- *Corresponding Author:
- Zhaojun Mei
Department of Pediatrics, Luzhou Maternal and Child Health Hospital (Luzhou Second People’s Hospital), Luzhou, Sichuan 646000, China
E-mail: 1059041571@qq.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “226-234” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Tea is a natural plant with important nutritional, health and clinical value. Tea has many natural active substances, such as catechins, epigallocatechin gallate and caffeine; these chemicals have a significant effect on the human body. Recently, some studies have suggested that tea intake may be related to childhood asthma. However, the relationship between those two factors remains controversial. In this study, two-sample Mendelian randomization was used to explore the causal effects of tea intake on childhood asthma. Genome-wide association studies have provided summary data on tea consumption and childhood asthma. Various Mendelian randomization methods were employed to evaluate the findings. To test the robustness of these outcomes, we applied different methods, including Cochran's Q test, Mendelian randomization-Egger regression and Mendelian randomization pleiotropy residual sum and outlier method. We also used several methods to test for heterogeneity and horizontal pleiotropy and the results were unaffected by heterogeneity and horizontal pleiotropy. Thus, our study suggests that there is no causal relationship between tea intake and childhood asthma, but the biochemical mechanisms between them need to be further explored.
Keywords
Caffeine, asthma, Mendelian randomization, epigallocatechin gallate, catechins
With the development of global civilization, tea has emerged as a common drink in daily life. This natural botanical plant possesses distinctive nutritional and health care attributes, alongside significant vitality and medical benefits (fig. 1). It has many physiologically active substances (fig. 2), such as Caffeine (CA), catechin polyphenols, saponins and theanine[1,2]. Tea has been reported to play an important role in various cardiopulmonary and other chronic diseases through various biological properties, such as antioxidant, antiinflammatory and immunomodulatory functions[3].
Asthma is a global public health challenge that affects individuals of all ages. However, the prevalence of asthma is significantly higher in children than in other age groups, affecting approximately one in five children worldwide[4]. It is a heterogeneous disease characterized by chronic inflammation of the airways, with respiratory symptoms such as wheezing, shortness of breath, chest tightness and cough, accompanied by variable expiratory airflow limitation, and the intensity and frequency of respiratory symptoms change over time[5]. The escalating prevalence of asthma has resulted in a significant upsurge in the global health care burden. In Western countries, individuals afflicted with asthma face an annual financial burden that varies between $300 and $1300, contingent upon the severity of the condition and treatment choices[6]. Nevertheless, the exact mechanisms underlying childhood asthma pathogenesis remain incompletely understood. It is speculated that dietary factors may contribute to the development of asthma in children. A recent study indicated a potential link between mild-tomoderate consumption of tea and coffee and a reduction in asthma attacks among adults[7]. An initial observation was made in another study linking tea intake with asthma. However, after controlling for confounding variables, this linkage did not sustain its statistical significance[8]. Nevertheless, the causal relationship between tea intake and childhood asthma has yet to be established. Further research is needed to determine if such a relationship exists.
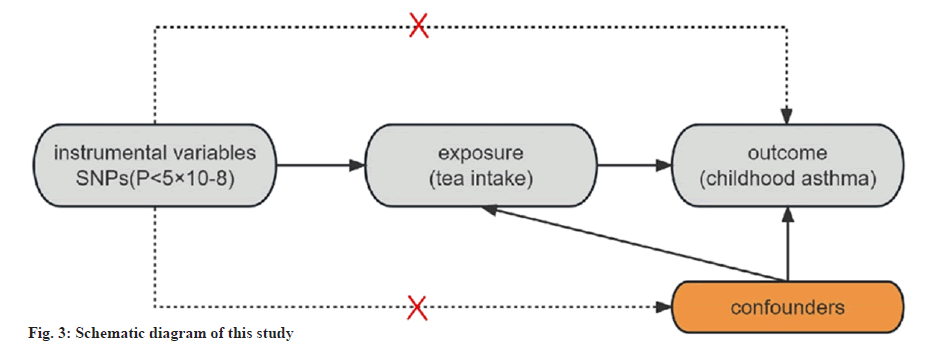
Compared to the observational research approaches, Mendelian Randomization (MR) analysis, which uses genetic variation as an Instrumental Variable (IV), has the benefit of reducing the impact of confounding and reverse causality[9]. As a result, we decided to perform an MR study to evaluate the possible connection between tea consumption and asthma in children.
Materials and Methods
MR study:
In this study, we used MR method to explore the relationship between exposure and outcome. In MR studies, the inclusion of IVs mainly follows three principles[10] (fig. 3). Initially, we identified significant IVs linked to tea consumption at the genome-wide level (p<5×10-8, r2<0.001, genetic distance=10 000 kb). Subsequently, to meet the requirements for the independence of genetic variation and confounders, we made sure that each IV included had no known relationship to any of the confounders by searching the catalog and PhenoScanner databases. Finally, to prevent the bias caused by weak IVs, we finally computed the F-statistics to ensure that weak IVs had no effect on these outcomes.
Data source:
Tea consumption data was obtained from the Medical Research Council (MRC) Integrative Epidemiology Unit (IEU) consortium which included 447 485 individuals and information on 9 851 867 Single Nucleotide Polymorphism (SNP) loci. Every individual in the sample was of European descent. Data for the Genome-Wide Association Study (GWAS) on childhood asthma were taken from the 9th release of the FinnGen project. A total of 208 264 participants were included in this GWAS (Number of individuals were 5685 and number of control individuals were 202 399).
Analytical study:
In this research, MR analysis was conducted using three distinct methods. The advantage of contrasting the outcomes of these three methods is that the uniformity of the three methodologies adds credibility to the results. The primary analysis was carried out by making use of an Inverse-Variance Weighting (IVW) method, which resulted in the most precise estimations possible but made the assumption that all SNPs were able to operate as IVs[11]. MR-Egger involves a weighted linear regression of exposure findings using Instrument Strength is Independent of the Direct Effect (InSIDE) assumption; however, it may be affected by specific IVs[12]. The use of a Weighted Median (WM) strategy has the potential to enhance the capacity to identify causal effects and decrease type I errors. MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) is a variant of IVW that eliminates IVs and its causal estimates differ significantly from those of other IVs[13]. It provides an accurate analysis if horizontal pleiotropy occurs in <50 % of IVs. The MR-PRESSO global test is utilized to assess horizontal pleiotropy. Identification of outliers and subsequent estimation of corrections are accomplished through the MR-PRESSO outlier test (p<0.05). Additionally, the MR-PRESSO distortion test is employed to ascertain significant distinctions both prior to and subsequent to the removal of outlier corrections.
Pleiotropy and heterogeneity analysis:
We used a number of different approaches to evaluate the possible impacts of the IVs to guarantee the independence of the IVs from the result[12]. Cochran’s Q statistics and MR-Egger regression were used to provide an explanation for the heterogeneity and pleiotropy seen in the research. Then, we also analyzed each SNP using the leave-one-out method and funnel plots to avoid the influence of a single SNP on the outcomes. Finally, MR-PRESSO was also used for outlier and distortion testing in order to ensure the robustness of the outcomes[13].
Functional analysis for pleiotropic genes:
We performed differential expression analysis and gene set enrichment analysis with Functional Mapping and Annotation (FUMA) GWAS[14]. The gene expression dataset was primarily obtained from Genotype Tissue Expression (GTEx) v8 dataset, which contains 54 different tissue types and includes a total of 15 201 samples[15].
Statistical analysis:
Differentially Expressed Gene (DEG) sets were pre-calculated by performing a two-tailed t-test for any gene against all others. Prior to this, expression values were normalized using a log2 transformation expression values. Genes with a Bonferroni-corrected p-value≤0.05 and an absolute log change≥0.58 were defined as the DEG set for a given tissue. Based on the DEG set, the up- and down-regulated genes were further calculated by considering the t-statistics.
Results and Discussion
The effect of consumption of CA and tea was studied. As shown in Table 1, we strictly followed the principles of MR, preliminarily screened 41 SNPs, deleted 8 palindromic SNPs and finally included 33 SNPs as IVs. Their F-statistics ranged from 30 to 493, and there was no influence of weak IVs; likewise, no IVs were affected by other known confounders. Notably, we found no overlap in the data sources for tea consumption and childhood asthma in the samples.
| SNPs | Chromosome (CHR) | Base pair position (POS) | Beta | Standard Error (SE) | p | A1 | A2 | F |
|---|---|---|---|---|---|---|---|---|
| rs10741694 | 11 | 16286183 | 1.50E-02 | 2.19E-03 | 7.90E-12 | C | T | 46.78 |
| rs10752269 | 10 | 12692902 | -1.29E-02 | 2.12E-03 | 1.30E-09 | A | G | 36.88 |
| rs10764990 | 10 | 129152608 | -1.22E-02 | 2.17E-03 | 1.90E-08 | A | G | 31.59 |
| rs11164870* | 1 | 93552187 | -1.20E-02 | 2.18E-03 | 4.20E-08 | G | C | 30.04 |
| rs1156588 | 2 | 58515375 | -1.55E-02 | 2.60E-03 | 2.90E-09 | G | A | 35.24 |
| rs11587444 | 1 | 150722844 | 1.40E-02 | 2.17E-03 | 1.00E-10 | G | A | 41.79 |
| rs12591786 | 15 | 60902512 | -1.84E-02 | 2.94E-03 | 3.70E-10 | T | C | 39.27 |
| rs13282783 | 8 | 22088975 | -1.36E-02 | 2.35E-03 | 7.90E-09 | T | C | 33.29 |
| rs132904* | 22 | 41798896 | 1.66E-02 | 2.55E-03 | 7.80E-11 | C | G | 42.3 |
| rs141071726 | 7 | 17558580 | 4.07E-02 | 6.81E-03 | 2.20E-09 | A | G | 35.75 |
| rs1453548* | 11 | 59192089 | -1.33E-02 | 2.25E-03 | 3.00E-09 | A | T | 35.17 |
| rs1481012 | 4 | 89039082 | -2.62E-02 | 3.36E-03 | 5.30E-15 | G | A | 61.15 |
| rs149805207 | 6 | 137095269 | -7.19E-02 | 1.26E-02 | 1.10E-08 | G | A | 32.68 |
| rs17245213 | 11 | 1679769 | -1.46E-02 | 2.61E-03 | 2.00E-08 | A | G | 31.52 |
| rs17576658 | 13 | 100272019 | -1.35E-02 | 2.46E-03 | 4.10E-08 | A | G | 30.12 |
| rs17685 | 7 | 75616105 | 2.31E-02 | 2.36E-03 | 1.60E-22 | A | G | 95.36 |
| rs2117137 | 3 | 89587262 | 1.30E-02 | 2.16E-03 | 1.70E-09 | G | A | 36.34 |
| rs2273447* | 20 | 62900120 | 1.75E-02 | 2.63E-03 | 3.30E-11 | T | A | 43.99 |
| rs2279844 | 17 | 40819809 | -1.20E-02 | 2.18E-03 | 4.00E-08 | A | G | 30.15 |
| rs2351187 | 10 | 86850616 | 1.29E-02 | 2.28E-03 | 1.60E-08 | A | G | 31.96 |
| rs2472297 | 15 | 75027880 | 5.33E-02 | 2.40E-03 | 2.30E-109 | T | C | 493.65 |
| rs2478875 | 6 | 51283110 | 2.19E-02 | 2.61E-03 | 5.10E-17 | G | A | 70.3 |
| rs2645929 | 13 | 56444529 | -1.50E-02 | 2.72E-03 | 3.50E-08 | G | A | 30.42 |
| rs2783129* | 13 | 80168720 | -1.17E-02 | 2.13E-03 | 3.80E-08 | G | C | 30.25 |
| rs34619 | 5 | 60465365 | 1.17E-02 | 2.14E-03 | 4.30E-08 | A | G | 30.02 |
| rs4410790 | 7 | 17284577 | 4.06E-02 | 2.20E-03 | 3.40E-76 | C | T | 341.27 |
| rs4808193 | 19 | 19410622 | 1.51E-02 | 2.25E-03 | 1.70E-11 | C | T | 45.24 |
| rs4817505 | 21 | 34343828 | 1.51E-02 | 2.17E-03 | 4.20E-12 | C | T | 48.01 |
| rs56188862 | 1 | 174189269 | -1.58E-02 | 2.17E-03 | 4.30E-13 | C | T | 52.5 |
| rs56348300* | 9 | 7054124 | 1.59E-02 | 2.73E-03 | 6.10E-09 | G | C | 33.8 |
| rs57462170 | 3 | 50239803 | 1.92E-02 | 3.41E-03 | 1.90E-08 | A | G | 31.62 |
| rs57631352 | 19 | 4338173 | -1.31E-02 | 2.32E-03 | 1.70E-08 | G | A | 31.87 |
| rs6829 | 13 | 111531264 | -1.19E-02 | 2.17E-03 | 3.70E-08 | T | C | 30.28 |
| rs713598* | 7 | 141673345 | 1.34E-02 | 2.16E-03 | 5.20E-10 | G | C | 38.59 |
| rs72797284 | 5 | 152031650 | -1.71E-02 | 2.38E-03 | 7.00E-13 | G | A | 51.56 |
| rs7757102 | 6 | 137222671 | -1.18E-02 | 2.13E-03 | 3.10E-08 | G | A | 30.62 |
| rs9302428* | 16 | 24717600 | 1.22E-02 | 2.20E-03 | 2.60E-08 | G | C | 30.95 |
| rs9624470 | 22 | 24820268 | 2.52E-02 | 2.15E-03 | 1.30E-31 | A | G | 136.84 |
| rs9648476 | 7 | 39293033 | 1.25E-02 | 2.19E-03 | 1.10E-08 | A | G | 32.72 |
| rs977474 | 12 | 11284772 | 2.18E-02 | 2.86E-03 | 2.40E-14 | T | C | 58.18 |
| rs9937354 | 16 | 53799847 | -1.41E-02 | 2.14E-03 | 4.90E-11 | A | G | 43.23 |
Note: (*): palindromic SNP which did not participate as an IV in the MR analysis, (A1): Effect allele; (A2): Other allele
Table 1: Characteristics of Tea Consumption and Its Associated Genetic Variants
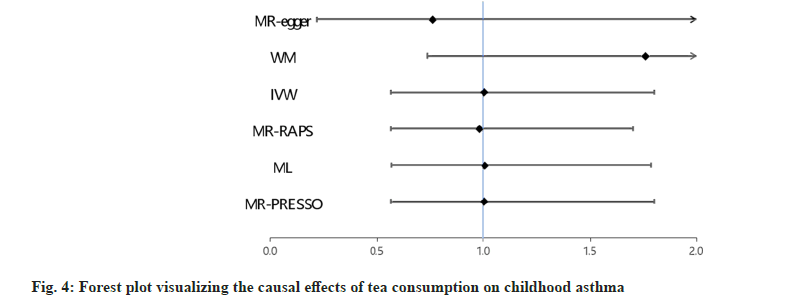
Regarding the IVW method, our findings indicated no significant association between tea intake and the risk of CA (Odds Ratio (OR)=1.005; 95 % Confidence Interval (CI): 0.562-1.799 and p=0.98) (fig. 4). Furthermore, consistent results were obtained through additional MR methods, including MR-Egger (OR=0.762; 95 % CI: 0.219- 2.656 and p=0.673); MR-Robust Adjusted Profile Score (RAPS) method (OR=0.980; 95 % CI: 0.565- 1.699 and p=0.941); WM method (OR=1.761; 95 % CI: 0.736-4.211 and p=0.204); maximum likelihood method (OR=1.006; 95 % CI: 0.566-1.785 and p=0.985) and MR-PRESSO method (OR=1.005; 95 % CI; 0.562-1.799 and p=0.985).
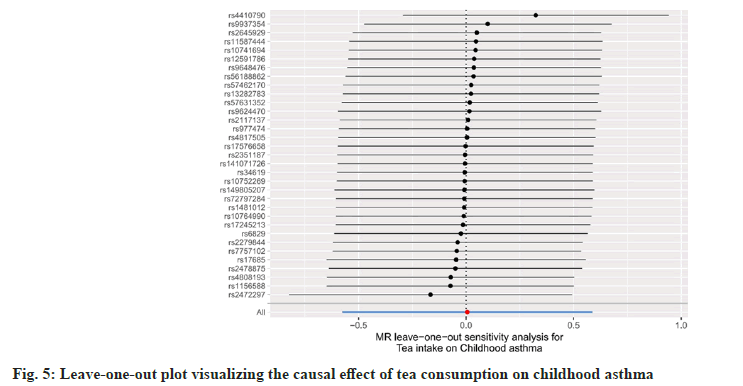
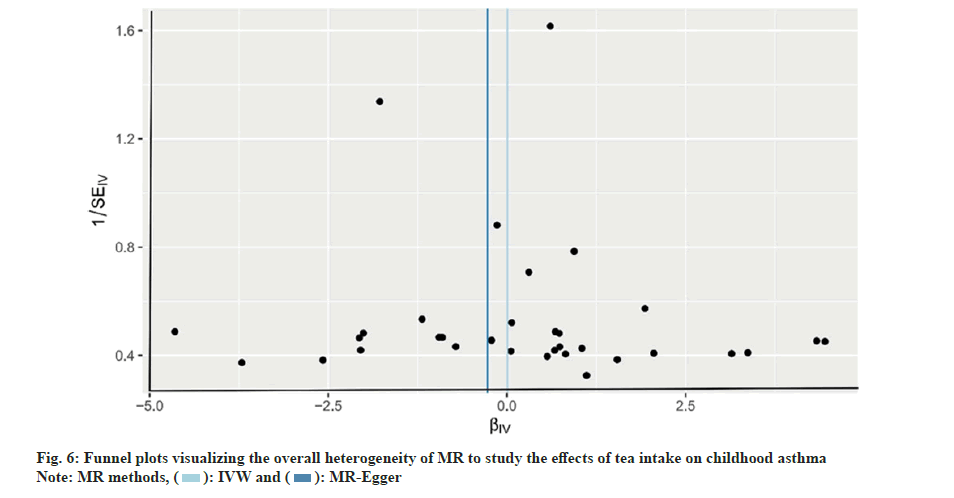
To comprehensively assess MR heterogeneity and pleiotropy, we employed a variety of methods. No significant heterogeneity was detected in our analysis using Cochran's Q test (p=0.363). Furthermore, we used MR-Egger regression, which demonstrated that horizontal pleiotropy had no effect on the MR analysis (p=0.626). Furthermore, the MR-PRESSO analysis revealed no significant outliers among the included IVs. Finally, the funnel plot and leave-one-out sensitivity analysis showed that our study conclusions were not significantly affected by the inclusion of any individual IV (fig. 5 and fig. 6).
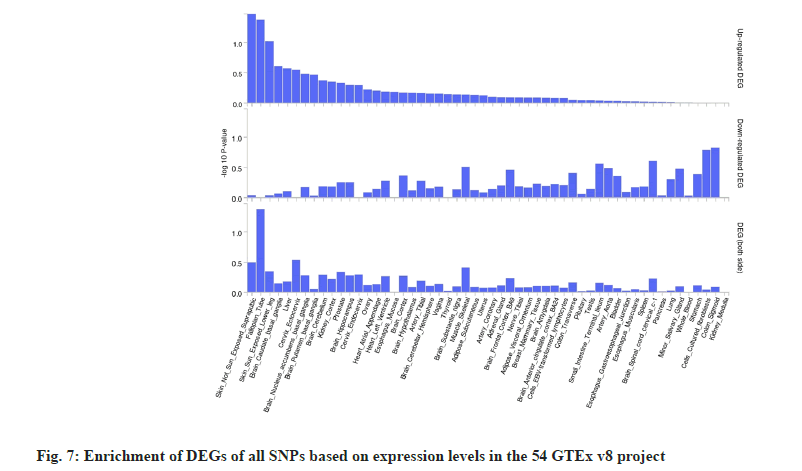
Further, gene set functional analysis was carried out where we performed gene enrichment analysis using FUMA GWAS for the identified 33 SNPs. The results indicated that DEGs were predominantly enriched in tissues such as skin, fallopian tube, brain and liver based on the analysis of expression levels from GTEx v8 (fig. 7).
Our study primarily used MR methods to explore the relationship between tea consumption and childhood asthma. There was no evidence of an increase in the risk of childhood asthma with tea intake. However, many more studies are needed to prove whether they are causal or not.
At present, the pathogenesis of childhood asthma remains incompletely elucidated, with airway inflammation, airway remodeling and heightened airway reactivity as its fundamental characteristics. Airway inflammation lies at the crux of disease progression, while the hypothesis of "free radical-induced injury" and "oxidative/ antioxidant disequilibrium" are deemed crucial underpinnings[16]. The contribution of dietary factors to the genesis of childhood asthma is yet to be comprehensively discerned.
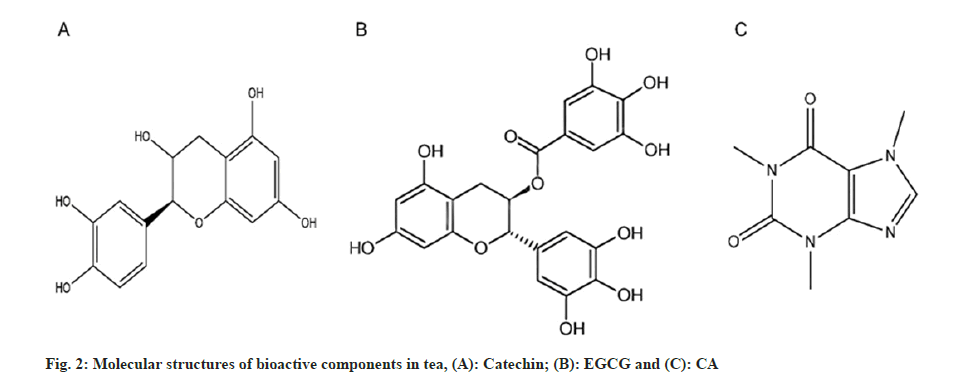
The verdant foliage of the tea plant has an abundance of vital bioactive compounds. Tea polyphenols is a comprehensive term encompassing a host of polyphenolic compounds, primarily constituted by flavanols, with the catechin group being the most important (fig. 2A). These components play a pivotal role in the chemical composition, accounting for a substantial 24 %-36 % of the dehydrated weight of tea leaves[17]. Catechins were shown to account for approximately 70 % of the total tea polyphenols, and among this class, Epigallocatechin Gallate (EGCG) is considered superior to other catechins, demonstrating greater biological potency (fig. 2B)[18].
EGCG can effectively scavenge Reactive Oxygen Species (ROS), inhibit pro-oxidative enzymes, invigorate antioxidant systems and boost cellular survivability. It suppresses cellular apoptosis induced by hydrogen peroxide and inhibits the abundant production of nitric oxide metabolites through the induction of nitric oxide synthase[19,20]. Moreover, ECGC possesses potent anti-inflammatory effects through the modulation of various intracellular signaling cascades. For example, in an in vitro model of rat asthma, EGCG attenuates the expression of High Mobility Group Box (HMGB) 1 and receptor for advanced glycation end products messenger Ribonucelic Acid (mRNA), attenuates the infiltration of inflammatory cells and improves the inflammatory cascade response associated with asthma[21]. However, the biological effects of EGCG are clearly dependent on the concentration[22]. At lower EGCG concentrations, antioxidant properties are observed[23], while at higher concentrations, EGCG may hasten the death of cells[24].
Another important active ingredient in tea is alkaloids, among which CA (otherwise known as 1,3,7-trimethylxanthine) (fig. 2C) has the highest content, accounting for approximately 2 %-5 % of the total dry mass. It is accompanied by theophylline and theobromine through the transformation of hypoxanthine nucleotides and transforms them into each other via purine metabolism. Primarily, methylxanthines promote the mobilization of intracellular calcium, inhibit Phosphodiesterases (PDEs), modulate Gamma (γ)-aminobutyric acid receptors and antagonize adenosine receptors[25]. Furthermore, methylxanthines increase cyclic Adenosine Monophosphate (cAMP) levels through their inhibitory effect on PDEs, causing smooth muscle relaxation in the airways[25]. In addition, a study demonstrated that CA has anti-inflammatory and antioxidant properties[26]. However, the precise mechanism of CA’s effect on pediatric asthma is still unknown and additional research is needed to determine the precise mechanism.
However, the mechanisms of action of tea polyphenols and CA on asthma are theoretical. The bioavailability of the active substances in actual tea is unknown. After consumption of tea, only a very tiny amount of catechins are accessible to be absorbed by the body as a whole, which might be attributed to the very acidic pH levels of the digestive fluids and digestive enzymes, as well as the lack of specific receptors to carry EGCG into enterocytes, thereby limiting the passage of EGCG into the bloodstream[27]. Further, the amount of these active ingredients in tea depends on the type of tea, its growing environment, the time of harvesting, the processing method and the degree of fermentation.
A previous study has shown that CA at 5 mg/ kg body weight reaches peak bronchodilatory effects within 2 h[28]. Therefore, it seems that a high intake of CA-containing products would be needed to achieve beneficial bronchodilatory effects; however, there may be greater side effects if excessive CA is consumed. It has been shown that CA intake >300 mg may lead to toxic effects such as agitation, euphoria, increased urination, disorganized thinking and speech, cardiac arrhythmia and even suicidal tendencies[29].
We would like to highlight some of the strengths in this study. Primarily, this is the first study to assess causality in the association between tea consumption and CA using a two-sample MR design that minimizes residual confounding, reverse causal bias and non-differential exposure compared with observational studies. Next, other MR approaches were also utilized to confirm our findings, and their consistency made our results more robust. Similarly, the selected IVs were derived from the most extensive GWASs and were closely related to the exposure, thereby minimizing weak IV bias and enhancing statistical power.
This study also has some limitations. First, our research was only conducted on people of European ancestry; thus, more research is needed to evaluate whether these findings are applicable to other groups. Second, no other available GWAS with a sufficient number of patients was reported. Third, there are multiple bioactive components in tea that may be associated with CA, and due to the lack of relevant GWASs, we are unable to perform MR analysis for each active component. Therefore, further studies on the biological mesachanism underlying the association between tea intake and childhood asthma may be needed in the future.
In summary, our research revealed no evidence of a causal relationship between tea consumption and the development of asthma in children. However, future studies should focus on the relationship between the number of bioactive components of tea absorbed in the body and childhood asthma.
Conflict of interests:
The authors declared no conflict of interests.
References
- Yang CS, Hong J. Prevention of chronic diseases by tea: Possible mechanisms and human relevance. Ann Rev Nutr 2013;33:161-81.
[Crossref] [Google Scholar] [PubMed]
- Suzuki Y, Miyoshi N, Isemura M. Health-promoting effects of green tea. Proc Jpn Acad Ser B Phys Biol Sci 2012;88(3):88-101.
[Crossref] [Google Scholar] [PubMed]
- Tang GY, Meng X, Gan RY, Zhao CN, Liu Q, Feng YB, et al. Health functions and related molecular mechanisms of tea components: An update review. Int J Mol Sci 2019;20(24):1-38.
[Crossref] [Google Scholar] [PubMed]
- Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front pediatr 2019;7:1-15.
[Crossref] [Google Scholar] [PubMed]
- Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Am J Respir Crit Care Med 2022;205(1):17-35.
[Crossref] [Google Scholar] [PubMed]
- Seaton A, Godden DJ, Brown K. Increase in asthma: A more toxic environment or a more susceptible population? Thorax 1994;49(2):171-4.
[Crossref] [Google Scholar] [PubMed]
- Lin F, Zhu Y, Liang H, Li D, Jing D, Liu H, et al. Association of coffee and tea consumption with the risk of asthma: A prospective cohort study from the UK biobank. Nutrients 2022;14(19):1-16.
[Crossref] [Google Scholar] [PubMed]
- Wee JH, Yoo DM, Byun SH, Song CM, Lee HJ, Park B, et al. Analysis of the relationship between asthma and coffee/green tea/soda intake. Int J Environ Res Public Health 2020;17(20):1-12.
[Crossref] [Google Scholar] [PubMed]
- Skrivankova VW, Richmond RC, Woolf BA, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement. JAMA 2021 26;326(16):1614-21.
[Crossref] [Google Scholar] [PubMed]
- VanderWeele TJ, Tchetgen EJ, Cornelis M, Kraft P. Methodological challenges in mendelian randomization. Epidemiology 2014;25(3):427-35.
[Crossref] [Google Scholar] [PubMed]
- Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013;37(7):658-65.
[Crossref] [Google Scholar] [PubMed]
- Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 2017;32:377-89.
[Crossref] [Google Scholar] [PubMed]
- Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018;50(5):693-8.
[Crossref] [Google Scholar] [PubMed]
- Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun 2017;8(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, et al. The Genotype-Tissue Expression (GTEx) project. Nat Genet 2013;45(6):580-5.
[Crossref] [Google Scholar] [PubMed]
- Papi A, Brightling C, Pedersen SE, Reddel HK. Pathogenesis of asthma. Lancet 2018;391:783-800.
[Crossref] [Google Scholar] [PubMed]
- Xing L, Zhang H, Qi R, Tsao R, Mine Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J Agric Food Chem 2019;67(4):1029-43.
[Crossref] [Google Scholar] [PubMed]
- Chakrawarti L, Agrawal R, Dang S, Gupta S, Gabrani R. Therapeutic effects of EGCG: A patent review. Expert Opin Ther Pat 2016;26(8):907-16.
[Crossref] [Google Scholar] [PubMed]
- Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018;10(11):1-23.
[Crossref] [Google Scholar] [PubMed]
- André DM, Horimoto CM, Calixto MC, Alexandre EC, Antunes E. Epigallocatechin-3-gallate protects against the exacerbation of allergic eosinophilic inflammation associated with obesity in mice. Int Immunopharmacol 2018;62:212-9.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Chen L, Guo F, Cao Y, Hu W, Shi Y, et al. Effects of epigallocatechin-3-gallate on the HMGB1/RAGE pathway in PM2. 5-exposed asthmatic rats. Biochem Biophys Res Commun 2019;513(4):898-903.
[Crossref] [Google Scholar] [PubMed]
- Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol 2014;2:187-95.
[Crossref] [Google Scholar] [PubMed]
- Meng Q, Velalar CN, Ruan R. Regulating the age-related oxidative damage, mitochondrial integrity, and antioxidative enzyme activity in Fischer 344 rats by supplementation of the antioxidant epigallocatechin-3-gallate. Rejuvenation Res 2008;11(3):649-60.
[Crossref] [Google Scholar] [PubMed]
- Mukhtar H, Ahmad N. Tea polyphenols: Prevention of cancer and optimizing health. Am J Clin Nutr 2000;71(6):1-5.
[Crossref] [Google Scholar] [PubMed]
- Oñatibia-Astibia A, Martínez-Pinilla E, Franco R. The potential of methylxanthine-based therapies in pediatric respiratory tract diseases. Respir Med 2016;112:1-9.
[Crossref] [Google Scholar] [PubMed]
- Azam S, Hadi N, Khan NU, Hadi SM. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med Sci Monit 2003;9(9):1-7.
[Google Scholar] [PubMed]
- Cai ZY, Li XM, Liang JP, Xiang LP, Wang KR, Shi YL, et al. Bioavailability of tea catechins and its improvement. Molecules 2018;23(9):1-18.
[Crossref] [Google Scholar] [PubMed]
- Welsh EJ, Bara A, Barley E, Cates CJ. Caffeine for asthma. Cochrane Database Syst Rev 2010(1):CD001112.
[Crossref] [Google Scholar] [PubMed]
- Rodak K, Kokot I, Kratz EM. Caffeine as a factor influencing the functioning of the human body-friend or foe? Nutrients 2021;13(9):1-29.
[Crossref] [Google Scholar] [PubMed]