<10 mmHg. The Overall Response Rate (ORR) refers to the sum of marked response rate, response rate and improvement rate.
Blood pressure and Heart Rate (HR) control: Systolic and Diastolic Blood Pressure (SBP/DBP), and HR in all the patients were assessed by using blood pressure meter before and after treatment and was recorded.
Complication rate: Adverse events such as dyspnea, accelerated HR, nausea and vomiting were observed and recorded by comparing the two groups and the rate of incidence was calculated.
Umbilical Artery Resistance Index (UARI) and Blood Viscosity (BV): UARI was detected with an ultrasonic detector while the BV was measured by a hemorheometer. Both the groups were compared and analyzed for these indices.
Adverse pregnancy outcomes: We mainly paid attention to observe the number of adverse pregnancy outcomes such as postpartum hemorrhage, placental abruption and cesarean section, and then we calculated the incidence rate.
Statistical analysis:
The collected data were statistically described as mean±Standard Error of Mean (SEM). The interand intra-group information was analyzed by preand post-treatment comparisons were made using independent sample and paired t-tests respectively. Thus calculated ratio (percentage) was used for statistical description of count data, and the comparison between the two groups carried out by the Chi-square (χ2) test. The collected experimental data were analyzed by Statistical Package of Social Sciences (SPSS) version 19.0 where p<0.05 was found to be statistically significant.
Results and Discussion
Comparative analysis of the general data such as age, weight, gestational age, delivery time, degree of PIH and gestational period was compared between two groups which showed no significant difference between them (p>0.05) (Table 1).
| Factors | Research group (n=54) | Control group (n=46) | χ 2/t | p |
|---|---|---|---|---|
| Age (y) | 28.81±5.91 | 29.74±4.63 | 0.865 | 0.389 |
| Weight | 53.83±6.05 | 53.20±4.81 | 0.569 | 0.571 |
| Gestational weight | 35.85±2.33 | 36.09±2.46 | 0.500 | 0.618 |
| Gender parity | 2.018 | 0.156 | ||
| Primipara | 47 (87.04) | 35 (76.09) | ||
| Multipara | 7 (12.96) | 11 (23.91) | ||
| Degree of PIH | 0.547 | 0.459 | ||
| Moderate | 35 (64.81) | 33 (71.74) | ||
| Severe | 19 (35.19) | 13 (28.26) | ||
| Gestation period | 1.717 | 0.190 | ||
| 2nd trimester | 14 (25.93) | 7 (15.22) | ||
| 3rd trimester | 40 (74.07) | 39 (84.78) |
Table 1: General Information of Patients in the two Groups
Clinical efficacy of PIH patients was evaluation between the two groups. The ORR in the research group was found to be 90.74 % while it was found to be 71.74 % in the control group. These results suggested markedly higher efficacy in the research group treated by MS therapy with EBC (p<0.05) (Table 2).
| Factors | Research group (n=54) | Control group (n=46) | χ2/t | p |
|---|---|---|---|---|
| Marked response | 28 (51.85) | 18 (39.13) | - | - |
| Response | 11 (20.37) | 9 (19.57) | - | - |
| Improvement | 10 (18.52) | 6 (13.04) | - | - |
| Non-response | 5 (9.26) | 13 (28.26) | - | - |
| ORR | 49 (90.74) | 33 (71.74) | 6.076 | 0.014 |
Table 2: Clinical Evaluation of Patients with PIH
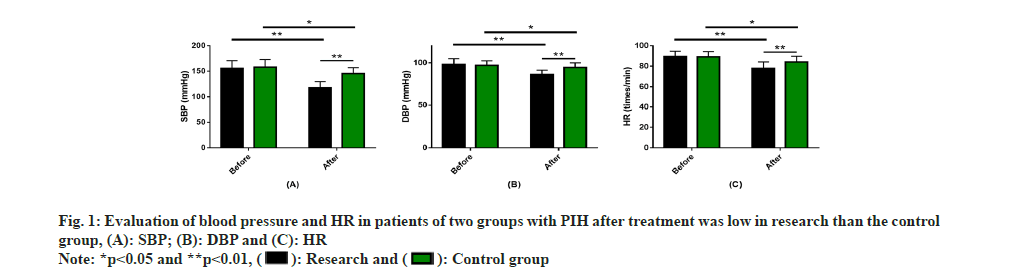
Evaluation of blood pressure and HR control in PIH patients was carried out. The two groups had similar pre-treatment SBP, DBP and HR levels (p>0.05). An obvious reduction in these 3 indices was observed after treatment (p<0.05), with even lower levels of SBP, DBP and HR in the research group (P<0.05) (fig. 1).
Evaluation of complication rate in PIH patients was evaluated. The incidence of complications such as dyspnea, increased HR, nausea and vomiting in the research group was 9.26 %, which was notably lower than the control group with 32.61 % (p<0.05) (Table 3).
| Factors | Research group (n=54) | Control group (n=46) | χ2/t | p |
|---|---|---|---|---|
| Dyspnea | 1 (1.85) | 3 (6.52) | - | - |
| Increased HR | 2 (3.70) | 5 (10.87) | - | - |
| Nausea | 1 (1.85) | 4 (8.70) | - | - |
| Vomiting | 1 (1.85) | 3 (6.52) | - | - |
| Total | 5 (9.26) | 15 (32.61) | 8.464 | 0.004 |
Table 3: Evaluation of Complication Rate in Patients With PIH
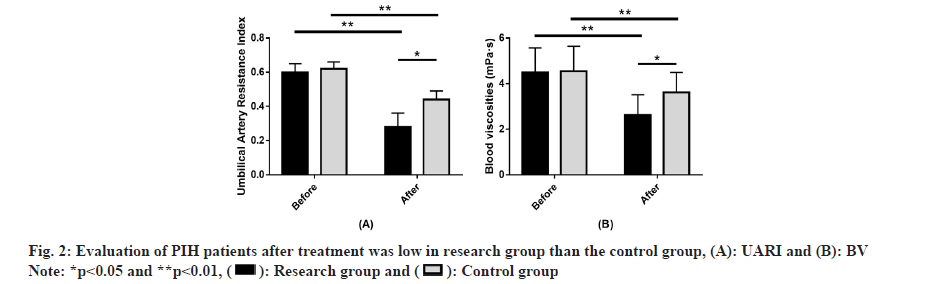
Evaluation of UARI and BV in PIH patients between the two groups was studied. The two groups did not differ significantly in pre-treatment UARI and BV (p>0.05). Evidently decreased UARI and BV were found in both groups after treatment (p<0.05), with even lower levels of the two in the research group (p<0.05) (fig. 2). Evaluation of adverse pregnancy outcomes in PIH patients was observed. The incidence of adverse pregnancy outcomes in the research group was 5.56 %, significantly lower than the 19.57 % in the control group (p<0.05) (Table 4).
| Factors | Research group (n=54) | Control group (n=46) | χ2/t | p |
|---|---|---|---|---|
| Postpartum hemorrhage | 2 (3.70) | 3 (6.52) | - | - |
| Placental abruption | 0 (0.00) | 2 (4.35) | - | - |
| Caesarean section | 1 (1.85) | 4 (8.70) | - | - |
| Total | 3 (5.56) | 9 (19.57) | 4.617 | 0.032 |
Table 4: Evaluation of Adverse Pregnancy Outcomes in Patients with PIH
The pathogenic factors of PIH are complex and the factors such as gender parity, age, increased body mass index during pregnancy and salty diet may increase the risk of the disease[19]. Among pregnant women, impact of the disease can involve the kidneys, liver, heart, nervous system and basal decidua, resulting in short- and long-term adverse effects[20]. Therefore, it is important to take timely and effective treatment to manage such patients.
In our study, the research group (90.74 %) showed an obviously higher ORR than the control group (71.74 %), suggesting that MS+NIF is beneficial to improve the curative effect of PIH patients receiving EBC.
In the research of Chang et al.[21], MS also reduced stress responses and vascular endothelial loss in PIH patients by regulating the Placental Growth Factor (PLGF), serum Heat shock protein 70 (Hsp70), Pentraxin related protein 3 (PTX3), Endothelin-1 (ET-1), Nitric Oxide (NO) and other indicators, thus playing an important therapeutic role in the disease. While NIF mainly inhibits the flow of calcium ions to cells, thereby increasing the coronary blood flow and the myocardial tolerance to ischemia, thereby playing a role in lowering the blood pressure[22,23]. In the study of Yu et al.[24],
MS+NIF in the treatment of PIH patients significantly improved the total clinical response rate, enhanced the coagulation function and alleviated oxidative stress damage of patients, similar to our study. In addition, the use of drugs for the treatment of PIH combined with EBC in this study is to ensure a certain therapeutic effect of drugs while reducing potential complications after medication. The therapeutic concentration of MS is very close to the toxic concentration, so it is necessary to completely understand and pay special attention to the medication methods and toxic effects[25]. In addition, EBC for patients with gestational diabetes has been shown not only to effectively control blood sugar and blood pressure, but also exerts a favorable impact on maternal and infant outcomes (lower overall complication rate)[26].
SBP, DBP and HR detection denoted lower levels of these three indices in the research group compared with thes control group after treatment, indicating that MS+NIF therapy has more advantages in blood pressure and HR control in PIH receiving EBC, similar to the findings of Xiang et al.[27]. According to the statistics, major complication in both groups was mainly increased HR, followed by nausea, vomiting and dyspnea, which was consistent with the research results of Shi et al.[28]. In addition, an obviously lower complication rate was determined in the research group (9.26 %) vs. control group (32.61 %), indicating that MS+NIF is helpful to prevent complications in PIH patients receiving EBC.
As reported by Zhao et al.[29], combination of NIF on the basis of MS therapy has a certain preventive effect on adverse reactions in PIH patients, exerting a good inhibitory effect on inflammatory reactions, which supports our research results. In terms of UARI and BV, both of them reduced markedly in the research group after treatment, lower compared with the control group, suggesting that MS+NIF has a significant positive impact on UARI and BV in PIH patients receiving EBC. Later, statistics of the incidence of adverse pregnancy outcomes such as postpartum hemorrhage, placental abruption, and cesarean section revealed a markedly lower incidence in the research group (5.56 %) vs. control group (19.57 %), demonstrating that MS+NIF for PIH patients receiving EBC is beneficial to reduce the risk of adverse pregnancy outcomes.
Conclusively, MS+NIF is effective in treating PIH patients receiving EBC, which can help the patients to control blood pressure and HR in better way, prevent the adverse pregnancy outcomes to a certain extent and lower UARI and BV with clinical promotion value.
Conflict of interests:
The authors declared no conflict of interests.
References
- Li L, Wilson C, Morton A. Maternal bradycardia heralding deteriorating HELLP syndrome (pregnancy hypertension). Pregnancy Hypertens 2022;27:115-6.
[Crossref] [Google Scholar] [PubMed]
- Zeng L, Yang K, Ge J. Uncovering the pharmacological mechanism of Astragalus Salvia compound on pregnancy-induced hypertension syndrome by a network pharmacology approach. Sci Rep 2017;7(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Coggins N, Lai S. Hypertensive disorders of pregnancy. Emerg Med Clin North Am 2023;41(2):269-80.
[Crossref] [Google Scholar] [PubMed]
- Sutton ALM, Harper LM, Tita ATN. Hypertensive disorders in pregnancy. Obstet Gynecol Clin North Am 2018;45(2):333-47.
[Crossref] [Google Scholar] [PubMed]
- Chen H, Zhang J, Qin F, Chen X, Jiang X. Evaluation of the predictive value of high sensitivity C-reactive protein in pregnancy-induced hypertension syndrome. Exp Ther Med 2018;16(2):619-22.
[Crossref] [Google Scholar] [PubMed]
- Ren Y, Wang H, Qin H, Yang J, Wang Y, Jiang S, et al. Vascular endothelial growth factor expression in peripheral blood of patients with pregnancy induced hypertension syndrome and its clinical significance. Pak J Med Sci 2014;30(3):634-7.
[Crossref] [Google Scholar] [PubMed]
- Zhuang C, Gao J, Liu J, Wang X, He J, Sun J, et al. Risk factors and potential protective factors of pregnancy-induced hypertension in China: A cross-sectional study. J Clin Hypertens 2019;21(5):618-23.
[Crossref] [Google Scholar] [PubMed]
- Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis 2013;20(3):229-39.
[Crossref] [Google Scholar] [PubMed]
- Falcao S, Bisotto S, Michel C, Lacasse AA, Vaillancourt C, Gutkowska J, et al. Exercise training can attenuate preeclampsia-like features in an animal model. J Hypertens 2010;28(12):2446-53.
[Crossref] [Google Scholar] [PubMed]
- Rothberger S, Carr D, Brateng D, Hebert M, Easterling TR. Pharmacodynamics of clonidine therapy in pregnancy: A heterogeneous maternal response impacts fetal growth. Am J Hypertens 2010;23(11):1234-40.
[Crossref] [Google Scholar] [PubMed]
- Shafik AN, Khattab MA, Osman AH. Magnesium sulfate vs. esomeprazole impact on the neonates of preeclamptic rats. Eur J Obstet Gynecol Reprod Biol 2018;225:236-42.
[Crossref] [Google Scholar] [PubMed]
- Korish AA. Magnesium sulfate therapy of preeclampsia: An old tool with new mechanism of action and prospect in management and prophylaxis. Hypertens Res 2012;35(10):1005-11.
[Crossref] [Google Scholar] [PubMed]
- Chen C, Tao R. The impact of magnesium sulfate on pain control after laparoscopic cholecystectomy: A meta-analysis of randomized controlled studies. Surg Laparosc Endosc Percutan Tech 2018;28(6):349-53.
[Crossref] [Google Scholar] [PubMed]
- Lamarca B, Brewer J, Wallace K. IL-6-induced pathophysiology during pre-eclampsia: Potential therapeutic role for magnesium sulfate? Int J Interferon Cytokine Mediat Res 2011;2011(3):59-64.
[Crossref] [Google Scholar] [PubMed]
- Ma L, Li L, Han P, Meng F, Jiao C, Zhang H. Effect of the drug combination of magnesium sulfate and phentolamine on homocysteine and C-reactive protein in the serum of patients with pregnancy-induced hypertension syndrome. Exp Ther Med 2019;17(5):3682-8.
[Crossref] [Google Scholar] [PubMed]
- Webster LM, Myers JE, Nelson-Piercy C, Harding K, Cruickshank JK, Watt-Coote I, et al. Labetalol vs. nifedipine as antihypertensive treatment for chronic hypertension in pregnancy: A randomized controlled trial. Hypertension 2017;70(5):915-22.
[Crossref] [Google Scholar] [PubMed]
- Sharma KJ, Greene N, Kilpatrick SJ. Oral labetalol compared to oral nifedipine for postpartum hypertension: A randomized controlled trial. Hypertens Pregnancy 2017;36(1):44-7.
[Crossref] [Google Scholar] [PubMed]
- Easterling T, Mundle S, Bracken H, Parvekar S, Mool S, Magee LA, et al. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: An open-label, randomised controlled trial. Lancet 2019;394(10203):1011-21.
[Crossref] [Google Scholar] [PubMed]
- de Castro I, Easterling TR, Bansal N, Jefferson JA. Nephrotic syndrome in pregnancy poses risks with both maternal and fetal complications. Kidney Int 2017;91(6):1464-72.
[Crossref] [Google Scholar] [PubMed]
- Hauspurg A, Countouris ME, Catov JM. Hypertensive disorders of pregnancy and future maternal health: How can the evidence guide postpartum management? Curr Hypertens Rep 2019;21(12):1-20.
[Crossref] [Google Scholar] [PubMed]
- Chang R, Miao H, Cui A, Jiang L, Yang L, Miao C. Clinical effect of nimodipine combined with magnesium sulfate on pregnancy-induced hypertension syndrome. J Healthc Eng 2022:1-12.
[Crossref] [Google Scholar] [PubMed]
- Bi X, Jia H, Wang Q. The effect of pre-procedural sublingual nifedipine on radial artery diameter. Coron Artery Dis 2020;31(3):206-7.
[Crossref] [Google Scholar] [PubMed]
- Mulrenin IR, Garcia JE, Fashe MM, Loop MS, Daubert MA, Urrutia RP, et al. The impact of pregnancy on antihypertensive drug metabolism and pharmacokinetics: Current status and future directions. Expert Opin Drug Metab Toxicol 2021;17(11):1261-79.
[Crossref] [Google Scholar] [PubMed]
- Yu X, Zhou Q. Effects of nifedipine tablets combined with magnesium sulfate on blood coagulation index, oxidative stress, NO and ET-1 levels in patients with pregnancy hypertension. Front Surg 2022;9:1-12.
[Crossref] [Google Scholar] [PubMed]
- Leetheeragul J, Boriboonhirunsarn D, Reesukumal K, Srisaimanee N, Horrasith S, Wataganara T. A retrospective review of on-admission factors on attainment of therapeutic serum concentrations of magnesium sulfate in women treated for a diagnosis of preeclampsia. J Matern Fetal Neonatal Med 2020;33(2):258-66.
[Crossref] [Google Scholar] [PubMed]
- Zhong W, Li C, Liu J, Zhou J, Xiao Z, Li C, et al. Effect and significance of high-quality nursing on blood glucose, pregnancy outcome, and neonatal complications of patients with gestational diabetes mellitus. Comput Math Methods Med 2022;1-8.
[Crossref] [Google Scholar] [PubMed]
- Xiang C, Zhou X, Zheng X. Magnesium sulfate in combination with nifedipine in the treatment of pregnancy-induced hypertension. Pak J Med Sci 2020;36(2):21-5.
[Crossref] [Google Scholar] [PubMed]
- Shi DD, Wang Y, Guo JJ, Zhou L, Wang N. Vitamin D enhances efficacy of oral nifedipine in treating preeclampsia with severe features: A double blinded, placebo-controlled and randomized clinical trial. Front Pharmacol 2017;8:1-10.
[Crossref] [Google Scholar] [PubMed]
- Zhao F, Ai F, Wu J, Dong X. Changes and clinical significance of serum inflammatory factors in the treatment of pregnancy hypertension syndrome with magnesium sulfate combined with nifedipine. Exp Ther Med 2020;20(2):1796-802.
[Crossref] [Google Scholar] [PubMed]