- *Corresponding Author:
- S. Lodhi
Guru Ramdas Khalsa Institute of Science and Technology (Pharmacy), Kukrikheda, Barela, Jabalpur-483 001, India
E-mail: srlodhi78@gmail.com
| Date of Submission | 01 February 2015 |
| Date of Revision | 14 December 2015 |
| Date of Acceptance | 21 February 2016 |
| Indian J Pharm Sci, 2016;78(1):94‑102 |
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Lichens produce variety of secondary metabolites including depsides, depsidones and dibenzofurans having multifunctional activity in response to external environmental condition. Present study provides evidence for in vitroantibacterial and in vivoantiinflammatory activity of acetone and ethanol extracts of Parmotrema reticulatum. In vitro antibacterial activity was investigated against gram positive and gram negative bacteria. Cotton pellet-induced granuloma, xylene-induced ear swelling, carragennan-induced edema, histamine-induced and carboxymethylcellulose sodium-induced leukocyte emigration in mice models were used to quantify the antiinflammatory activity. Acetone and ethanol extracts were showed antibacterial activity against Bacillus subtilis (minimal inhibitory concentration: 100 and 150 μg/ml) and Staphylococcus aureus(minimal inhibitory concentration: 100 and 200 μg/ml), Escherichia coli (minimal inhibitory concentration: 200 and 250 μg/ml), and Pseudomonasa eruginosa(minimal inhibitory concentration: 200 and 300 μg/ml). Acetone extract was inhibited edema significantly at 200 mg/kg with xylene, cotton pellet, carragennan and histamine induced edema in vivo models. Ethanol extract was found effective at dose of 300 mg/kg with all in vivo antiinflammatory models. The results showed significant (P<0.01) antiinflammatory effects at 200 and 300 mg/kg dose of acetone and ethanol extracts, respectively, which can be concluded that significant activity may be due to presence of flavanoids in ethanol extract and usnic acid in acetone extract.
Keywords
Parmotrema reticulatum, antibacterial, antiinflammatory, usnic acid, carragennan
Lichens, are symbiotic organisms of fungi and algae and synthesize characteristic secondary compounds. The genus Parmotrema is typically characterized by large foliose thalli with broad lobes, pored epicortex, broad marginal zone, thick-walled hyaline ellipsoid ascospores, sublageni form and with or without marginal cilia. The distribution of more than 220 species were found out of 350 known species in tropical regions [1]. Species of Parmotrema have also been reported for antimicrobial [2] and antioxidant [3,4] properties. Parmotrema reticulatum, was first described by Taylor (1836; as Parmelia reticulate Taylor) based on the reticulated maculae of the upper surface, and the species was characterized by marginal soralia, a black lower surface and simple to squarrose rhizines [5]. P. reticulatum occur in abundance in Dima Hasao Hills district of Assam, North-East, India. Besides, lichen metabolites exert a wide variety of biological actions including antiviral, antiinflammatory, antimycobacterial, antipyretic, analgesic, antiproliferative and cytotoxic effects [6]. The natural source of antibacterial components always has low toxicity and side effects to the human body and they produce effects similar to the body mechanisms. Usnic acid is an important constituent of lichen products. It is a yellow pigment, dibenzofuran derivative produced in upper cortex of many species. Usnic acid is effective antibiotic against Gram-positive bacteria including, Pneumococcus, Streptococcus and other bacteria Mycobacterium tuberculosis [7]. In previous reported work, a HPLC analysis was reported to identify the presence of flavonoids by comparing the retention time of reference compounds. The four major compounds were identified and having same retention time corresponded to the chromatographic patterns of purpurin, catechin, tannic acid and reserpine in alcoholic extract [4]. The amount of flavonoids in the lichen extracts was reported as 1.54 and 1.49 μg for ethanol and methanol extracts [8]. The aim of present study was to evaluate in vitro antibacterial activity on gram negative and gram positive bacteria and antiinflammatory activity of P. reticulatum extracts on various in vivo mice models.
Materials and Methods
The lichens specimen was collected from the trees growing around Amarkantak town and was identified from Tropical Forest Research Institute, Jabalpur (M.P.), India (fig. 1). The dried lichens were powdered and extracted in soxhlet apparatus for defatting with petroleum ether. The defatted lichen material was dried and then exhaustively extracted with acetone and ethanol by continuous soxhlet extraction method. The extracts were concentrated under reduced pressure to yield semisolid mass and were stored in well closed container for further study. Standard procedures for qualitative chemical screening were undertaken to characterize chemical constituents in petroleum ether, acetone and ethanol extracts i.e., alkaloids, glycosides, terpenoids, saponins, tannins and flavonoids [9].
All reagents and chemicals were of analytical grade. Usnic acid, carrageenan, histamine, and dexamethasone were purchased from Sigma Chemical Co. (USA). Indomethacin was procured from Zydus Cadila Healthcare Ltd, India. Bacillus subtilis and Staphylococcus aureus, Escherichia coli and Pseudomonasa eruginosa were procured from Microbial Type Culture Collection and gene bank (MTCC); Institute of Microbial Technology, Chandigarh, India.
HPLC analysis of extracts
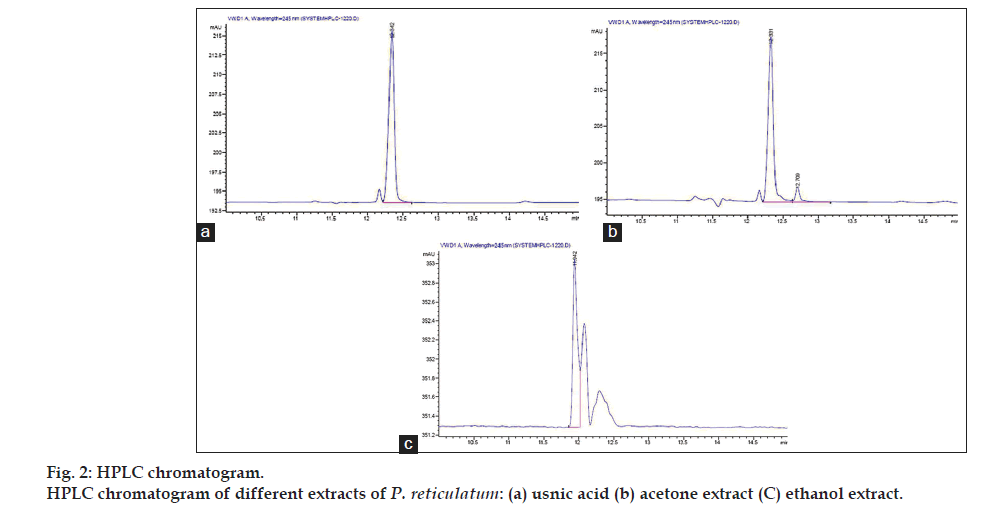
The HPLC analysis of acetone and ethanol extracts were performed on Agilent 1220 Infinity LC system equipped with a reverse phase C18 column and a diode array detector. The separation was carried out using a mixture of methanol and pH 7.4 phosphate buffer (70:30 v/v) as mobile phase with a flow rate of 1.0 ml/min. The mobile phase was filtered under vacuum through a 0.45 mm membrane filter and degassed before use. The test solution was prepared by dissolving 20 mg of extract in 50 ml of methanol and solutions were passed through 0.45 μm filters then injected 20 μl into the HPLC system. Each analysis was carried out in triplicate. A stock solution of 1 mg/ml usnic acid was prepared in methanol and appropriate dilutions of this stock solution were made. Quantification was performed on the basis of a linear calibration plot of peak area against concentrations of the standard. Identification of usnic acid was made at 245 nm by the comparison of the retention times with pure standard.
Antibacterial activity
The antibacterial activity was investigated against two Gram positive bacteria: Bacillus subtilis and Staphylococcus aureus, two Gram negative bacteria: Escherichia coli and Pseudomonasa eruginosa. The minimal inhibitory concentration (MIC) was determined by the broth tube dilution method. A series of dilutions with concentrations ranging from 50 to 0.004 mg/ml were made in dimethylsulfoxide (DMSO, Sigma-Aldrich) for each extract against every microorganism tested. Two fold dilutions of extracts were prepared in Muller-Hinton broth for bacterial cultures in test tubes. The MIC of petroleum ether, acetone and ethanol extracts of P. reticulatum on various bacterial strains were determined using a rapid p-Iodonitrotetrazolium chloride (INT; Sigma-Aldrich) colorimetric assay [10]. Briefly, the test samples were dissolved in DMSO, and the solution was added to Mueller-Hinton Broth (MHB; Sigma-Aldrich) and serially diluted two fold (in a 96-well microtilter plate). One hundred microliters of inoculums (1.5×106 CFU/ml) prepared in MHB were then added. The plates were covered with a sterile plate sealer and then agitated with a shaker to mix the contents of the wells and incubated at 37° for 18 h. Wells containing MHB, 100 μl of inoculum, and DMSO at a final concentration of 2.5% served as the negative control. Streptomycin was used as positive reference standard for all bacterial strains. The MIC of each extract was detected after 18 h of incubation at 37° following addition of 40 μl INT (0.2 mg/ml) and incubation at 37° for 30 min. Viable bacteria reduced this yellow dye to pink. The MIC of each sample was defined as its lowest concentration that prevented this change and then resulted in the complete inhibition of microbial growth [11]. Each assay was performed three independent times in triplicate.
Experimental animals
The healthy albino mice of either sex (20-25 g) with no prior drug treatment were used for in vivo studies. The animals were fed on a commercial pellet diet (Hindustan Lever, Bangalore, India), and water ad libitum. The mice were acclimatized to laboratory hygienic conditions for 7 d before starting the experiment. The animal experimentation was performed according to permission of Institutional animal ethical committee (Registration No. GRKIST(P)/406/02/IAEC/16A).
Acute toxicity study
The acute toxicity study of P. reticulatum was conducted according to standard guideline [12]. The animals of female sex mice were divided into 4 groups (3 mice per group) for each extract after a single oral dose. Animals were kept in their cages to allow for acclimatization to the laboratory conditions for at least 5 days prior to dosing. Each extract (0.5, 1, 2, and 4 g/kg) was mixed in 0.5% Tween-80 solution and administered orally. All experimental animals were fed with standard diet and clean water ad libitum and kept under observation. The mortality or behavioral changes, including hyperactivity, tremors, ataxia, convulsions, salivation, diarrhea, sleep, and coma, were regularly observed for 14 d.
Cotton pellet-induced granuloma
For study of antiinflammatory activity, animals were divided into eight groups of five mice in each group for each in vivo model. Group I acted as control and fed only vehicle. Group II and III were given petroleum ether extract in 200 and 300 mg/kg, respectively. Group IV and V were given acetone extract in 200 and 300 mg/kg, respectively. Group VI and VII received ethanol extract in 200 and 300 mg/kg, respectively. Group VIII was given indomethacin, (10 mg/kg, p.o.) for comparison. All extracts were suspended in distilled water for oral administration.
The antiinflammatory activity against chronic inflammation was tested using cotton pellet granuloma method in mice [13]. Granulomatous lesions were induced by surgically inserting sterile cotton pellets (15±1 mg) subcutaneously in axilla region of each mouse following a single incision which was thereafter closed by interrupted sutures. After implantation of pellets, the petroleum ether, acetone and ethanol extracts (200 and 300 mg/kg) were orally administered once a daily for 7 consecutive days. Indomethacin (10 mg/kg, p.o.) was also given daily to standard group while the control group received the same volume of vehicle. On day 8th, the cotton pellets were dissected out under ether anesthesia, cleaned of extraneous tissue, weighed and dried at 50° to a constant weight. The mean weight of tissues from different groups was determined. The increase in dry weight of the pellets was taken as the measure of the granuloma formation.
Xylene-induced ear swelling in mice
The antiinflammatory activity against acute inflammation was tested by using xylene-induced ear edema method in mice [14]. The treated groups of mice were same as in cotton pellet model and were administered test samples 1 h before xylene application. Ear edema was induced by applying carefully a drop of xylene (0.03 ml) to the anterior and posterior surfaces of the right ear. The left ear was considered as control. One hour after xylene application, the animals were killed under ether anesthesia and 6 mm punches were made in the right and left ear of each mouse using a borer. Each ear punch was weighed and differences between the weight of the right and left ear punches of mice were recorded. The increase in weight caused by the irritant was measured by subtracting the weight of the control left ear section from that of the test right ear section.
Carrageenan-induced paw edema
The carrageenan induced edema in the hind paw of mice was produced by the subplantar injection of 0.1 ml of a 1% carrageenan suspension in normal saline [15,16]. Test extracts (200 and 300 mg/kg) and vehicle (10 ml/kg) were given orally 1 h prior to carrageenan injection. Paw volume was measured immediately after the carrageenan injection, at 30 min interval for 3 h later and the difference from initial volume was taken as the edema volume, which was compared to standard.
The inhibition of inflammation was calculated using the following formula: Percent inhibition = (1-Vt/Vc)× 100, where, Vt is the paw volume of test group and Vc is the paw volume of control group.
Histamine-induced paw edema
Paw edema was induced after 1 h by subplantar administration of 0.1 ml histamine (0.001 mg/ml) on the right hand paw [17]. The linear paw circumference was measured initially and after every 30 min up to 3 h. Test groups were treated with the petroleum ether, acetone and ethanol extracts (200 and 300 mg/kg, p.o.), reference group with indomethacin (10 mg/kg, p.o.), and control group with vehicle prior to the histamine administration. The inhibition of inflammation was calculated using the following formula:
Percent inhibition = (1-Vt/Vc)× 100 , Where, Vt is the paw volume of test group and Vc is the paw volume of control group.
Carboxymethylcellulose sodium-induced leukocyte emigration in mice
In the study of carboxymethylcellulose sodium (CMC Na)-induced leukocyte emigration, initially petroleum ether, acetone and ethanol extracts (200 and 300 mg/kg) were given orally to the respective group of mice and after 1 h, 1.5% CMC Na solution (375 mg/kg) in normal saline were injected intraperitoneally to all mice. The mice were sacrificed by cervical displacement after 4 h and 5 ml of normal saline was injected into the peritoneal cavity. The washing solution was collected. The peritoneal fluid (50 μl) was mixed with 0.45 ml of Turk’s solution (0.01% crystal violet in acetic acid) and leukocyte cells were counted under a microscope. Dexamethasone (20 mg/kg) was administrated as a positive control [18].
Statistical methods
In statistical analysis four independent groups with three mice per group for each extract was compared. The data were expressed as mean values ±SEM using InStat software and tested with analysis of variance followed by the multiple comparison test of Tukey–Kramer with P<0.01 were considered significant.
Results and Discussion
The petroleum ether, acetone and ethanol extracts were taken for phytochemical and biological screening. The phytochemical results showed the presence of glycosides, terpenoids in the acetone and glycosides and flavonoids in ethanol extracts. Petroleum ether extract contains steroidal constituents. Chromatograms obtained from different extracts and standard solution (fig. 2) reveals the presence of usnic acid in standard and sample solutions with having same retention time of 12.331 min. The presence of usnic acid was confirmed only in acetone extract by HPLC analysis. The amount of usnic acid was found 0.46% w/w in acetone extract of P. reticulatum. Usnic acid was found to be absent in ethanol extract.
The results of antibacterial activity of P. reticulatum are shown in Table 1. The acetone extract showed antibacterial activity against Bacillus subtilis with MIC value of 100 μg/ml; S. aureus with MIC value of 100 μg/ml; Escherichia coli MIC value with 200 μg/ml and Pseudomonasa eruginosa MIC value was 200 μg/ml. The ethanol extract of P. reticulatum showed the antibacterial activity against Bacillus subtilis with MIC value of 150 μg/ml; S. aureus with MIC value of 200 μg/ml; Escherichia coli MIC value with 250 μg/ml and Pseudomonasa eruginosa MIC value was 300 μg/ml. The petroleum ether extract was showed highest MIC than other extracts. The results of the present study support the use of P. reticulatum to control infectious diseases caused by gram-positive and gram-negative bacteria. Some pprevious studies reported that usnic acid has antiviral activity due to transcription inhibition [19]. In another study the antibacterial activity of usnic acid is reported mainly due to inhibition of DNA and RNA synthesis in all bacterial cells [20]. Therefore, we suggest that antibacterial activity of acetone extract may be due to presence of usnic acid in P. reticulatum acetone extract.
| Extracts | MIC (µg/ml) | |||
|---|---|---|---|---|
| Bacillus subtilis | Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | |
| Petroleum ether extract | 350 ± 16.3 | 430 ± 19.7 | 450 ± 21.1 | 410 ± 20.4 |
| Acetone extract | 100 ± 4.8 | 100 ± 4.5 | 200 ± 8.2 | 200 ± 8.8 |
| Ethanol extract | 150 ± 6.2 | 200 ± 8.6 | 250 ± 11.4 | 300 ± 12.8 |
| Streptomycin | 5 ± 0.02 | 6 ± 0.04 | 5 ± 0.03 | 4 ± 0.03 |
Each value is the mean±SEM. (n=5), MIC: Minimal inhibitory concentration. SEM: standard error of mean
Table 1: Minimal Inhibitory Concentration Of Different Extracts Of Parmotrema Reticulatum And Standard Antibioticdetermined By Broth Dilution Method
Acetone extract of P. reticulatum significantly reduced (P<0.01) the granuloma induced by cotton pellets in mice in a dose-dependent manner (Table 2). The acetone extract at the doses of 200 mg/kg and ethanol extract at 300 mg/kg were showed maximum reduction on the granuloma tissue formation on implanted cotton pellets with inhibition of 73.0 and 69.0%, respectively which was compared with Indomethacin (10 mg/kg) produced inhibition 70.5%.
| Groups | Doses | Granuloma | Percent |
|---|---|---|---|
| weight (mg) | inhibition | ||
| Control (ml/kg) | 10 | 19.7 ± 0.09 | 0 |
| Petroleum ether extract | 200 | 12.6 ± 0.03 | 36.0 |
| (mg/kg) | 300 | 13.1 ± 0.05 | 33.5 |
| Acetone extract (mg/kg) | 200 | 5.3 ± 0.02 | 73.0* |
| 300 | 10.8 ± 0.03 | 45.1 | |
| Ethanol extract (mg/kg) | 200 | 9.7 ± 0.01 | 50.7* |
| 300 | 6.1 ± 0.02 | 69.0* | |
| Indomethacin (mg/kg) | 10 | 5.8 ± 0.01 | 70.5* |
Each value is the mean±SEM. (n=5). *P<0.01 compared with standard. SEM: Standard error of mean
Table 2: Effect of Different Extracts of Parmotrema Reticulatumon Cotton Pellet Induced Granuloma in Mice
The effect of different extracts of P. reticulatum on xylene-induced ear edema in mice was shown in Table 3. Oral administration of the acetone and ethanol extracts were significantly (P<0.01) inhibited the development of ear edema in mice at a dose dependent manner. The inhibition produced by 300 mg/kg of ethanol extract was 69.0% and it was comparable to the inhibition produced by indomethacin (70.5%). Acetone extract showed significant inhibition at 200 mg/kg but petroleum ether does not showed significant edema inhibition.
| Group | Doses | Edema | Percent |
|---|---|---|---|
| (mg) | inhibition | ||
| Control (ml/kg) | 10 | 11.2 ± 0.04 | 0.0 |
| Petroleum ether extract (mg/kg) | 200 | 8.7 ± 0.03 | 22.3 |
| 300 | 7.9 ± 0.01 | 29.4 | |
| Acetone extract (mg/kg) | 200 | 4.1 ± 0.02 | 63.3* |
| 300 | 8.6 ± 0.03 | 41.0 | |
| Ethanol extract (mg/kg) | 200 | 5.2 ± 0.01 | 53.5* |
| 300 | 3.8 ± 0.02 | 66.0* | |
| Indomethacin | 10 | 4.0 ± 0.02 | 64.2* |
Each value is the mean±SEM. (n=5). *P<0.01 compared with standard. SEM: Standard error of mean
Table 3: Effect Of The Different Extracts Of Parmotrema Reticulatum On Xylene Inducedear Edema In Mice
Lichens are symbiotic organisms consisting of fungus and a photosynthetic partner which can be either an alga or cyanobacterium. Inflammation constitutes body’s response to injury and is characterized by a series of events that mainly occur in three distinct phases. The first phase is caused by an increase in vascular permeability resulting in exudation of fluids from the blood into the interstitial space; the second phase involves the infiltration of leukocytes from the blood into the tissue and third phase is characterized by granuloma formation and tissue repair [21]. Therefore, it is vital to estimate the activities of the test substance in different phases of inflammation, while evaluating the antiinflammatory effect. Accordingly, acetone and ethanol extracts of P. reticulatum were investigated for antiinflammatory potential using acute exudative (xylene induced ear edema) and chronic proliferative (cotton pellet granuloma) inflammation models [22]. Moreover, the ear edema associated with xylene involves inflammatory mediators such as histamine, kinin, and fibrinolysin [23]. The significant inhibition of xylene-induced ear edema in mice treated with lichen provides a probability that the active principles in the extract could reduce the release of inflammatory mediators such as histamine, kinin and fibrinolysin or antagonize the actions. The present results were indicated that acetone and ethanol extracts of P. reticulatum were significantly inhibited edema and granuloma mass in xylene induced ear edema and cotton pellet study, respectively. This leads to the dilation of arterioles and venules and may increase vascular permeability [24].
Cotton pellet granuloma model has been widely used to evaluate the transudative, exudative, and proliferative components of chronic inflammation. Transudate phase causes increase in the wet weight of the cotton pellet while hosting inflammatory response to the implanted cotton pellet between 3 and 6 d causes granuloma formation. Therefore, increase in dry weight is considered as a measure of proliferative component of inflammation [25]. Acetone and ethanol extracts were showed antiinflammatory effects in acute inflammatory tests with different efficacy. This lichen may have a membrane stabilizing effect that reduces capillary permeability or has inhibitory effects on the release of mediators. In higher doses, the antiinflammatory efficacy, especially for the acetone extract, was decreased. This might be related to some constituents in the extracts that oppose against antiinflammatory activity. The extracts reduced cotton pellet-induced granuloma, thereby suggested its activity in the proliferative phase of the inflammation. Other studies have demonstrated that various flavonoids such as quercetin, luteolin, hesperidin produce significant and antiinflammatory activities [26,27]. Therefore, it could be suggested that the antibacterial and antiinflammatory effects of the ethanol extract of P. reticulatum may be due to their contents of flavenoids.
The paw edema of mice increased progressively and reached its maximum after 120 min of carrageenan injection. The ethanol extract was reduced carrageenan-induced inflammation significantly (P<0.01) at dose of 300 mg/kg as compared to the control group, but reduced low percent at a dose of 200 mg/kg (Table 4). The acetone extract was showed a significant inhibition of paw edema at 200 mg/kg.
| Animal groups | Carrageenan induced paw edema mean ± SEM (percentage inhibition of paw volume) | |||||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | 150 min | 180 min | |
| Control | 0.68 ± 0.04 | 0.81 ± 0.07 | 0.97 ± 0.05 | 1.18 ± 0.13 | 1.10 ± 0.27 | 0.97 ± 0.12 |
| Petroleum ether extract | 0.63 ± 0.02 (7.3) | 0.78 ± 0.03 (3.7) | 0.85 ± 0.04 (12.3) | 0.96 ± 0.12 (18.6) | 0.91 ± 0.08 (17.2) | 0.85 ± 0.14 (12.3) |
| (200 mg/kg, p.o.) | ||||||
| Petroleum ether extract | 0.61 ± 0.01 (10.2) | 0.75 ± 0.12 (7.4) | 0.82 ± 0.05 (15.4) | 0.89 ± 0.02 (24.5) | 0.85 ± 0.07 (22.7) | 0.8 ± 0.05 (17.5) |
| (300 mg/kg, p.o.) | ||||||
| Acetone extract | 0.41 ± 0.014 (39.7) | 0.45 ± 0.06 (44.4) | 0.48 ± 0.06 (50.5) | 0.51 ± 0.05 (56.7)* | 0.50 ± 0.05 (54.5)* | 0.48 ± 0.05 (50.5) |
| (200 mg/kg, p.o.) | ||||||
| Acetone extract | 0.63 ± 0.02 (7.3) | 0.71 ± 0.07 (12.3) | 0.76 ± 0.06 (21.6) | 0.85 ± 0.07 (27.9) | 0.81 ± 0.06 (26.3) | 0.77 ± 0.06 (20.6) |
| (300 mg/kg, p.o.) | ||||||
| Ethanol extract | 0.50 ± 0.05 (26.4) | 0.55 ± 0.05 (32.1) | 0.59 ± 0.04 (39.18) | 0.65 ± 0.03 (44.92) | 0.63 ± 0.04 (42.73) | 0.59 ± 0.06 (39.18) |
| (200 mg/kg, p.o.) | ||||||
| Ethanol extract | 0.40 ± 0.01 (41.1) | 0.43 ± 0.01 (46.9) | 0.46 ± 0.03 (52.5) | 0.48 ± 0.05 (59.3)* | 0.45 ± 0.07 (59.0)* | 0.43 ± 0.07 (55.6) |
| (300 mg/kg, p.o.) | ||||||
| Indomethacin | 0.44 ± 0.04 (38.2) | 0.47 ± 0.05 (41.9) | 0.49 ± 0.09 (49.4) | 0.50 ± 0.06 (57.6)* | 0.48 ± 0.04 (56.0)* | 0.47 ± 0.02 (51.5) |
Each value is the mean±SEM. (n=5), *P<0.01 compared with control and standard. SEM: Standard error of mean
Table 4: Effect Of The Different Extracts Of Parmotrema Reticulatum On Carrageenan Inducedpaw Edema In Mice
Carrageenan-induced paw edema in mice has been accepted as a useful phlogistic tool for investigating antiinflammatory agents. Serious signs of inflammation develop immediately following subcutaneous injection, resulting from action of pro-inflammatory agents. The development of edema is a biphasic event: The early phase (0–2.5 h after carrageenan injection) involves the release of inflammatory mediators like histamine, serotonin and bradykinins; the late phase (3–6 h post-injection) is associated with the release of prostaglandins [28]. In the present study, both extracts and standard significantly inhibited the paw edema during the early phase of inflammation, indicating that the extract and standard may be blocks histamine and serotonin release within the early phase. This suggested that the acetone and ethanol extracts were exhibits its antiinflammatory action by inhibiting the synthesis, release or action of histamine.
Histamine is a potent vasodilator substance which increases vascular permeability. In order to confirm the results obtained from the carrageenan-induced edema test, the effect of all extracts at the dose of 200 and 300 mg/kg were investigated using histamine-induced edema models. The results were showed that the ethanol extract at dose of 300 mg/kg was significantly (P<0.01) suppressed the edema produced by histamine but had a low effect on the edema by dose of 200 mg/kg (Table 5). The acetone extract was reduced edema significantly at 200 mg/kg. Petroleum ether extract was not inhibited significantly paw edema. Thus an increase in percent inhibition by the acetone extract may be due to presence of usnic acid into the extract, whereas the effect of ethanol extract may be due to synergistic effect of flavonoids constituents in the extract.
| Animal groups | Histamine‑induced paw edema mean ± SEM (percentage inhibition of paw volume) | |||||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | 150 min | 180 min | |
| Control | 0.95 ± 0.08 | 1.05 ± 0.09 | 1.16 ± 0.14 | 1.25 ± 0.15 | 1.21 ± 0.017 | 1.15 ± 0.16 |
| Petroleum ether extract | 0.90 ± 0.05 (5.26) | 0.87 ± 0.07 (17.04) | 0.85 ± 0.9 (26.72) | 0.83 ± 0.07 (33.6) | 0.87 ± 0.08 (28.09) | 0.85 ± 0.07 (26.08) |
| (200 mg/kg, p.o.) | ||||||
| Petroleum ether extract | 0.85 ± 0.06 (10.53) | 0.82 ± 0.06 (21.9) | 0.79 ± 0.06 (31.89) | 0.77 ± 0.04 (38.4) | 0.79 ± 0.06 (34.71) | 0.81 ± 0.07 (29.56) |
| (300 mg/kg, p.o.) | ||||||
| Acetone extract | 0.72 ± 0.05 (24.21) | 0.68 ± 0.05 (35.24) | 0.65 ± 0.05 (43.97) | 0.62 ± 0.03 (50.4)* | 0.67 ± 0.06 (44.63) | 0.69 ± 0.06 (40.0) |
| (200 mg/kg, p.o.) | ||||||
| Acetone extract | 0.88 ± 0.04 (7.37) | 0.85 ± 0.07 (19.04) | 0.82 ± 0.07 (29.31) | 0.79 ± 0.07 (36.8) | 0.76 ± 0.04 (33.91) | 0.78 ± 0.05 (32.17) |
| (300 mg/kg, p.o.) | ||||||
| Ethanol extract | 0.81 ± 0.04 (14.74) | 0.79 ± 0.07 (24.76) | 0.77 ± 0.06 (33.62) | 0.75 ± 0.05 (40.0) | 0.77 ± 0.04 (36.36) | 0.79 ± 0.07 (31.3) |
| (200 mg/kg, p.o.) | ||||||
| Ethanol extract | 0.73 ± 0.08 (23.16) | 0.70 ± 0.04 (33.33) | 0.67 ± 0.05 (42.24) | 0.59 ± 0.03 (52.8)* | 0.63 ± 0.04 (48.76) | 0.60 ± 0.04 (47.82) |
| (300 mg/kg, p.o.) | ||||||
| Indomethacin | 0.57 ± 0.06 (40.0) | 0.48 ± 0.02 (54.2) | 0.43 ± 0.03 (62.9) | 0.41 ± 0.02 (67.2)* | 0.46 ± 0.02 (61.9) | 0.50 ± 0.02 (56.5) |
Each value is the mean±SEM. (n=5), *P<0.01 compared with control and standard. SEM: Standard error of mean
Table 5: Effect Of The Different Extracts Of Parmotrema Reticulatum On Histamine Induced Paw Edema In Mice
Usnic acid is a chemical class of dibenzofurandiones commonly occurring in Lichens. Additionally, usnic acid has antiinflammatory activity comparable to the synthetic agent ibuprofen [29]. It has already reported that usnic acid could inhibit TNF-α, IL-1β and IL-6 in LPS-stimulated cell lines. These pro-inflammatory cytokines and mediators have potential role in acute or chronic inflammatory disorders [30].
It was observed that acetone and ethanol extracts were capable of inhibiting edema induced by histamine. It can be suggested from these studies that the effectiveness for suppression of edema is due to the ability of extract to either inhibit the synthesis, release or action of histamine involved in the inflammation. Chronic inflammation is the reaction arising when the acute response is insufficient to eliminate the proinflammatory agents. Chronic inflammation includes proliferation of fibroblasts and infiltration of neutrophils with exudation of fluid. It occurs by means of development of proliferative cells which can either spread or form granuloma [31].
CMC Na significantly enhanced leukocyte emigration in the mice peritoneal cavity. Ethanol extract of P. reticulatum dose-dependently inhibited leukocyte emigration with inhibition percentage of 66.20% at the dose of 300 mg/kg, which was comparable to the reference group (Table 6). Acetone extract was produce 60.83% inhibition at dose of 300 mg/kg.
| Animal groups | Doses | Leucocyte | Inhibition |
|---|---|---|---|
| (mg/kg) | count (×104) | (%) | |
| Normal | ‑ | 53.25 ± 4.35 | ‑ |
| Control | ‑ | 172.64 ± 16.28 | ‑ |
| Petroleum ether extract | 200 | 161.43 ± 14.62 | 6.47 |
| 300 | 153.24 ± 13.85 | 11.23 | |
| Acetone extract | 200 | 112.15 ± 13.46 | 35.03 |
| 300 | 67.62 ± 7.29 | 60.83* | |
| Ethanol extract | 200 | 71.20 ± 8.61 | 58.75* |
| 300 | 58.35 ± 6.43 | 66.20* | |
| Dexamethasone | 20 | 48.52 ± 5.41 | 71.89* |
Each value is the mean±SEM. (n=5), *P<0.01 compared with control and standard. SEM: Standard error of mean
Table 6: Effect Of Extracts On Carboxymethyl Cellulose Sodium-Induced Leukocyte Emigration In Mice
For the consideration of essential role of the migration of leukocytes to inflammatory area, CMC Na-induced leukocytes emigration was also used in this study. The data showed that the acetone and ethanol extract of P. reticulatum significantly suppressed on leukocytes emigration, which was comparable to that of dexamethasone, and the inhibition was also obviously enhanced with the dose raised. In future, detail pharmacological and chemical studies are needed in order to characterize the mechanism responsible for the antiinflammatory action and also to identify other active constituents present in P. reticulatum.
The results were showed significant antiinflammatory activity at 200 and 300 mg/kg dose of acetone and ethanol extracts, respectively. The results can be concluded that the significant activity may be due to presence of flavanoids in ethanol and usnic acid in acetone extract, respectively. In conclusions, these results were revealed the acetone and ethanol extracts of P. reticulatum showed antiinflammatory activity significantly, via inhibiting vascular permeability and leukocyte transmigration, which might be connected with the reduction of release of inflammatory mediators.
Acknowledgements
The authors are thankful to All India Council for Technical Education (AICTE), New Delhi, for financial support (letter No 8023/RID/RPS/038/11/12) for this research work.
Financial support and sponsorship
The authors are thankful to All India Council for Technical Education (AICTE), New Delhi, for financial support (letter No 023/RID/RPS/038/11/12) for this research work
Conflicts of interest
There are no conflicts of interest.
References
- Balaji P, Hariharan GN. In vitro antimicrobial activity of Parmotrema praesorediosum. Res J Bot 2007;2:54-9.
- Sati SC, Joshi S. Antibacterial activity of the Himalayan Lichen Parmotrema nilgherrense extracts. Br Microbiol Res J 2011;1:26-32.
- Stanly C, Hag Ali DM, Keng CL, Boey PL, Bhatt A. Comparative evaluation of antioxidant activity and total phenolic content of selected lichen species from Malaysia. J Pharm Res 2011;4:2824.
- Ghate NB, Chaudhuri D, Sarkar R, Sajem AL, Panja S, Rout J, et al. An antioxidant extract of tropical lichen, parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF-7. PLoS One 2013;8:e82293.
- Divakar PK, Blanco O, Hawksworth DL, Crespo A. Molecular phylogenetic studies on the Parmotrema reticulatum (syn. Rimelia reticulata) complex, including the confirmation of P. pseudoreticulatum as a distinct species. Lichenologist 2005;37:55-65.
- Müller K. Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol 2001;56:9-16.
- Cansaran D, Kahya D, Yurdakulola E, Atakol O. Identification and quantitation of usnic acid from the lichen Usnea species of Anatolia and antimicrobial activity. J Biosci 2006;61:773-6.
- Sharma BC, Kalikotay S. Screening of antioxidant activity of lichens Parmotrema reticulatum and Usnea sp. From Darjeeling hills, India.IOSR J Pharm 2012;2:54-60.
- Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 45th ed. Pune: Nirali Prakashan; 2010. p. 6.19.
- Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 1998;64:711-3.
- Sahu MC, Padhy RN. In vitro antibacterial potency of Butea monosperma Lam. Against 12 clinically isolated multidrug resistantbacteria. Asian Pac J Trop Dis 2013;3:217-26.
- OECD. Guidelines for Acute Toxicity of Chemicals. No. 423. Paris: Organization for Economic Co-operation and Development; 2001.
- Wu XL, Li CW, Chen HM, Su ZQ, Zhao XN, Chen JN, et al. Anti-inflammatory effect of supercritical-carbon dioxide fluid extract from flowers and buds of Chrysanthemum indicum Linnén. Evid Based Complement Alternat Med 2013;2013:413237.
- Eddouks M, Chattopadhyay D, Zeggwagh NA. Animal models as tools to investigate antidiabetic and anti-inflammatory plants. Evid Based Complement Alternat Med 2012;2012:142087.
- Saleem TK, Azeem AK, Dilip C, Sankar C, Prasanth NV, Duraisami R. Anti-inflammatory activity of the leaf extacts of Gendarussa vulgaris Nees. Asian Pac J Trop Biomed 2011;1:147-9.
- Santos JA, Arruda A, Silva MA, Cardoso CA, Vieira Mdo C, Kassuya CA, et al. Anti-inflammatory effects and acute toxicity of hydroethanolic extract of Jacaranda decurrens roots in adult male rats. J Ethnopharmacol 2012;144:802-5.
- Amann R, Schuligoi R, Lanz I, Donnerer J. Histamine-induced edema in the rat paw – Effect of capsaicin denervation and a CGRP receptor antagonist. Eur J Pharmacol 1995;279:227-31.
- Zhou M, Wang H, Suolangjiba, Kou J, Yu B. Antinociceptive and anti-inflammatory activities of Aquilaria sinensis (Lour.) Gilg. Leaves extract. J Ethnopharmacol 2008;117:345-50.
- Campanella L, Delfini M, Ercole P, Iacoangeli A, Risuleo G. Molecular characterization and action of usnic acid: A drug that inhibits proliferation of mouse polyomavirus in vitro and whose main target is RNA transcription. Biochimie 2002;84:329-34.
- Maciag-Dorszynska M, Wegrzyn G, Guzow-Krzeminska B. Antibacterial activity of lichen secondary metabolite usnic acid is primarily caused by inhibition of RNA and DNA synthesis. FEMS Microbiol Lett 2014;353:57-62.
- Badgujar VB, Jain PS, Patil RR, Haswani NG, Chaudhari SG. Antiinflammatory activity of Helicteres isora L. stem bark extracts in rats. Asian J Pharm Clin Res 2009;2:63-5.
- Igbe I, Ching FP, Eromon A. Anti-inflammatory activity of aqueous fruit pulp extract of Hunteria umbellata K. Schum in acute and chronic inflammation. Acta Pol Pharm 2010;67:81-5.
- Carlson RP, O’Neill-Davis L, Chang J, Lewis AJ. Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions 1985;17:197-204.
- Vogel HG, Vogel WH. Drug Discovery and Evaluation, Pharmacological Assay. Berlin: Springer; 1997. p. 370,382,402-3.
- Swingle KF, Shideman FE. Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain anti-inflammatory agents. J Pharmacol Exp Ther 1972;183:226-34.
- Küpeli E, Yesilada E. Flavonoids with anti-inflammatory and antinociceptive activity from Cistus laurifolius L. leaves through bioassay-guided procedures. J Ethnopharmacol 2007;112:524-30.
- Guvenc A, Okada Y, Akkol EK, Duman H, Okuyama T, Calis I. Investigations of antiinflammatory, antinociceptive, antioxidant and aldose reductase inhibitory activities of phenolic compounds from Sideritis brevibracteata. Food Chem 2010;118:686-92.
- Wang Y, Chen P, Tang C, Wang Y, Li Y, Zhang H. Antinociceptive and anti-inflammatory activities of extract and two isolated flavonoids of Carthamus tinctorius L. J Ethnopharmacol 2014;151:944-50.
- Huang Z, Tao J, Ruan J, Li C, Zheng G. Antiinflammatory effects and mechanisms of usnic acid, a compound firstly isolated from lichen Parmelia saxatili. J Med Plant Res 2014;8:197-207.
- Francolini I, Norris P, Piozzi A, Donelli G, Stoodley P. Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob Agents Chemother 2004;48:4360-5.
- Gupta M, Mazumder U, Ramanathan SK, Thangavel SK. Studies on antiinflammatory, analgesic and antipyretic properties of methanol extract of Caesalpinia bonducella leaves in experimental animal models. Iran J Pharmacol Ther 2003;2:30-4.