- *Corresponding Author:
- Kunhikatta V
Department of Pharmacy Practice, Manipal College of Pharmaceutical Sciences, India
E-mail: vijayanarayana.k@manipal.edu
| Date of Received | 24 May 2021 |
| Date of Revision | 05 December 2021 |
| Date of Acceptance | 07 February 2023 |
| Indian J Pharm Sci 2023;85(1):119-127 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A retrospective study was conducted in a tertiary care teaching hospital to evaluate the drug utilization pattern in pregnant women. A total of 876 pregnant women were enrolled in the study. Parameters such as demography, gravidity, comorbid illness, neonatal outcome, drugs prescribed and their utilization details were collected. The mean age of the study population was found to be 29.4±4.4 y (mean±standard deviation). Majority of them underwent caesarean section (68.2 %) compared to normal vaginal delivery (31.8 %). Most of the neonates were healthy (94.3 %) whereas 0.8 % of the neonates expired during the postnatal period. Diabetes mellitus (33.6 %) was the most common comorbidity, followed by hypertension (28.3 %) and thyroid disorders (18.3 %). Folic acid (15.7 %) and calcium (15.2 %) were the most prescribed drugs, followed by ferrous fumarate (7.1 %), vitamin B12 (5.3 %) and vitamin B6 (3.4 %). In terms of defined daily dose/100 bed d, levothyroxine (369.3) was the most frequently utilized drug, followed by digoxin (53.6), dydrogesterone (27.4) and salbutamol (20.5). Estradiol was only the food and drug administration pregnancy category X drug prescribed, as estradiol supplement as this was required to improve pregnancy rates after in vitro fertilization.
Keywords
Pregnancy, gravidity, gestational age, drug utilization in pregnancy, defined daily dose
Pregnancy is a conglomerate condition where transformation of maternal physiology affects the Pharmacokinetics (PK) and Pharmacodynamics (PD) which characterize drug dosing and physiological complications in pregnancy[1]. Complete changeover of maternal physiology during the pregnancy period determines a drug to be utilized or not during this complicated period. Pernicious drug administered during the organogenesis phase of pregnancy may lead to teratogenic effect and birth deformities[2,3]. One such vintage example was “thalidomide disaster” where thalidomide was administered during pregnancy for morning sickness.
Gobs of such cases have occurred which embossed the alert on administration of drug during pregnancy. As a result of which many classification systems were developed. United States-Food and Drug Administration (US-FDA) was one among those classification systems where all therapeutic agents were categorized according to the peril they bear to fetuses exposed during pregnancy. A total of 645 drugs are categorized by the US-FDA[4]. But these classification systems were mostly based on human experience and much less on well-designed studies conducted on pregnant women. Very limited number of studies is available in this regard. Barely any studies are available which would quantify the burden of each drug utilized during pregnancy.
Continuous supervision of all drugs prescribed during pregnancy is necessary to check whether the potential benefits to mother overshadow the threat to the foetus. Inadequate drug monitoring in pregnant women can lead to consequences such as improper treatment, adverse effects, teratogenic effects and increased drug burden on pregnant women.
Our study will manoeuvre some of the methods such as drug utilization studies and Defined Daily Dose (DDD) which recuperate the appropriate and effective use of pharmaceuticals. World Health Organization (WHO) has defined drug utilization research as “marketing, distribution, prescription and use of drugs in a society, with special emphasis on the resulting medical, social and economic consequences”. It can be used to assess the number of patients exposed to specify drugs within a given time span, to encourage the rational utilization of medications in populations and to what extent drugs are legitimately utilized, overused, or underused. Each drug is given with an Anatomical Therapeutic Chemical (ATC) code and DDD which helps in analyzing the changes in drug utilization over time, prescription pattern and the quality usage of medicine and health outcomes[5,6].
Our study will be helpful for the physician in understanding the prescribing patterns of various drugs in pregnant women in different trimesters which will aid in planning appropriate options for medications in pregnancy. The main objective of our study was to determine the utilization pattern of various FDA category drugs used in pregnancy and reporting it in terms of DDD/100 bed d. The secondary objective of the study was to assess the demographic characteristics of pregnant women and neonatal outcomes. The study will also highlight the committal to establish and implement systems to prevent exposures to drugs that are known to cause risk to both pregnant women and foetus.
Materials and Methods
Study design:
A retrospective observational study conducted in pregnant women who have been admitted during the period January 2014 to December 2017 in a South Indian tertiary care teaching hospital.
Study population:
A total of 1136 pregnant women were identified from the medical records files. Pregnant women who have been admitted during the period January 2014 to December 2017 in Obstetrics and Gynecology ward had been included in the study. However, abortion cases, human immunodeficiency virus positive pregnant women and cases with incomplete data were excluded from the study. Based on the above inclusion and exclusion criteria and sample size a total of 876 patients were included. This study was approved by the Institutional Ethical Committee (IEC 514/2018).
Data collection:
Cases were selected as per the ICD 10 Code: O00.0-O99.9 from Medical Records Department based on the inclusion and exclusion criteria. All the parameters were collected from patient medical records and online discharge summary reports. The data collected was recorded in a standard Case Record Form (CRF) which was designed particularly for this study. The CRF contained data on demography, gravidity, comorbid illness, neonatal outcome, drugs prescribed and their utilization details (total number of drugs prescribed, generic name, dose, grams per unit dosage, number of doses per package, number of packages consumed, duration and route of administration) and length of hospitalization.
Statistical analysis:
Nominal data were expressed in frequency and percentage. Parametric data were expressed as mean with Standard Deviation (SD). Data entry and statistical analysis were done using IBM Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM Corp. Armonk, NY). Percentage (%) of prescribed drugs from each trimester was calculated by the number of prescriptions of a drug in that trimester divided by total number of prescriptions in that trimester multiplied by 100. DDD/100 bed was used to calculate the total usage of drugs in pregnant women. For the calculation of DDD, “ATC classification system” was used to categorize the drugs and WHO DDD for each drug was obtained from “WHOCC-ATC/DDD Index 2019. Combination of vitamins and minerals, for which WHO, DDD is not assigned, DDD/100 bed d was not calculated. DDD/100 bed d was calculated by using the below mentioned formula using Microsoft Excel® (2016).
DDD/100 bed d=(Number of grams of drug used×100)/ (WHO DDD Units (g)×Number of bed days)
Number of bed days=Number of beds in the hospital×Occupancy index×Number of days (during the study period)
Results and Discussion
A total of 1136 pregnant women were identified and their files were reviewed from the medical records, from which 876 were included as per inclusion and exclusion criteria to evaluate the drug utilization pattern among the pregnant women. Demography and other pregnancy related characteristics of the study population are described in Table 1. The mean age of pregnant women was 29.4±4.4 (mean±SD) y. Majority of the study population was in the age group of 21 y-30 y (60.6 %), followed by 31 y-40 y (37.1 %). Multigravida was the commonest (54.4 %) and 5.4 % women had In Vitro Fertilization (IVF). Most of the pregnant women in study population were in 3rd trimester of their pregnancy (65.2 %). Out of 876 pregnant women, 789 delivered and the data on delivery of the remaining 87 pregnant women were missing. Among the 789 delivered pregnant women, 538 (68.2 %) had caesarean section, remaining by normal vaginal delivery (31.8 %).
| Parameter | Frequency (%), n=876 |
|---|---|
| Age in years (mean±SD) | 29.4±4.4 |
| Age group in year | |
| Under 20 | 8 (0.9) |
| 21-30 | 531 (60.6) |
| 31-40 | 325 (37.1) |
| 41 and over | 12 (1.4) |
| Gravidity | |
| Primigravida | 399 (45.5) |
| Multigravida | 477 (54.4) |
| Mode of fertilization | |
| Normal | 829 (94.6) |
| IVF | 47 (5.4) |
| Gestational age | |
| First Trimester | 122 (13.9) |
| Second Trimester | 183 (20.9) |
| Third Trimester | 571 (65.2) |
| Type of delivery (n=789) | |
| Vaginal delivery | 251 (31.8) |
| Caesarean Section | 538 (68.2) |
| Neonatal outcomes (n=789) | |
| Alive and healthy | 744 (94.3) |
| Still birth | 18 (2.3) |
| NICU | 16 (2) |
| Expired during postnatal period | 6 (0.8) |
| Macerated fetus | 2 (0.3) |
| Cleft lip | 1 (0.1) |
| Multiple external anomalies | 1 (0.1) |
| Serratia and Klebsiella infection | 1 (0.1) |
Table 1: Demography and Other Characteristics of Study Population
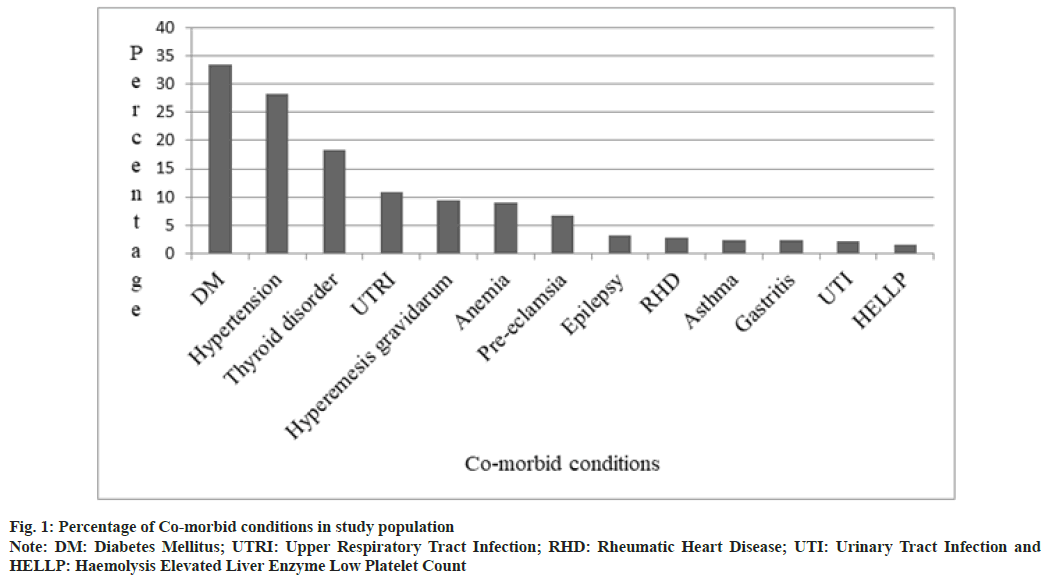
The most common comorbid conditions in the study population are depicted in fig. 1. Gestational Diabetes Mellitus (GDM) (33.6 %) was the commonest, followed by Gestational Hypertension (GHTN) (28.3 %) and thyroid disorders (18.3 %). Neonatal outcomes of study population are shown in Table 1. Majority of the neonates 744 (94.3 %) were healthy, 18 (2.3 %) of the neonates were stillborn, 15 (1.9 %) were admitted to Neonatal Intensive Care Unit (NICU) due to prematurity and other reasons, 6 (0.8 %) neonates expired during postnatal period.
Prescription pattern of drugs in study population is analyzed as per FDA category of each of these drugs and shown with respect to trimester of pregnancy are shown in Table 2. The total number of prescriptions was 821, 1310 and 2956 in 1st, 2nd and 3rd trimester respectively. The most prescribed drug in this study is folic acid (15.7 %) which belongs to FDA drug category A, followed by calcium (15.2 %) and ferrous fumarate (7.1 %). Prescription of folic acid, iron preparations, calcium and vitamin B-complexes were comparatively more in 3rd trimester of pregnancy. The FDA drug category D drugs prescribed in study population were carbamazepine (0.2 %), propylthiouracil (0.2 %), carbimazole (0.04 %), phenytoin (0.02 %) and clonazepam (0.02 %). Except carbamazepine and propylthiouracil FDA category D drugs were mostly prescribed in 3rd trimester of pregnancy, compared to carbimazole, propylthiouracil is safer in pregnant women. But there is no safer alternative drug for carbamazepine. Estradiol (0.8 %) was the only drug belonging to FDA drug category X, prescribed in study population and its use was steadily increased and more than doubled in 2017.
| Drug | FDA drug category | Prescriptions, n (%) | |||
|---|---|---|---|---|---|
| 1st Trimester (n=821) | 2nd Trimester (n=1310) | 3rd Trimester (n=2956) | Total (n=5087) | ||
| Folic Acid | A | 115 (14) | 166 (12.7) | 520 (17.6) | 801 (15.7) |
| Calcium | NA | 70 (8.5) | 164 (12.5) | 541 (18.3) | 775 (15.2) |
| Ferrous Fumarate | NA | 56 (6.8) | 141 (10.7) | 166 (5.6) | 363 (7.1) |
| Vitamin B12 | A | 45 (5.5) | 64 (4.9) | 162 (5.5) | 271 (5.3) |
| Vitamin B6 | A | 41 (5) | 47 (3.6) | 87 (3) | 175 (3.4) |
| Levothyroxine | A | 21 (2.5) | 47 (3.6) | 96 (3.2) | 164 (3.2) |
| Pantoprazole | B | 53 (6.4) | 45 (3.4) | 65 (2.2) | 163 (3.2) |
| Dydrogesterone | B | 42 (5.1) | 33 (2.5) | 64(2.2) | 139 (2.7) |
| Ranitidine | B | 28 (3.4) | 32 (2.4) | 56 (1.9) | 116 (2.3) |
| Hydroxyprogesterone | B | 19 (2.3) | 25 (2) | 62 (2.1) | 106 (2.1) |
| Amoxicillin | B | 19 (2.3) | 32 (2.4) | 53 (1.8) | 104 (2.04) |
| Metformin | B | 8 (1) | 26 (2) | 69 (2.3) | 103 (2) |
| Ondansetron | B | 52 (6.3) | 28 (2.1) | 20 (0.7) | 100 (1.96) |
| Azithromycin | B | 15 (1.9) | 27 (2.1) | 52 (1.7) | 94 (1.8) |
| Methyldopa | B | 1 (0.1) | 18 (1.4) | 44 (1.5) | 63 (1.2) |
| Acetaminophen | B | 9 (1.1) | 18 (1.4) | 32 (1.1) | 59 (1.1) |
| Clotrimazole | B | 5 (0.6) | 14 (1.1) | 32 (1.1) | 51 (1) |
| Cetirizine | B | 7 (0.8) | 14 (1.1) | 22 (0.7) | 43 (0.85) |
| Betamethasone | C | 10 (1.2) | 34 (2.6) | 124 (4.2) | 168 (3.3) |
| Oseltamivir | C | 18 (2.2) | 26 (2) | 45 (1.5) | 89 (1.7) |
| Labetalol | C | 2 (0.2) | 17 (1.3) | 63 (2.1) | 82 (1.6) |
| Aspirin | C | 18 (2.2) | 43 (3.3) | 75 (2.5) | 136 (2.7) |
| Dexamethasone | C | 14 (1.7) | 22 (1.7) | 94 (3.2) | 130 (2.5) |
| Hydroxychloroquine | C | 5 (0.6) | 4 (0.31) | 9 (0.3) | 18 (0.3) |
| Levetiracetam | C | 2 (0.2) | 4 (0.31) | 9 (0.3) | 15 (0.3) |
| Fexofenadine | C | 0 (0) | 5 (0.38) | 8 (0.3) | 13 (0.2) |
| Albendazole | C | 0 (0) | 4 (0.31) | 4 (0.1) | 8 (0.1) |
| Carbimazole | D | 0 (0) | 0 (0) | 2 (0.06) | 2 (0.04) |
| Carbamazepine | D | 3 (0.4) | 3 (0.23) | 6 (0.2) | 12 (0.2) |
| Propylthiouracil | D | 2 (0.2) | 0 (0) | 10 (0.34) | 12 (0.2) |
| Phenytoin | D | 0 (0) | 0 (0) | 1(0.03) | 1 (0.02) |
| Clonazepam | D | 0 (0) | 0 (0) | 1 (0.03) | 1 (0.02) |
| Estradiol | X | 13 (1.6) | 11 (0.8) | 16 (0.5) | 40 (0.8) |
Table 2: Commonly Prescribed Drugs during Different Trimester of Pregnancy and Their FDA Category
Drug utilization was calculated as per WHO, DDD methodology and DDD/100 bed d of the commonly used drugs are summarized in Table 3 and Table 4 according to their classes. Levothyroxine (369.3) was the highly utilized drug in terms of DDD/100 bed d and levothyroxine use was very high (309.5) in 2016 compared to 2014, 2015 and 2017, being a retrospective study, we don’t have corresponding thyroid disorder prevalence data in that year to correlate disease prevalence drug usage. The changes in physiology, PK and PD properties of the drugs during pregnancy make pregnant women different from normal women. Due to this reason, not all drugs are safe during pregnancy. The objective of our work was to understand the drug utilization pattern in pregnancy.
| Drug | FDA drug category | ATC class | DDD/100 bed d | ||||
|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | Total | |||
| Levothyroxine | A | H03AA01 | 3.2 | 50.2 | 309.5 | 6.4 | 369.3 |
| Dydrogesterone | B | G03DB01 | 1.8 | 3.1 | 10.7 | 11.7 | 27.4 |
| Methyldopa | B | C02AB01 | 4.3 | 3.9 | 3.1 | 0.1 | 11.4 |
| Enoxaparin | B | B01AB05 | 0.3 | 0.2 | 3.2 | 2 | 5.7 |
| Metformin | B | A10BA02 | 0.8 | 1.6 | 1.4 | 1.3 | 5.1 |
| Insulin | B | A10AE01 | 1.5 | 0.7 | 0.8 | 1.2 | 4.2 |
| Hydroxyprogesterone | B | G03DA03 | 0.5 | 0.7 | 1.04 | 1.1 | 3.3 |
| Pantoprazole | B | A02BC02 | 0.3 | 0.5 | 0.5 | 1.9 | 3.2 |
| Cetirizine | B | R06AE07 | 0.1 | 0.2 | 0.6 | 0.5 | 1.4 |
| Meclizine | B | R06AE05 | 0.4 | 0.2 | 0.3 | 0.6 | 1.5 |
| Ethambutol | B | J04AK02 | 0.4 | 0.1 | 0.3 | 0.5 | 1.3 |
| Ranitidine | B | A02BA02 | 0.4 | 0.4 | 0.3 | 0.2 | 1.3 |
| Azithromycin | B | J01FA10 | 0.2 | 0.3 | 0.4 | 0.4 | 1.3 |
| Acetaminophen | B | N02BE01 | 0.03 | 0.03 | 0.1 | 0.05 | 0.2 |
Table 3: DDD/100 Bed d of FDA Category Drugs (A and B) Used in Study Population
| Drug | FDA drug category | ATC class | DDD/100 bed d | ||||
|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | Total | |||
| Digoxin | C | C01AA05 | 0 | 14.3 | 39.3 | 0 | 53.6 |
| Salbutamol | C | R03AC02 | 2.2 | 1.8 | 0.1 | 16.4 | 20.5 |
| Amlodipine | C | C08CA01 | 3.9 | 1.05 | 3.02 | 1.8 | 9.8 |
| Dexamethasone | C | H02AB02 | 4.2 | 0.3 | 1.2 | 0.6 | 6.4 |
| Labetalol | C | C07AG01 | 0.1 | 0.8 | 0.7 | 0.8 | 2.5 |
| Levetiracetam | C | N03AX14 | 0.6 | 0.02 | 0.01 | 1.7 | 2.3 |
| Isoniazid | C | J04AC01 | 0.6 | 0.1 | 0.4 | 0.6 | 1.7 |
| Rifampicin | C | J04AB02 | 0.5 | 0.1 | 0.4 | 0.5 | 1.5 |
| Hydroxychloroquine | C | P01BA02 | 0.2 | 0.3 | 0.02 | 0.2 | 0.7 |
| Carbimazole | D | H03BB01 | 0 | 0.4 | 0.1 | 0 | 0.5 |
| Phenytoin | D | N03AB02 | 0 | 0.1 | 0 | 0 | 0.1 |
| Clonazepam | D | N03AE01 | 0 | 0 | 0.02 | 0 | 0.02 |
| Carbamazepine | D | N03AF01 | 0.02 | 0.1 | 0.4 | 0.7 | 1.2 |
| Propylthiouracil | D | H03BA02 | 1.2 | 3.6 | 0.5 | 0 | 5.3 |
| Estradiol | X | G03CA03 | 0.8 | 0.8 | 1.9 | 4.9 | 8.4 |
Table 4: DDD/100 Bed d of FDA Category Drugs (C, D and X) Used in Study Population
In a study conducted in California hospitals, hypertension, diabetes, asthma and thyroid disorders were found in increasing rates among pregnant women[7]. In another study conducted, it was observed that urinary tract infection, GHTN, gastritis, GDM, diarrhea, malaria, Upper Respiratory Tract Infection (URTI), hyper emesis graviduram and anemia were the commonly associated illnesses during pregnancy[8]. Our study results were in accordance to both the studies mentioned above, where GDM, GHTN and thyroid disorders were the most common comorbid conditions. The occurrence of diabetes during pregnancy is due to the hormones that are released by the placenta which causes insulin-desensitizing effects. Usually, body responds to this by producing more insulin but in some cases, it does not happen which leads to GDM[9]. GHTN generally occur after 20th w and can be due to the overstimulation of Renin-Angiotensin-Aldosterone System (RAAS) where all of elements in RAAS chain are also found to be high. Usually, renal blood flow and Glomerular Filtration Rate (GFR) are increased during pregnancy, but GHTN is associated with decrease in renal blood flow and GFR[10].
In a study conducted by Bánhidy et al.[11] it is mentioned that folic acid, vitamins, iron, calcium, multivitamins were given to prevent pregnancy complications and failed pregnancy outcomes particularly congenital abnormalities. Similar kind of results were found in our study population i.e., 15.7 % of prescriptions were of folic acid. In another study conducted by Selvaraj et al.[12] the most commonly prescribed drugs in 1st, 2nd, and 3rd trimester were iron, calcium and folic acid which we can see in our results too i.e., 15.2 % calcium and 7.1 % ferrous fumarate were prescribed.
Vitamin B12 defiance in pregnant women was having higher risk of neural tube defects in their infants where 5.3 % of the prescriptions were of vitamin B12[13]. Our finding showed heterogeneous results. This may be due to the fact that pregnant women in our study population have normal levels of vitamin B12. A study by Lockshin et al.[14] specified that corticosteroids are used to induce lung maturity in infants that are expected to be premature and no adverse effects were found with these drugs. Concordance results were found in our study and the most common corticosteroid used was betamethasone, which was administered in divided doses and was prescribed mostly in 3rd trimester.
Risks may appear if thyroid disorders are not treated during pregnancy. In a study conducted by Negro et al.[15] there was a decrease in the adverse effects in pregnant women who were treated for hypothyroidism or hyperthyroidism. According to a study conducted by Alexander et al.[16] levothyroxine is the best choice of drug for hypothyroidism in pregnant women and is not associated with any adverse effects. Our study population which includes pregnant women was also prescribed with levothyroxine for hypothyroidism. Sasidharan et al.[17] study states that the most prescribed antiulcer drug was ranitidine but, pantoprazole was mostly prescribed in our study group.
In a study conducted by Omar et al.[18] it was stated that dydrogesterone decreased the occurrence of pregnancy loss in threatened abortion through the first trimester. In our study, 3.6 % of them had threatened abortion and 37.5 % of them were prescribed dydrogesterone and majority of them had healthy neonatal outcomes. The most commonly prescribed cardiovascular drug was low dose of aspirin (75 mg) which was in homogenous to a study conducted by Sibai et al.[19] stating that low dose of aspirin (60 mg) can be effectively used to reduce pre-eclampsia in pregnant women.
Hydroxyprogesterone prevents the risk of premature labor and similar kind of results were seen in our study population where 2.1 % of the prescriptions had hydroxyprogesterone[20]. Study conducted by Japsen et al.[21] states that use of amoxicillin during pregnancy, reduces the risk of adverse pregnancy outcome and similar prescription pattern was seen in our study population.
In a study conducted by Feig et al.[22], it was stated that glyburide and metformin can be used in the treatment of gestational diabetes and have no teratogenic effects in human. A study by de Veciana et al.[23], concluded that insulin therapy recovers glycemic control and reduces the threat of neonatal hypoglycemia, macrosomia and cesarean delivery. Likewise, in our study, metformin was the major anti-diabetic drug prescribed. Even though diabetes mellitus was the most common comorbidity, anti-diabetic drugs were not the most prescribed drugs as most of the diabetic pregnant women were managed with diet.
In a study conducted by Asker et al.[24] the most frequently used anti-emetic drug is meclizine and only a few women were using ondansetron. Women who used anti-emetics had a better neonatal outcome and adverse neonatal outcomes were low. Different scenario was seen in our study population where ondansetron was mostly prescribed.
Identical results were observed between our study and Sarkar et al.[25], study where azithromycin was mostly prescribed as it does not have greater risk of major malformation during pregnancy. In our study azithromycin was the second largest prescribed antibiotic.
In our study, oseltamivir is the most commonly used antiviral in all the three trimesters which is in accordance to the study conducted by Beauet et al.[26], which concludes no association of oseltamivir with any adverse pregnancy outcomes. No adverse neonatal outcomes pertaining to oseltamivir was seen in our study.
Labetalol and methyldopa are the commonly prescribed anti-hypertensive drugs in this study and is supported by a study conducted by Podymow et al.[27] stating that methyldopa and labetalol are the first-line antihypertensive drugs that can be used commonly in pregnancy. In another study conducted in pregnant women who was administered with methyldopa by Jones and Cummings in 1978, highlighted that traces of methyldopa were found in the blood of fetus around the time of delivery and observed no adverse effects on the fetus. This study concluded that methyldopa is safe in pregnancy[28].
In a study conducted by Rebordosa et al.[29] 26 424 children who were exposed to acetaminophen during first trimester was compared with 61 718 unexposed children stated that no association of congenital abnormality with acetaminophen was observed. In accordance to the above study acetaminophen is the main analgesic and antipyretic drug used in our study population.
In a study by Czeizel et al.[30] that included 38 151 newborn infants in which 8.1 % of them were born to mothers who received clotrimazole management throughout pregnancy, stated that the mean gestational age was increased by the use of clotrimazole. Identical results were seen in our study where, clotrimazole was used for the treatment of candidiasis and was also found with long gestational age.
The most commonly prescribed antihistamine is cetirizine and fexofenadine. No adverse neonatal outcomes were observed in mothers who were administered with these drugs in our population. Our study results were supported by Seto et al.[31] and Son et al.[32] research which states that no teratogenic effects were observed on this class of drug during pregnancy and neither first generation nor second generation antihistamines are linked with any adverse fetal effects. Hydroxychloroquine is used to treat systemic lupus erythematosus during pregnancy[33]. Alike results were found in our study also.
Valproate and phenobarbital are related with major malformations when compared to lamotrigine and levetiracetam. Topiramate was also associated with risk of cleft lip[34]. In a study conducted by Tomson et al.[35] it was concluded that lamotrigine, levetiracetam and oxcarbazepine are having low risk of adverse neonatal outcomes when administered during pregnancy. No drugs that caused malformations were given to pregnant women in our study. Levetiracetam and carbamazepine was the most prescribed drug in this study and no adverse effects were observed in association with the anti-epileptic drugs given in this study.
WHO has recommended using albendazole and mebendazole for the deworming in pregnant women[36]. In a study conducted in Nepal by Christian et al.[37] it was found that the use of albendazole increased the birth weight and infant survival. Similar results found in our study. The drug utilization pattern among pregnant women was studied by in terms of DDD/100 bed d. Our study showed that thyroxine is the highest prescribed drug in this study among pregnant women with DDD/100 bed d of 53.6 which is a thyroid drug. Other highly prescribed drugs from different classes were digoxin from cardiovascular drugs, dydrogesterone from sex hormones, salbutamol from the class of asthmatic drugs, methyldopa from cardiovascular drugs, amlodipine from cardiovascular drugs, metformin from hypoglycemic drugs, cetirizine from antihistamines, oseltamivir from antiviral drugs, isoniazid from antitubercular drugs, azithromycin which is an antibiotic, acetaminophen which is an analgesic and antipyretic drug, clotrimazole which is an antifungal, albendazole which is from anthelmintic drugs, meclizine from antiemetic class, pantoprazole an anti-ulcer drug, lactulose from laxatives, levetiracetam which is an anti-epileptic drug, hydroxychloroquine which is an immunemodulator and ursodeoxycholic acid which is a bile acid derivative. Even though estradiol is a category X drug, it was prescribed to the pregnant women in this study. Studies have suggested that estradiol supplementation in the luteal phase improves the pregnancy rate in women undergoing IVF cycles[38]. In our study, about 5.4 % had IVF assisted pregnancy in which 44.4 % was given with estradiol. It was found that in our study, no adverse neonatal outcomes were observed in mothers who were administered with estradiol.
In conclusion in this study, we have analyzed the drug utilization pattern among pregnant women in a tertiary care teaching hospital. We observed that most of the pregnant women are prescribed with medications due to the associated illnesses in circumstances where it is difficult to avoid drugs. Many drugs from different classes like cardiovascular drugs, antibiotics, antiemetic drugs, anti-fungal drugs, vitamins, antiepileptics, corticosteroids etc. are prescribed among the pregnant women. Levothyroxine was one of the most utilized drugs among the study population, because 18.3 % of the pregnant women had thyroid disorders. It is essential to treat thyroid disorders during pregnancy, otherwise both pregnant women and fetus will have adverse consequences. Study reveals that highly teratogenic drugs like angiotensin converting enzyme inhibitors, warfarin, lithium and methotrexate were not prescribed in pregnant women and there were no reports of fetal abnormality due to drug usage, from this we can conclude that there are systems to monitor and prevent exposures to drugs that are known to cause risk to both pregnant women and foetus in the hospital and they are functioning well. Estradiol was only the FDA pregnancy category X drug prescribed and its use almost doubled in 2017 (may number of IVF pregnancy was more in that year). But estradiol supplement is required to improve pregnancy rates after, however frequent monitoring of foetus is essential when estradiol is prescribed. Being a retrospective study, we do not have categorized information on utilization of particular class of drug in the respective disease condition. This is one of the limitations of our study. However, we believe that the information presented in this study will be useful for healthcare administrators to plan stock medicines required for the treatment of pregnant women in similar healthcare setting. This study also may help the health care professionals in the selection of suitable and safe drugs when the pregnancy is accompanied with one or more disease conditions.
Acknowledgements:
We would like to thank the Manipal Academy of Higher Education (MAHE), Manipal College of Pharmaceutical Sciences and the Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal for providing necessary facilities for carrying out this study.
Conflict of interests:
The authors declared no conflict of interests.
References
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015;39(7):512-9.
[Crossref] [Google Scholar] [PubMed]
- Schaefer C, Peters P, Miller R. Drugs during pregnancy and lactation. 3rd ed. Amsterdam: Elsevier; 2001.
- Sachdeva P, Patel BG, Patel BK. Drug use in pregnancy; a point to ponder! Indian J Pharm Sci 2009;71(1):1.
[Crossref] [Google Scholar] [PubMed]
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: Pregnancy rating classifications and controversies. Clin Dermatol 2016;34(3):401-9.
[Crossref] [Google Scholar] [PubMed]
- Introduction to Drug Utilization Research. World Health Organization. 2013
- Defined Daily Dose (DDD). World Health Organization. 2014
- Fridman M, Korst LM, Chow J, Lawton E, Mitchell C, Gregory KD. Trends in maternal morbidity before and during pregnancy in California. Am J Public Health 2014;104(1):S49-57.
[Crossref] [Google Scholar] [PubMed]
- Dhar M, Komaram RB. Assessment of drug utilization pattern and teratogenicity risk among pregnant women attending a tertiary care hospital, Andhra Pradesh. Int J Pharm Sci Res 2017;8(12):5291-7.
- Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005;115(3):485-91.
- Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens 2001;14(3):178S-85S.
[Crossref] [Google Scholar] [PubMed]
- Bánhidy F, Lowry RB, Czeizel AE. Risk and benefit of drug use during pregnancy. Int J Med Sci 2005;2(3):100-6.
[Crossref] [Google Scholar] [PubMed]
- Selvaraj N, Sekar A, Gandhi R, Jayabalan N, Ganesan S, Mohammad MA. Drug utilization pattern in pregnancy at a tertiary care hospital in Puducherry: A cross sectional observational study. Int J Basic Clin Pharmacol 2018;7(5):900-5.
- Molloy AM, Kirke PN, Troendle JF, Burke H, Sutton M, Brody LC, et al. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 2009;123(3):917-23.
[Crossref] [Google Scholar] [PubMed]
- Lockshin MD, Sammaritano LR. Corticosteroids during pregnancy. Scand J Rheumatol 1998;27(107):136-8.
[Crossref] [Google Scholar] [PubMed]
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Universal screening vs. case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metabol 2010;95(4):1699-707.
[Crossref] [Google Scholar] [PubMed]
- Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med 2004;351(3):241-9.
[Crossref] [Google Scholar] [PubMed]
- Sasidharan P, Kolasani BP, Divyashanthi CM. An observational prospective study on prescribing pattern of drugs among pregnant women admitted in antenatal ward of a tertiary care teaching hospital in coastal town of South India. Natl J Physiol Pharm Pharmacol 2017;7(1):25.
- Omar MH, Mashita MK, Lim PS, Jamil MA. Dydrogesterone in threatened abortion: Pregnancy outcome. J Steroid Biochem Mol Biol 2005;97(5):421-5.
[Crossref] [Google Scholar] [PubMed]
- Sibai BM, Caritis SN, Thom E, Klebanoff M, McNellis D, Rocco L, et. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. N Engl J Med 1993;329(17):1213-8.
[Crossref] [Google Scholar] [PubMed]
- Meis PJ. 17 Hydroxyprogesterone for the prevention of preterm delivery. Obste Gynecol 2005;105(1):1128-35.
[Crossref] [Google Scholar] [PubMed]
- Jepsen P, Skriver MV, Floyd A, Lipworth L, Schønheyder HC, Sørensen HT. A population?based study of maternal use of amoxicillin and pregnancy outcome in Denmark. Br J Clin Pharmacol 2003;55(2):216-21.
[Crossref] [Google Scholar] [PubMed]
- Feig DS, Briggs GG, Koren G. Oral antidiabetic agents in pregnancy and lactation: A paradigm shift? Ann Pharmacother 2007;41(7-8):1174-80.
[Crossref] [Google Scholar] [PubMed]
- de Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, et al. Postprandial vs. preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med 1995;333(19):1237-41.
[Crossref] [Google Scholar] [PubMed]
- Asker C, Norstedt Wikner B, Källén B. Use of antiemetic drugs during pregnancy in Sweden. Eur J Clin Pharmacol 2005;61:899-906.
[Crossref] [Google Scholar] [PubMed]
- Sarkar M, Woodland C C, Koren G, Einarson A. Pregnancy outcome following gestational exposure to azithromycin. BMC Pregnancy and Childbirth 2006;6(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Beau AB, Didier A, Hurault-Delarue C, Montastruc JL, Lacroix I, Damase-Michel C. Prescription of asthma medications before and during pregnancy in France: An observational drug study using the EFEMERIS database. J Asthma 2017;54(3):258-64.
[Crossref] [Google Scholar] [PubMed]
- Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension 2008;51(4):960-9.
[Crossref] [Google Scholar] [PubMed]
- Jones HM, Cummings AJ. A study of the transfer of alpha-methyldopa to the human foetus and newborn infant. Br J Clin Pharmacol 1978;6(5):432.
[Crossref] [Google Scholar] [PubMed]
- Rebordosa C, Kogevinas M, Horváth-Puhó E, Nørgård B, Morales M, Czeizel AE, et al. Acetaminophen use during pregnancy: Effects on risk for congenital abnormalities. Am J Obstet Gynecol 2008;198(2):178-e1.
[Crossref] [Google Scholar] [PubMed]
- Czeizel AE, Fladung B, Vargha P. Preterm birth reduction after clotrimazole treatment during pregnancy. Eur J Obstet Gynecol Reprod Biol 2004;116(2):157-63.
[Crossref] [Google Scholar] [PubMed]
- Seto A, Einarson T, Koren G. Pregnancy outcome following first trimester exposure to antihistamines: Meta-analysis. Am J Perinatol 1997;14(03):119-24.
[Google Scholar] [PubMed]
- So M, Bozzo P, Inoue M, Einarson A. Safety of antihistamines during pregnancy and lactation. Can Fam Physician 2010;56(5):427-9.
[Google Scholar] [PubMed]
- Buchanan NM, Toubi E, Khamashta MA, Lima F, Kerslake S, Hughes GR. Hydroxychloroquine and lupus pregnancy: Review of a series of 36 cases. Ann Rheum Dis 1996;55(7):486-8.
[Crossref] [Google Scholar] [PubMed]
- Hernandez-Diaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78(21):1692-9.
[Crossref] [Google Scholar] [PubMed]
- Tomson T, Battino D, Perucca E. Teratogenicity of antiepileptic drugs. Curr Opin Neurol 2019;32(2):246-52.
[Crossref] [Google Scholar] [PubMed]
- Deworming in pregnant women. World Health Organization. 2019
- Christian P, Khatry SK, West KP. Antenatal anthelmintic treatment, birthweight, and infant survival in rural Nepal. Lancet 2004;364(9438):981-3.
[Crossref] [Google Scholar] [PubMed]
- Gelbaya TA, Kyrgiou M, Tsoumpou I, Nardo LG. The use of estradiol for luteal phase support in in vitro fertilization/intracytoplasmic sperm injection cycles: A systematic review and meta-analysis. Fertil Steril 2008;90(6):2116-25.
[Crossref] [Google Scholar] [PubMed]