- *Corresponding Author:
- Rajendran Ananthan
Division of Food Chemistry, ICMR-National Institute of Nutrition, Hyderabad, Telangana 500007, India
E-mail: ananthan.nin@gmail.com
| Date of Received | 13 July 2023 |
| Date of Revision | 02 August 2024 |
| Date of Accepted | 25 October 2024 |
| Indian J Pharm Sci 2024;86(5):1821-1834 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Bamboo rice, an underutilised grain obtained as seeds of Bambusa arundinacea Willd., is consumed by many Indian population for its nutritional and other health benefits. Many of these health benefits are linked to the presence of bioactive phytonutrients. However, comprehensive quantification of different bioactive components has not been reported in bamboo rice. Thus, this study was intended to quantify its fatty acid profile, amino acid composition, phytosterols, polyphenols and measure the antioxidant potentials. Bamboo rice samples collected from six different regions of the Western ghats of India were analysed independently and compared with commonly consumed brown and polished rice. The total saturated, monounsaturated and polyunsaturated fatty acids in bamboo rice were 36.97 %±6.01 %, 28.59 %±2.01 % and 34.44 %±6.22 % fatty acid methyl esters, respectively. In bamboo rice, the total amino acid content was (96.76/100) g protein while the total essential amino acid content was (38.67/100) g with lysine being the limiting amino acid. The grain had higher concentrations of phytosterols, especially ergosterol and beta-sitosterol, when compared to brown and polished rice. The relative distribution of the polyphenols was in the order of catechinin>protocatechuic acid>sinapic acid>4-coumaric acid>myricetin>ferulic acid>2-coumaric acid>luteolin 7-O-glucoside>4-hydroxy benzoic acid>gallic acid>caffeic acid>ellagic acid. Ferric reducing antioxidant power and 2,2-diphenyl-1-picrylhydrazyl assay expressed more activity in the methanolic extract while the aqueous extract showed better activity for 2,2-azino-bis(3-ethylbenzothiazoline-6- sulphonic acid assay. This study has clearly shown that bamboo rice is rich in bioactive phytonutrients when compared to brown and polished rice. It also highlighted the need for comprehensive research on this grain to explore its pharmacological and therapeutic effects.
Keywords
Bamboo rice, phytosterols, fatty acids, amino acids, polyphenols, antioxidant activity, biotherapeutics
Bioactive Compounds (BACs) are specific compounds in foods known to be biologically and physiologically active, that can be of immense benefit to human health besides meeting the body's basic nutritional needs. These compounds can interact with constituents of living tissue and exhibit various potential effects[1]. Major elements, including vitamins and phytochemicals, which are obtained naturally during food or plant processing, are considered BACs[2]. Moreover, food components like proteins, peptides, amino acids, flavonoids and other phenolics are vital in improving the body's natural resistance to oxidative damage[3]. These compounds can provide protection against high amounts of free radicals and reactive oxygen species which can induce cell damage by interacting with other molecules[4]. Reportedly, serum cholesterol levels are significantly reduced in populations consuming diets that are particularly rich in natural phytosterols. Phytosterols such as sterols and stanols are also known to be beneficial in preventing colon cancer [5]. Among all the vital phytochemicals, phenolic compounds are considered key phytoconstituents having strong antimicrobial, hepatoprotective, antiinflammatory, anticancer and antioxidant potential[6]. These biological activities exhibited by plant polyphenols increase their potential to treat diseases, including diabetes, cancer and Alzheimer's disease[7]. Studies have also shown that polyphenols, mainly flavonoids are potential inhibitors of severe acute respiratory syndrome coronavirus-2 infection[8]. Applying proper packaging techniques to foods rich in these BACs can aid in retaining their phenolic content and antioxidant activity[9].
Rice bran contains several nutraceutical compounds, including γ-oryzanol, tocopherols, tocotrienols and phenolics. Brans of pigmented rice contains higher nutraceutical components when compared to nonpigmented rice bran[10]. Generally, some nutraceutical components exist in bound forms in rice bran. They are usually bound chemically to the cell wall constituents like pectin, cellulose, hemicellulose and arabinoxylans[11]. These BACs in cereal-based foods aid in reducing chronic diseases related to ageing, including heart diseases and various cancers[12]. Since the toxicity and possible carcinogenicity of synthetic antioxidants have raised concerns regarding their safety, food manufacturers prefer to attain antioxidative effects from natural sources, like extracts of plant materials, including cereal grains[13].
Traditional rice varieties possess unique medicinal and nutritional properties. However, these old rice landraces are nearing extinction because they are deemed less economical than the newly introduced short-duration rice varieties[14]. Modern generations have a limited understanding of traditional food systems and their significance. In rural areas, people often rely on ethnomedical practitioners, although they are quite scarce. The poor nutritional status of vulnerable groups is often linked to the loss of local traditions and knowledge systems. Therefore, documenting information about indigenous foods, particularly those with nutritional and medicinal benefits, is essential. This study focuses on the bioactive components of bamboo rice. Bamboo rice, part of the cereal family Gramineae, closely resembles rice grains and is widely consumed by many tribal populations in Southern India[15,16]. Previous studies have quantified a few nutrients in bamboo rice[17-19]. Recent research by Sebastian et al.[20] revealed prominent protein molecules in bamboo rice and confirmed a superior nutrient profile, including protein, dietary fiber, vitamins B2 and B5, zinc, calcium, copper and iron, with lower phytic acid content compared to brown and polished rice[20]. Manohari et al.[21] conducted a preliminary phytochemical analysis of bamboo rice from Tamil Nadu, identifying phytochemicals such as cardiac glycosides, reducing sugars, tannins, phlobatannins, phenols and flavonoids, while saponins, alkaloids, terpenoids, and anthraquinones were absent[21].
So far, there has been very limited quantitative analytical literature on the concentrations of the vital components such as fatty acids, phytochemicals and associated antioxidant activity in bamboo rice. Therefore, this study focuses on the analysis of key nutrients, including amino acids, fatty acids, phytosterols and polyphenols, in bamboo rice and evaluation of its in vitro free radical scavenging potential. The current study aims to promote the wider utilization of this underutilized grain in functional food development by food industries and by nutritionists for estimation of dietary nutrient intakes. This study enriches the current understanding and records of indigenous foods and traditional food systems, thereby aiding in the preservation and promotion of local culture and biodiversity.
Materials and Methods
Sample collection:
De-husked bamboo rice (Bambusa arundinacea Willd.) samples (Barcode: BSID0001202, Botanical Survey of India, Indian Virtual Herbarium) were procured from six different regions of South Western Ghats of India. Samples were collected from the indigenous population of Berambadi State Forest (BRB) [11°46'23"N 76°30'04"E] and Kodagu (BRK) [12°22'58"N 75°46'38"E] belonging to the state of Karnataka; Vythiri (BRV) [11°32'59"N 76°03'01"E], Sulthanbathery (BRS) [11°37'56"N 76°15'23"E] and a local market (BRM) located in the Wayand district of Kerala [11°42'54"N 76°06'28"E]; Gudalur [11°29'12"N 76°29'16"E] from Nilgiri district (BRG) located in Tamil Nadu. Bamboo rice samples were collected from 3-4 different sources in each region and combined to create a composite sample. All the bamboo rice samples were hand-pounded, where the husk was removed while retaining the bran, ensuring consistency with commercial brown rice. The collected grains were cleaned thoroughly after separating foreign matter like stones, straw and dirt. A widely consumed rice variety, sona masoori, was sourced from a local market in Hyderabad in the forms of brown and polished (10 % Degree of Milling (DoM)) rice and analysed for comparison with bamboo rice. All the rice samples were finely powdered using a commercial cyclone mill (UDY Corporation, United States of America (USA)), and stored in an airtight container at -20° until further analysis.
Fatty acid composition:
The samples were analysed for their fatty acid profile as per the Fallon et al.[22] method with slight modification. 0.5 g of the sample was weighed into the Pyrex culture tube and 0.5 ml of heptadecanoic acid (C17) (1 mg/ml) was added and dried under a stream of Nitrogen (N2). Then 0.7 ml of 10 N Potassium hydroxide (KOH) and 5.3 ml of methanol (with 0.05 % butylated hydroxytoluene) were added and vortexed for 30 s. The tubes were placed in water bath at 55° for 1.5 h and were allowed to shake vigorously for 5-20 s every 20 min. After cooling the tubes to room temperature, 0.58 ml of 24 N Sulfuric acid (H2SO4) was added. The tubes were vortexed and incubated at 55° for 1.5 h with vigorous shaking at 20 min intervals. After proper vortexing, 3 ml of n-hexane was added to it and centrifuged for 5 min at 2000 revolutions per minute (rpm) (~600 g). The hexane layer was collected in 5 ml test tubes containing 0.1 g of Sodium sulfate (Na2SO4), vortexed, and allowed to settle. It was then dried under a stream of N2 and 1.5 ml of Suprasolv® dichloromethane was added. The dichloromethane layer containing Fatty Acid Methyl Esters (FAME) was transferred into a labelled Gas Chromatography (GC) auto-sampler vial and dried under N2. Suprasolv® dichloromethane was used to make the final volume up to 1 ml. The samples were then analysed in GC (7890A GC System, Agilent Technologies Inc., USA) with a column (Phenomenex BPX-70 fused silica capillary column) of dimension 30×0.32 mm Internal Diameter (ID). Peaks obtained were identified for individual fatty acids by comparing their retention time with external standards (SupelcoTM37 component FAME Mix- Certified Reference Material (CRM47885)) procured from Sigma Aldrich Co. St. Louis, USA.
Amino acid composition:
The rice sample equivalent to 10 mg protein was taken and hydrolysed with 6 N Hydrochloric acid (HCl) in sealed ampoules[23]. The ampoules were kept in oven (Bio Technics, India) for 22 h at 110°. Continuous flash evaporation was carried out under pressure (Buchi, Switzerland) to remove excess acid and the sample was mixed with citrate buffer (pH 2.2). An automated amino acid analyser (Biochrom-30, Cambridge, UK) was used for the analysis, where an aliquot (20 μl) of the sample was taken. Methionine and cysteine were separately quantified after their conversion by performic acid oxidation into methionine sulfone and cysteic acid[24]. A standard hydrolysis procedure was then further carried out. Individual amino acids were detected and quantified with authentic standards (Standard Reference Material (SRM) 2389) from the National Institute of Standards and Technology (Gaithersburg, Maryland, USA) and later provided in g in 100 g of protein. The amino acid score was computed as per Food and Agriculture Organization/World Health Organization/United Nations University (1985), where amino acids required for preschool children of age 2 to 5 y were considered.
Phytosterols:
Phytosterols were analysed following the methods described by Sorenson et al.[25] and Toivo et al.[26]. Acid hydrolysis was performed in 3-5 g of sample using absolute ethanol and 6 M HCl. The samples were kept in a water bath at 80° for 60 min with vigorous shaking every 10 min. Further, 5 ml of absolute ethanol and 20 ml of hexane:diethyl ether mixture were added at a ratio of 1:1 (v/v) and vortexed. The organic phase (approximately 15 ml) was collected into another tube after centrifugation for 10 min at 2000 rpm (~600 g). The organic solvent was evaporated using a nitrogen evaporator at 35°. For saponification, 20 ml of 95 % ethanol and 4 ml of 50 % KOH (w/v) were added to the samples and kept in a water bath at 80° for 30 min with vigorous shaking every 3 min. After cooling, 20 ml of cyclohexane and 10 ml of deionised water were added to the tubes and vortexed. The organic phase (approximately 10 ml) was collected and transferred into fresh centrifuge tubes and was evaporated using a nitrogen evaporator at 35°. The samples were derivatised by dissolving the dry extract in 4-5 ml of cyclohexane, transferred into new tubes and evaporated under nitrogen. Chloroform (1 ml), 0.05 ml (50 μl) of hexamethyldisilazane and 0.1 ml (100 μl) of trimethylchlorosilane was added and made to stand for 15 min at room temperature after proper vortexing. 10 ml of Milli-Q water was added to this, vortexed and centrifuged at 2000 rpm (~600 g) for 2 min. The water layer was separated and the chloroform layer was collected into tubes containing 0.5 g of Na2SO4, filtered with 0.45 μm syringe and collected into GC vials for analysis in GC (7890A GC system, Agilent Technologies, USA) with a column (Phenomenex BPX-70 fused silica capillary column) of dimension 30 m×0.32 mm ID. Peaks obtained were identified for individual phytosterols by comparing their retention time with external standards, including ergosterol (Chemical Abstracts Service (CAS): 57-87-4), stigmasterol (CAS: 83-48-7), campesterol (CAS: 474-62-4) and Beta (β)-sitosterol (CAS: 83-46- 5) procured from Sigma Aldrich Co. St. Louis, USA. A standard stock solution was prepared separately for stigmasterol (5 mg/ml), β-sitosterol (5 mg/ml), campesterol (1 mg/ ml) and ergosterol (10 mg/ml). A standard mixture with all the individual phytosterols at 0.5 mg/ml concentration was prepared for the analysis.
Estimation of Total Phenolic Content (TPC):
The TPC was determined following the method described by Tachakittirungrod et al.[27]. 1 g of sample was weighed into two 15 ml centrifuge tubes and 10 ml of water was added into one tube and 10 ml of methanol into the other. The solution was vortexed for 2 min and centrifuged at 3000 rpm for 10 min. 5 ml of Folin-Ciocalteu reagent was mixed with one ml of the sample extract. The mixture was thoroughly vortexed, and 4 ml of Na2CO3 solution was added and kept for incubation for 60 min at room temperature. Absorbance was measured at 765 nm in a spectrophotometer (SPECORD S 600, Germany). TPC was expressed as Gallic Acid Equivalents (GAE) in g/100 g of sample. A stock solution of gallic acid was prepared. 1 ml of the stock solution was made up to 5 ml with water for the working standard. From this, different concentrations of gallic acid standards were prepared at 20, 40, 60, 80 and 100 μg/ml, respectively. The standard graph was plotted with concentration on the X-axis and absorbance on the Y-axis. The equation (y=mx+c) was obtained from the plot. The concentration of samples was calculated through the above equation observed from the graph by substituting the values[27].
Quantification of individual polyphenols:
Individual polyphenols were quantified following the method described by Giusti et al.[28]. High- Performance Liquid Chromatography (HPLC) grade reference standards for gallic acid (CAS: 149-91- 7), protocatechuic acid (CAS: 99-50-3), 4-hydroxy benzoic acid (CAS: 99-96-7), catechin (CAS: 154-23- 4), caffeic acid (CAS: 331-39-5), sinapic acid (CAS: 530-59-6), ferulic acid (CAS: 537-98-4), 4-coumaric acid (CAS: 501-98-4),ellagic acid (CAS:476-66- 4), 2-coumaric acid (CAS: 614-60-8), luteolin-7-Oglucoside (CAS: 5373-11-5), myricetin (CAS: 529-44- 2), resveratrol (CAS: 501-36-0), daidzein (CAS: 486- 66-8), quercetin (CAS: 522-12-3), luteolin (CAS: 491- 70-3), naringenin (CAS: 67604-48-2), apigenin (CAS: 520-36-5), kaempferol (CAS: 520-18-3), hesperetin (CAS: 69097-99-0) and flavone (CAS: 525-82-6) were purchased from Extrasynthese, Genay, France. A stock was prepared from each polyphenol standard (1 mg/ ml) and a working standard mix was prepared from the stock solution (25 ng/μl) for analysis. The samples were extracted by 10 ml of 90 % methanol with 0.5 % acetic acid. The samples (0.5 g) were vortexed for 5 min and the supernatants were separated by centrifugation at 4000 rpm for 15 min. After three extractions, the extracts were dried with a rotavapour (Buchi). The residue was dissolved in 3.0 ml of methanol. A 0.45 μm syringe filter (Whatman) was used to filter the samples, which were then analysed using Ultra HPLC (UHPLC) with Chromeleon software (Dionex UltiMate 3000 Rapid Separation Liquid Chromatography (RSLC) USA). The compounds were quantified using gradient elution with a mobile phase containing solvents A and B (consisting of 0.1 % formic acid in water v/v and 0.1 % formic acid in acetonitrile, respectively). Luna C18 column (150×2 mm ID; 3 μm particle size) was used for the separation. A column temperature of 40° was maintained. An injection volume of 4 μl was taken with a flow rate of 0.9 ml/min for a run time of 52 min. HPLC with diode-Array Detection (HPLC-DAD) analysis was performed at four wavelengths such as 250, 270, 320 and 370 nm. The ultraviolet spectra and retention times were compared with the corresponding standards to identify the specific phenolic compounds and the data was quantified using the corresponding calibration curves.
Measurement of total antioxidant activity:
The antioxidant activity was measured by 2,2-diphenyl- 1-picrylhydrazyl (DPPH) assay according to Charmforoshan et al.[29] with slight modifications whereas 2,2-azino-bis(3-ethylbenzothiazoline-6- sulphonic acid (ABTS) and Ferric Reducing Antioxidant Power (FRAP) assays were performed following the method described by Tachakittirungrod et al.[27] with slight modifications.
DPPH assay: 1 g sample was extracted with 10 ml of water or methanol, vortexed and centrifuged at 4000 rpm for 10 min. The supernatant obtained (150 μl) was mixed with 2 ml of methanolic DPPH solution. Methanol was added to make it up to 3 ml. The sample mixture was incubated for 40 min at room temperature and the Optical Density (OD) was measured at 517 nm using a spectrophotometer (SPECORD S 600, Germany)[29]. The free radical scavenging activity was expressed as a percentage of inhibition. The percentage of inhibition and half-maximal Inhibitory Concentration (IC50) was measured using the following formulas
% inhibition of DPPH=(control OD-extract OD)/ control OD×100 (1)
IC50=concentration of extract/% inhibition of DPPH×50 (2)
FRAP assay: About 1 g of sample was extracted either with water or methanol independently and centrifuged at 4000 rpm for 10 min after vortexing for 2 min. The supernatant was collected for analysis. FRAP reagent was prepared using sodium acetate buffer (300 mmol), 2,4,6-tripyridyl-s-triazine (TPTZ) solution (10 mmol/l), HCl (40 mmol/l) and iron (III) chloride (20 mmol/l) respectively, at a ratio of 10:1:1 v/v/v. Milli-Q water (240 μl) was taken in a screw cap tube and 2.4 ml of TPTZ buffer and 80 μl of the extracted sample was added into each tube and mixed using vortex. The OD readings were taken at 593 nm in a spectrophotometer (SPECORD S 600, Germany) after 4 min of incubation at 37°. TPTZ buffer (2.4 ml) was added into five screw cap tubes, followed by 20, 40, 60, 80 and 100 μl of Ferrous sulfate (FeSO4) solution as standards. The standard solutions were made up to 320 μl using Milli-Q water and mixed using a vortex. The OD readings were taken at 593 nm. The standard graph with different standard concentrations was plotted and the results were calculated and expressed as mM equivalents of FeSO4/100 g sample[27].
ABTS assay: To prepare the radical cation solution, 2.45 mM potassium persulphate was mixed with 7 mM ABTS stock solution. The mixture was left for 4-16 h until the completion of the reaction and a stable absorbance was obtained. ABTS solution was diluted using Milli-Q water till an absorbance of 0.700 was measured at 734 nm. The photometric assay was carried out on a mixture of 200 μl of sample extract and 1.8 ml of ABTS in a spectrophotometer (SPECORD S 600, Germany)[24]. The percentage of inhibition and IC50 was measured using the following formulas.
% inhibition of ABTS=(control OD-extract OD)/ control OD×100 (3)
IC50=concentration of the extract/% inhibition of ABTS×50 (4)
Statistical analysis:
Descriptive statistical analysis was conducted using the Statistical Package for Social Sciences (SPSS) software version 16.0 (Chicago, USA). To determine the significance of differences within the analysed samples, a one-way ANOVA was performed, followed by the Scheffe test as a post hoc analysis with a significance level set at p<0.05. The results for brown and polished rice were presented as the mean±Standard Deviation (SD) of triplicate analyses (n=3), while for bamboo rice, the mean±SD was based on six collected samples (n=6).
Results and Discussion
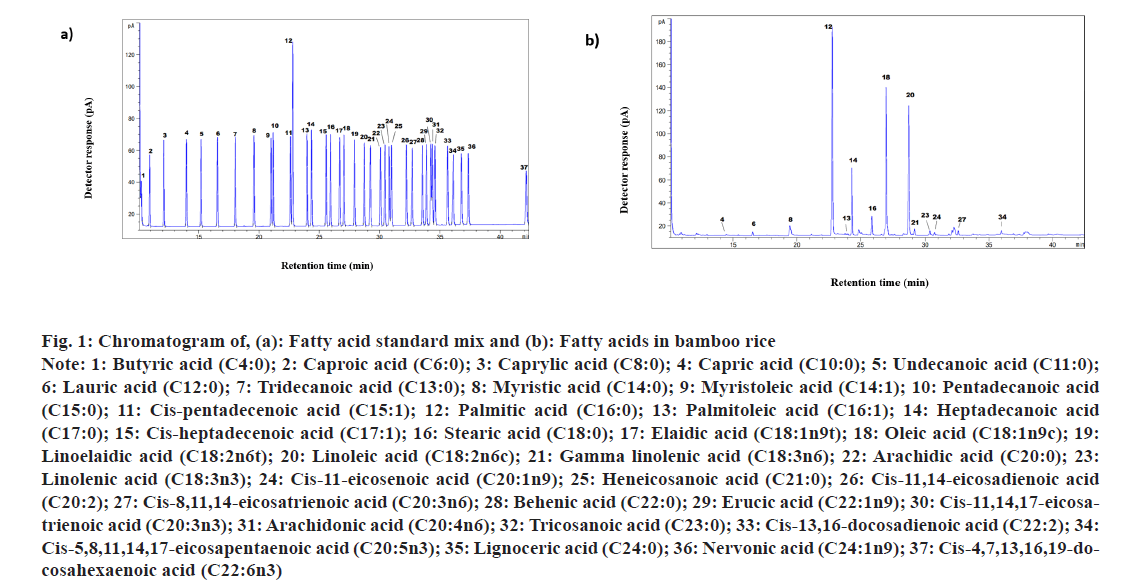
Dietary fats and fatty acids play an important role in human lipid metabolism. Each fatty acid follows its own metabolic pathway and therefore knowing the fatty acid composition of foods is essential[30,31]. In this study, we have quantified comprehensive fatty acid profiles of bamboo rice along with commonly consumed brown and polished rice (10 % DoM) (fig. 1), which are presented in Table 1. These data are particularly significant, because to the best of our knowledge, no prior studies have detailed the comprehensive fatty acid composition of bamboo rice (Bambusa arundinacea Willd.). The levels of Polyunsaturated Fatty Acids (PUFA) were similar across all the rice samples. Similarly, the difference in PUFA levels in polished rice and brown rice was not statistically significant as observed previously[32].
Fig. 1: Chromatogram of, (a): Fatty acid standard mix and (b): Fatty acids in bamboo rice
Note: 1: Butyric acid (C4:0); 2: Caproic acid (C6:0); 3: Caprylic acid (C8:0); 4: Capric acid (C10:0); 5: Undecanoic acid (C11:0); 6: Lauric acid (C12:0); 7: Tridecanoic acid (C13:0); 8: Myristic acid (C14:0); 9: Myristoleic acid (C14:1); 10: Pentadecanoic acid (C15:0); 11: Cis-pentadecenoic acid (C15:1); 12: Palmitic acid (C16:0); 13: Palmitoleic acid (C16:1); 14: Heptadecanoic acid (C17:0); 15: Cis-heptadecenoic acid (C17:1); 16: Stearic acid (C18:0); 17: Elaidic acid (C18:1n9t); 18: Oleic acid (C18:1n9c); 19: Linoelaidic acid (C18:2n6t); 20: Linoleic acid (C18:2n6c); 21: Gamma linolenic acid (C18:3n6); 22: Arachidic acid (C20:0); 23: Linolenic acid (C18:3n3); 24: Cis-11-eicosenoic acid (C20:1n9); 25: Heneicosanoic acid (C21:0); 26: Cis-11,14-eicosadienoic acid (C20:2); 27: Cis-8,11,14-eicosatrienoic acid (C20:3n6); 28: Behenic acid (C22:0); 29: Erucic acid (C22:1n9); 30: Cis-11,14,17-eicosatrienoic acid (C20:3n3); 31: Arachidonic acid (C20:4n6); 32: Tricosanoic acid (C23:0); 33: Cis-13,16-docosadienoic acid (C22:2); 34: Cis-5,8,11,14,17-eicosapentaenoic acid (C20:5n3); 35: Lignoceric acid (C24:0); 36: Nervonic acid (C24:1n9); 37: Cis-4,7,13,16,19-docosahexaenoic acid (C22:6n3)
| Fatty acids (% dw) | Brown rice | Polished rice | Bamboo rice |
|---|---|---|---|
| Caprylic acid (C10:0) | 0.02±0.005a | 0.08±0.007b | 0.07±0.01b |
| Lauric acid (C12:0) | 0.06±0.01a | 0.22±0.11a | 0.21±0.13a |
| Myristic acid (C14:0) | 0.76±0.05a | 2.18±0.45b | 0.59±0.19a |
| Palmitic acid (C16:0) | 26.86±0.12a | 36.03±0.55b | 30.62±5.08ab |
| Stearic acid (C18:0) | 1.81±0.05a | 2.66±0.48b | 3.06±0.46b |
| Arachidic acid (C20:0) | 0.42±0.01a | 0.30±0.04a | 0.97±0.14b |
| Methyl Behenate (C22:0) | 0.17±0.004a | 0.10±0.01a | 0.72±0.12b |
| Methyl Lignocerate (C24:0) | 0.28±0.01a | 0.22±0.03a | 0.74±0.10b |
| Palmitoleic acid (C16:1) | 0.22±0.001b | 0.25±0.008b | 0.14±0.03a |
| Oleic acid (C18:1n9c) | 35.95±0.18c | 21.34±0.56a | 27.55±1.97b |
| Methyl 11-Eicosenoate (C20:1) | 0.41±0.00b | 0.24±0.03a | 0.90±0.12c |
| Linoleic acid (C18:2n6c) | 31.97±0.29a | 35.11±1.01a | 33.76±6.07a |
| alpha linolenic acid (C18:3 n3) | 1.05±0.005b | 1.28±0.053b | 0.68±0.17a |
| TSFA | 30.39±0.17a | 41.80±1.53b | 36.97±6.01ab |
| TMUFA | 36.58±0.18c | 21.82±0.58a | 28.59±2.01b |
| TPUFA | 33.02±0.29a | 36.38±0.97a | 34.44±6.22a |
Note: The values are mean±SD (n=3 for brown and polished rice; n=6 for bamboo rice); Different superscript alphabets in the same row indicates the statistically significant (p<0.05) difference among rice samples.
Table 1: Fatty Acid Composition of Bamboo Rice and Commonly Consumed Brown and Polished Rice
In the present study, bamboo rice contained 23 % higher Monounsaturated Fatty Acids (MUFA) than polished rice. MUFA was significantly different in all the rice samples analysed with the highest content found in brown rice (36.58 % FAME). Similarly, in brown rice varieties, highest proportion of MUFA (45.5 % of total fatty acids) was observed previously among all fatty acids[33]. Kim et al.[32] also reported that MUFA in brown rice was higher by 27 % when compared to polished rice. MUFA was previously reported to significantly decrease upon polishing the rice to 10 % DoM[34]. Among the monounsaturated fatty acids, methyl 11-eicosenoic acid was found to be 0.90±0.12 % FAME in bamboo rice, which was 54 % and 73 % higher than brown rice and polished rice, respectively. These findings align with previously reported proportions of MUFA in brown rice where the relative proportion of oleic acid, eicosenoic acid and palmitoleic were in the order of 44.75, 0.6 and 0.15 % weight of total fatty acids, respectively[33]. Oleic acid in polished rice was lesser than bamboo rice and brown rice by 22 % and 40 %, respectively. Similarly, previous studies found that oleic acid decreased after milling of rice by 10 %[34].
The total Saturated Fatty Acids (SFA) in bamboo rice is comparable to both brown and polished rice while there was a significant difference between Total Saturated Fatty Acids (TSFA) in brown (30.39 % FAME) and polished rice (41.80 % FAME). Similarly, Chen et al.[35] reported that the TSFA concentrations in bamboo rice (P. edulis and D. asper) were comparable to those in the Chinese rice varieties Jiayou5, Fengliangyou4 and Liangyoupeijiu. Among SFA, caprylic acid in bamboo rice was lower than polished rice by 12 %. Myristic acid was lowest in bamboo rice (0.59±0.19 % FAME) when compared to both brown rice and polished rice. Compared to polished rice, myristic acid in bamboo rice was less by 73 %.
The proportion of palmitic acid was found to be higher by 25 % in polished rice which is in agreement with the findings of Longvah et al.[34] where it was observed that milling increases the proportion of palmitic acid in rice. Among all the analysed unsaturated fatty acids, bamboo rice was rich in oleic acid and linoleic acid. In the present study, polished rice contained higher levels of SFA than brown rice and bamboo rice. A similar increase in SFA content was observed in polished rice in previous studies[32,34]. Palmitic, oleic, myristic and stearic acids were higher in polished rice than in brown rice which is similar to the trend observed by Kim et al.[32]. The observed differences in fatty acids between polished rice, brown rice and bamboo rice may be attributed to milling, environmental factors, genetic factors and cultivation area which can significantly influence the lipid and fatty acid content in rice[36].
Amino acids, the fundamental components of proteins, are crucial for numerous biological functions, including growth, repair and maintenance of body tissues. Understanding the amino acid composition of rice is crucial for assessing its nutritional value[37]. Recent studies using inflammatory bowel disease models have identified peptides such as glutamine and arginine, which demonstrate chemoprotective and anti-apoptotic properties within the intestinal mucosa. These properties are crucial for reducing inflammation and restoring mucosal homeostasis[38]. In the present study, 18 different amino acids were quantified (Table 2). The total and essential amino acids in all the samples were similar but the Limiting Amino Acid (LAA) score (lysine) was significantly higher in bamboo rice (78) when compared to brown (68) and polished rice (61). The lysine content in brown, polished and bamboo rice were 3.96 g/100 g, 3.55 g/100 g and 4.52 g/100 g protein, respectively. Chen et al.[35] have observed that the lysine content in two different species of bamboo seeds namely Passiflora edulis (P. edulis) and Dendrocalamus asper (D. asper) were 4.55 g/100 g and 5.16 g/100 g protein, respectively. Lysine content in the International Rice Research Institute's world collection of rice showed a variability of not more than 3 g/100 g protein at any protein level[39]. In bamboo rice, the lysine content was positively correlated with the protein content[20].
| Amino acids (g/100 g Protein) | Brown rice | Polished rice | Bamboo Rice |
|---|---|---|---|
| Aspartic acid | 8.82 | 8.63 | 9.38 |
| Threonine | 3.63 | 3.45 | 3.73 |
| Serine | 5.09 | 5.08 | 4.54 |
| Glutamic acid | 17.70 | 18.44 | 16.74 |
| Proline | 6.23 | 6.66 | 6.48 |
| Glycine | 4.64 | 4.38 | 4.98 |
| Alanine | 5.62 | 5.39 | 5.52 |
| Cystine | 1.44 | 1.35 | 1.59 |
| Valine | 5.88 | 5.86 | 5.90 |
| Methionine-S | 1.70 | 1.69 | 1.73 |
| Isoleucine | 4.16 | 4.19 | 3.94 |
| Leucine | 7.55 | 5.98 | 6.87 |
| Tyrosine | 5.27 | 5.89 | 4.41 |
| Phenylalanine | 6.06 | 6.90 | 5.98 |
| Histidine | 2.85 | 3.01 | 2.56 |
| Lysine | 3.96 | 3.55 | 4.52 |
| Arginine | 7.88 | 5.48 | 7.88 |
| TAA | 98.48 | 95.92 | 96.76 |
| TEA | 39.66 | 38.86 | 38.67 |
| LAA Score (Lysine) | 68 | 61 | 78 |
Note: TAA: Total Amino Acid; TEA: Total Essential Amino acid and LAA: Limiting Amino Acid
Table 2: Amino Acid Composition of Bamboo Rice and Commonly Consumed Brown and Polished Rice
Similarly, Cagampang et al.[40] have observed that the relative increase in the lysine content was concomitant with the increase in the protein content of polished rice, but at a substantially lesser rate than other amino acids. Rice protein has gained popularity due to its lysine content being up to 30 % higher than that of other cereals and grains such as corn, wheat, millet and sorghum[41]. In this context, it is noteworthy that bamboo rice contains a relatively higher lysine content compared to commonly consumed brown and polished rice. The concentrations of essential amino acids, namely lysine and threonine were higher in bamboo rice by 12 % and 2.6 % when compared to brown rice and by 21 % and 7.5 % compared to polished rice respectively. In the present study, histidine and phenylalanine content in bamboo rice were 2.56 g/100 g and 5.98 g/100 g protein, respectively. Gopalan et al.[42], have also reported similar concentrations for histidine and phenylalanine which were 2.93 g/100 g and 6.09 g/100 g protein, respectively. However, the concentrations of arginine, lysine, methionine, threonine, leucine, isoleucine and valine were found to be comparatively lower than the values reported in the Nutritive Value of Indian Foods (NVIF) by Gopalan et al.[42]. This variation could be due to the difference in the analytical methodology used.
The Essential Amino Acids (EAAs) in bamboo rice were found to be in the decreasing order of leucine, phenylalanine, valine, lysine, isoleucine, threonine, histidine and methionine with respective concentrations of 6.87, 5.98, 5.9, 4.52, 3.94, 3.73, 2.56 and 1.73 g/100 g protein. Chen et al.[35] reported that the EAAs in P. edulis species of bamboo rice were present in the decreasing order of leucine, phenylalanine, valine, lysine, isoleucine, threonine, methionine and histidine with respective concentrations of 8.99 g/100 g, 7.25 g/100 g, 6.63 g/100 g, 4.55 g/100 g, 4.44 g/100 g, 3.93 g/100 g, 2.81 g/100 g and 2.75 g/100 g protein. However, in D. asper species of bamboo rice, the decreasing order of EAA was leucine, phenylalanine, valine, lysine, isoleucine, threonine, histidine and methionine with respective concentrations of 8.70 g/100 g, 6.85 g/100 g, 6.70 g/100 g, 5.16 g/100 g, 4.31 g/100 g, 3.85 g/100 g, 2.85 g/100 g and 2.39 g/100 g protein[35]. In the present study, EAAs in brown rice were distributed in the order of leucine, phenylalanine, valine, isoleucine, lysine, threonine, histidine, methionine with respective concentrations of 7.55 g/100 g, 6.06 g/100 g, 5.88 g/100 g, 4.16 g/100 g, 3.96 g/100 g, 3.63 g/100 g, 2.85 g/100 g and 1.7 g/100 g protein. Moongngarm et al.[43] reported the brown rice EAAs contents in the order leucine, valine, lysine, phenyalanine, threonine,histidine, isoleucine and methionine with respective concentrations of 9.96 g/100 g, 7.72 g/100 g, 4.74 g/100 g, 4.73 g/100 g, 3.65 g/100 g, 3.17 g/100 g, 1.95 g/100 g and 1.48 g/100 g protein. In this study, the concentrations of aspartic acid, valine, methionine and lysine were found to be higher in bamboo rice compared to brown and polished rice. Similarly, Chen et al.[35] observed that the concentrations of these essential amino acids in bamboo rice were significantly higher than those in the Chinese rice varieties Jiayou5, Fengliangyou4 and Liangyoupeijiu.
Arginine is known to be one of the most significant non-essential amino acids involved in synthesising other nitrogenous compounds vital for physiological viability[44]. In this study, bamboo rice contained arginine at 7.8 g/100 g protein which was similar to that in brown rice but higher by 30 % compared to polished rice. However, the concentrations of histidine and phenyl alanine in bamboo rice were lower than in brown and polished rice (Table 2). The concentrations of all amino acids analysed in brown and polished rice in this study were comparable to the values reported by Longvah et al.[34,45].
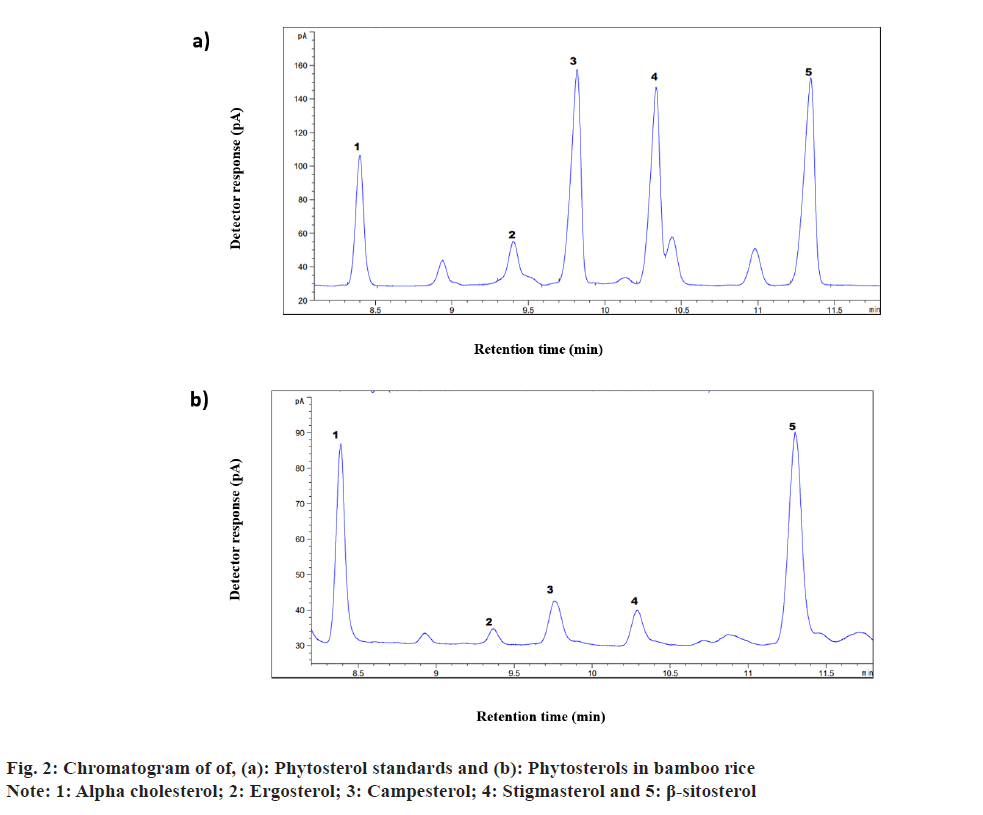
A combination of plant sterols and stanols make up phytosterols and are generally present in small quantities. They are structurally closer to cholesterol while it is different by the side chain i.e., the presence or absence of double bonds[46]. Cereals and their by-products are reported to be significant sources of phytosterols. Common cereals such as wheat, corn, rice and oats contain notable amounts of phytosterols particularly β-sitosterol, campesterol, and stigmasterol[47]. These phytosterols were quantified in brown, polished and bamboo rice (fig. 2 and Table 3). Among all the samples analysed, β-sitosterol (22.26 mg/100 g) was the predominant phytosterol in bamboo rice. β-sitosterol content in brown and polished rice were 19.72±0.28 mg/100 g and 7.90±0.11 mg/100 g, respectively. Piironen et al.[48] reported 37.5 mg/100 g and 19.5 mg/100 g of β-sitosterol in brown and polished rice, respectively. This finding confirms that β-sitosterol is the dominant phytosterol in brown rice compared to polished rice. The concentrations of campesterol and stigmasterol in brown rice were 8.18±0.27 mg/100 g and 6.03±0.19 mg/100 g, respectively which was double the amount in bamboo rice (4.10±0.20 mg/100 g and 3.20±0.39 mg/100 g, respectively). However, the concentration of β-sitosterol and ergosterol were not significantly different among bamboo rice (22.26±1.86 mg/100 g and 4.77±0.68 mg/100 g respectively), and brown rice (19.72±0.28 mg/100 g and 4.25±0.31 mg/100 g respectively), Although the concentration of ergosterol in polished rice (5.50±0.43 mg/100 g) was comparable with brown rice and bamboo rice, it had the lowest levels of all the other phytosterols analysed. The campesterol, sitosterol and stigmasterol content in rice were reported as 14.6 mg/100 g, 37.5 mg/100 g and 10. 4 mg/100 g previously[49]. Furthermore, the phytosterol content in brown rice and bamboo rice was 50 %-55 % higher than in polished rice. This is because phytosterols are predominantly located in the germ and bran layers of cereals, making wholegrain products excellent sources of these sterols[46,50]. Therefore,increasing the consumption of cereals, especially whole grains, can be considered a natural way to increase the intake of phytosterols.
| Sample | Phytosterols (mg/100 g) | |||
|---|---|---|---|---|
| Ergosterol | Campesterol | Stigmasterol | β-sitosterol | |
| Brown rice | 4.25±0.31a | 8.18±0.27c | 6.03±0.19c | 19.72±0.28b |
| Polished rice | 5.50±0.43a | 2.57±0.06a | 1.38±0.01a | 7.90±0.11a |
| Bamboo rice | 4.77±0.68a | 4.10±0.20b | 3.20±0.39b | 22.26±1.86b |
Note: The values are mean±SD (n=3 for brown and polished rice; n=6 for bamboo rice); Different superscript alphabets in the same column indicates the statistically significant (p<0.05) difference among rice samples
Table 3: Phytosterol Concentration in Bamboo Rice and Commonly Consumed Brown and Polished Rice
Phenolic acids include a group of cinnamic and benzoic acid derivatives that occur in both free and bound forms in plant-based foods[51]. Regular consumption of phytochemicals, particularly phenolic compounds found in cereals, has been shown to potentially reduce the risk of degenerative diseases, such as heart disease and cancer[46,52]. In the current study, the TPC was extracted with methanol and water independently, analysed and presented in Table 4.
| TPC (g GAE in 100 g) | Brown rice | Polished rice | Bamboo Rice |
|---|---|---|---|
| TPC in water extract | 0.091±0.001b | 0.025±0.000a | 0.09±0.010b |
| TPC in methanolic extract | 0.071±0.000a | 0.024± 0.001b | 0.04±0.010c |
| Individual polyphenols (mg/100 g) | |||
| Gallic acid | 0.008±0.006a | BDL | 0.043±0.015b |
| Protocatechuic acid | 0.068±0.01a | BDL | 0.426±0.259b |
| 4-hydroxy benzoic acid | 0.085±0.006a | 0.086±0.011a | 0.132±0.067a |
| Catechin | BDL | BDL | 0.963±0.563a |
| Caffeic acid | 0.032±0.003a | BDL | 0.037±0.02a |
| Sinapic acid | 0.146±0.013a | 0.043±0.003a | 0.349±0.245a |
| Ferulic acid | 0.516±0.012b | 0.066±0.003a | 0.266±0.242ab |
| 4-Coumaric acid | 0.117±0.002a | BDL | 0.292±0.231a |
| Ellagic acid | BDL | BDL | 0.024±0.018a |
| 2-Coumaric acid | 0.026±0.003a | BDL | 0.177±0.06b |
| Luteolin 7-O-Glucoside | BDL | BDL | 0.157±0.09a |
| Myricetin | BDL | BDL | 0.272±0.105a |
Note: The values are mean±SD (n=3 for brown and polished rice; n=6 for bamboo rice); different superscript alphabets in the same row indicate the statistically significant (p<0.05) difference among rice samples; BDL: Below Detectable Limit
Table 4: Tpc and Individual Polyphenol Concentration in Bamboo Rice and Commonly Consumed Brown and Polished Rice
It was found that water was more efficient than methanol in extracting polyphenols in brown, polished and bamboo rice. In aqueous medium extraction, the TPC content of bamboo rice was 0.090±0.010 g GAE/100 g, which is comparable to the TPC of brown rice (0.091±0.001 g GAE/100 g). In methanolic medium extraction, the TPC of brown rice (0.071 g GAE/100 g) was higher than bamboo rice (0.04 g GAE/100 g) and polished rice (0.024 g GAE/100 g). However, in a study by Haldipur et al.[16] bamboo rice showed significantly higher content of TPC (0.0647 g GAE/100g) when compared to brown rice (0.0485 g GAE/100 g) when extracted in a mixture of methanol, water and formic acid. This difference in the TPC of the bamboo rice in the present study could be due to the difference in the extraction medium. It has been proved that the amounts of phenolics and their antioxidant activity typically differ based on the extraction process and vary significantly among the aleurone, bran and whole grain with the aleurone layer containing the highest levels[53,54]. However, in this study, the TPC in brown rice is consistent with the findings of Gong et al.[55] who reported that the TPC of brown rice cultivars ranged from 0.072-0.120 g GAE/100 g. Polished rice had the least content of TPC in both water and methanolic extracts with 0.025±0.0004 g GAE/100 g and 0.024±0.001 g GAE/100 g, respectively. This reduction in phenolic concentration is attributed to the removal of the outer layers of the grain during polishing[56]. The observed variations in the TPC content in brown rice, polished rice and bamboo rice could be ascribed to the difference in cultivation conditions[57].
Polyphenols, naturally occurring in cereals like wheat, rice and oats, offer numerous health benefits including antioxidant and anti-inflammatory effects. Antioxidant compounds, predominantly phenolic compounds are located in both free and insoluble forms within the outer layers of cereals[58]. In cereals, the primary hydroxycinnamic acids present are sinapic acid, ferulic acid and p-coumaric acid[59]. In this study, 21 polyphenolic compounds were analysed in bamboo rice, brown rice and polished rice, out of which 12 compounds were detected and quantified (Table 4). The polyphenols that were detected and quantified in bamboo rice were catechin>protocatechuic acid>sinapic acid>4-coumaric acid>myricetin>ferulic acid>2-coumaric acid>luteolin7-o-glucoside>4-hydroxy benzoic acid>gallic acid>caffeic acid>ellagic acid. Among these, catechin (0.963±0.563 mg/100 g), ellagic acid (0.024±0.018 mg/100 g), luteolin 7-O-glucoside (0.157±0.09 mg/100 g) and myricetin (0.272±0.105 mg/100 g) were exclusively detected in bamboo rice, with catechin emerging as the predominant polyphenol. Similarly, in a study by Haldipur et al.[16] where an untargeted phenolic profiling of bamboo rice was conducted by quadrupole time-of-flight liquid chromatography with tandem mass spectrometry, gallic acid, 4-hydroxybenzaldehyde, caffeic acid, 4-O-glucoside and p-coumaric acid were reported to be the most abundant among the 63 different phenolic metabolites identified[16]. Certain polyphenols in bamboo rice such as gallic acid, protocatechuic acid and 2-coumaric acid were found to be over 80 % higher than brown rice. Sinapic acid, 4-coumaric acid, 4-hydroxybenzoic acid and caffeic acid in bamboo rice were also abundant by 58 %, 59 %, 35 % and 13 % respectively when compared to brown rice. Moreover, polyphenols such as protocatechuic acid, sinapic acid and ferulic acid which were detected in polished rice were approximately 75 %-85 % lower than in bamboo rice. It was found that the concentrations of polyphenols such as ferulic acid and caffeic acid were comparable with concentrations reported previously in brown rice and polished rice[60-62]. van Hung[63], also reported relatively higher concentrations of coumaric and ferulic acids and lower concentrations of gallic, caffeic and vanillic acids in brown and polished rice as observed in this study. These bioactive polyphenol compounds possess high antioxidant potential having significant pharmaceutical relevance due to their biological activities, such as anti-allergic, antimicrobial, anticancer and anti-inflammatory effects[6].
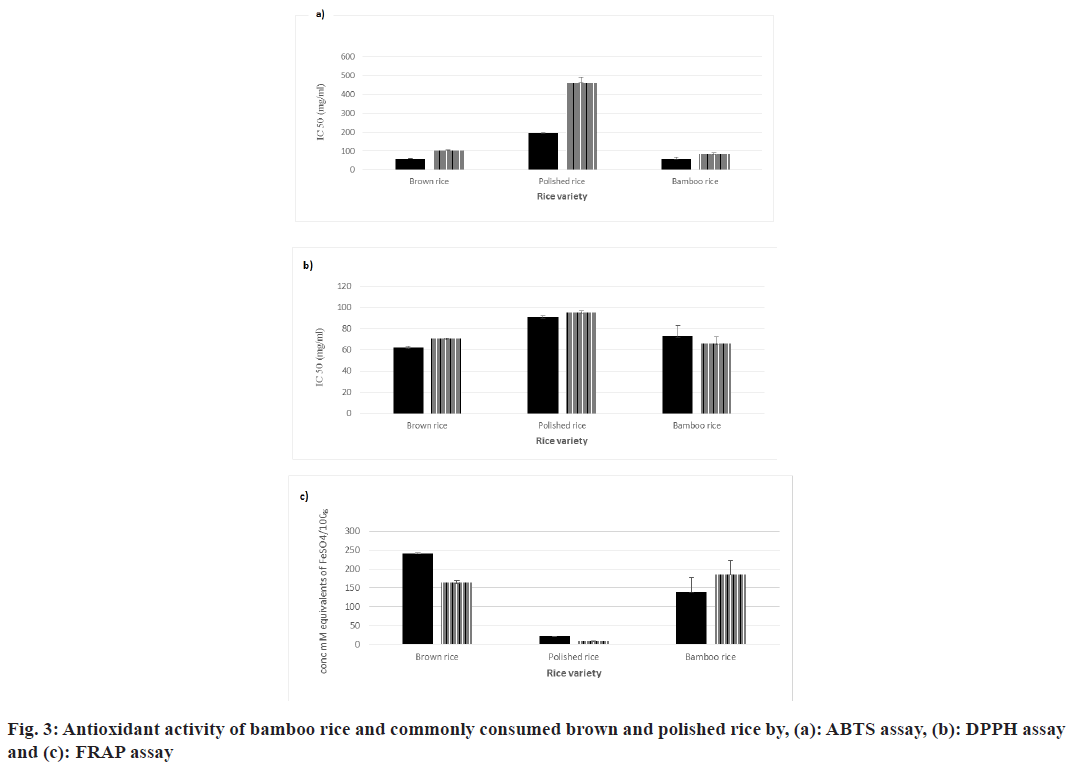
The antioxidant activities exhibited by the bamboo rice were investigated and compared with the commonly consumed polished and brown rice (fig. 3). The methanolic extract of bamboo rice exhibited 25 % higher antioxidant activity in the FRAP assay (185±36.79 mM FeSO4 equivalents/100 g) and a 10 % higher activity in the DPPH assay (IC50 of 65.52±7.03 mg/ml) compared to its water extract, which showed 139±38.13 mM FeSO4 equivalents/100 g and an IC50 of 73.07±9.89 mg/ml, respectively. However, the water extract of bamboo rice demonstrated approximately 32 % higher activity in the ABTS assay when compared to the methanolic extract.
Though bamboo rice exhibited a higher antioxidant activity with ABTS assay in both water and methanolic extracts (IC50 of 57.62±7.30 mg/ml and 84.81±7.75 mg/ ml, respectively), brown rice showed a better activity for both FRAP (241±1.82 mM equivalents of FeSO4/100 g and 163±5.92 mM equivalents of FeSO4/100 g, respectively) and DPPH assays (IC50 of 61.96±1.61 mg/ml and 70.34±0.50 mg/ml, respectively). Notably, bamboo rice exhibited superior antioxidant activity compared to polished rice across all the assays. This is in agreement with studies that have demonstrated that darker grains possess higher levels of total phenolics and exhibit enhanced antioxidant activity[64,65]. According to Galanakis[66], phenolic compounds such as sinapic, gallic, syringic, chlorogenic, cinnamic, caffeic, ferulic, protocatechuic and vanillic acids can be utilized as additives in meat products to inhibit lipid oxidation and prolong shelf life. This is attributed to their capacity to neutralize radicals through hydrogen donation. Furthermore, Karimi et al.[6], underscored the potential of phenolic compounds and fatty acids, including palmitic acid, oleic acid, linoleic acid and γ-linolenic acid in contributing to antioxidant activity[6]. Therefore, the antioxidant activity observed in bamboo rice is likely due to the presence of various polyphenols and fatty acids. The biological activities of these compounds may be linked to the ease with which an aromatic hydroxyl group can donate a hydrogen atom to a free radical and the capacity of an aromatic compound to stabilize an unpaired electron. This mechanism is crucial, as compounds with antioxidant activity can safeguard cellular systems from the detrimental effects of metabolic processes that cause excessive oxidation. Additionally, these antioxidants can disrupt free radical chain reactions, scavenge free radicals and thereby help prevent cancer, heart disease, vascular disorders, and neurodegenerative diseases[6]. Therefore, the significant antioxidant activity in bamboo rice, driven by its rich polyphenol content, underscores its potential to protect against oxidative stress and related chronic diseases.
Conclusion
Epidemiological data has demonstrated a positive correlation between the consumption of antioxidantrich foods and improved human well-being. This study aimed to explore the BACs in bamboo rice, revealing a rich profile of essential BACs beneficial for human health. Bamboo rice contained a higher proportion of total MUFA and a lower proportion of SFA compared to polished rice. The concentrations of essential amino acids namely lysine and threonine were higher in bamboo rice compared to both brown and polished rice. The significant antioxidant activity exhibited by bamboo rice can be attributed to the higher concentrations of various individual polyphenolic acids analysed, including protocatechuic, gallic, 4-hydroxybenzoic, catechin, caffeic, sinapic, ferulic, 4-coumaric, ellagic, 2-coumaric, luteolin 7-O-glucoside and myricetin. Moreover, few polyphenols were exclusively found in bamboo rice when compared to brown and polished rice. The findings of this study can significantly contribute to further research aimed at enhancing the extraction and utilization of these BACs from bamboo rice. The data gathered can also help explore the mechanisms and pathways of these BACs and their interactions with human metabolism and physiology. These improvements can lead to the development of new functional foods, supplements, and nutraceutical products that offer health benefits such as lowering cholesterol, preventing oxidative stress, and improving protein quality. Importantly, this study can also support the conservation and use of bamboo rice as a traditional and nutritious food source for indigenous and rural communities in India and other regions where it naturally grows.
Acknowledgements
The authors express their sincere gratitude to the Director, Indian Council of Medical Research (ICMR)- National Institute of Nutrition, for the scientific encouragement and financial support to carry out the study.
Conflict of interest:
The authors declare no conflict of interests.
References
- Guaadaoui A, Benaicha S, Elmajdoub N, Bellaoui M, Hamal A. What is a bioactive compound? A combined definition for a preliminary consensus. Int J Nutr Food Sci 2014;3(3):174-9.
- Verma DK, Thakur M, editors. Phytochemicals in food and health: Perspectives for research and technological development. CRC Press; 2021.
- Shahidi F. Antioxidants in food and food antioxidants. Nahrung 2000;44(3):158-63.
[Crossref] [Google Scholar] [PubMed]
- Shetty K, Sarkar D. Introduction: Metabolic-driven ecological rationale to advance biotechnological approaches for functional foods. In: Functional Foods and Biotechnology. CRC Press 2019:1-4.
- Oakenfull D. Saponins in food-a review. Food Chem 1981;7(1):19-40.
- Karimi E, Oskoueian E, Karimi A, Noura R, Ebrahimi M. Borago officinalis L. flower: A comprehensive study on bioactive compounds and its health-promoting properties. Food Measure 2018;12:826-38.
- Galanakis CM, Aldawoud TM, Rizou M, Rowan NJ, Ibrahim SA. Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: A comprehensive review. Foods 2020;9(11):1701.
[Crossref] [Google Scholar] [PubMed]
- Omidfar F, Gheybi F, Davoodi J, Amirinejad M, Badiee A. Nanophytosomes of hesperidin and of hesperetin: Preparation, characterization, and in vivo evaluation. Biotechnol Appl Biochem 2023;70(2):846-56.
[Crossref] [Google Scholar] [PubMed]
- Esmaeili Y, Zamindar N, Mohammadi R. The effect of polypropylene film containing nano-hydroxyapatite on physicochemical and microbiological properties of button mushrooms (Agaricus bisporus) under Modified atmosphere packaging. Food Measure 2023;17(1):773-86.
- Friedman M. Rice brans, rice bran oils, and rice hulls: Composition, food and industrial uses, and bioactivities in humans, animals, and cells. J Agri Food Chem 2013;61(45):10626-41.
[Crossref] [Google Scholar] [PubMed]
- Adom KK, Liu RH. Antioxidant activity of grains. J Agri Food Chem 2002;50(21):6182-7.
- Miller HE, Rigelhof F, Marquart L, Prakash A, Kanter M. Whole-grain products and antioxidants. J Am Coll Nutr2000;19(3 Suppl):312S-9S.
[Crossref] [Google Scholar] [PubMed]
- Branen AL. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J Am Oil Chem Soc 1975;52(2):59-63.
[Crossref] [Google Scholar] [PubMed]
- Hegde S, Yenagi NB, Kasturiba B. Indigenous knowledge of the traditional and qualified ayurveda practitioners on the nutritional significance and use of red rice in medications; 2013.
- Vasudeva S, Ali SZ, Kartha KR. Physico-chemical properties of Bamboo seed and its starch. Trends Carbohydr Res 2011;3(2):54-9.
- Haldipur AC, Srividya N. A comparative evaluation of in vitro antihyperglycemic potential of Bamboo seed rice (Bambusa arundinacea) and Garudan samba (Oryza sativa): An integrated metabolomics, enzymatic and molecular docking approach. J Cereal Sci 2021;99:103200.
- Rao ML, Subramanian N, Srinivasan M. Nutritive value of Bamboo seeds (Bambusa arundinacea, Willd.). Curr Sci 1955;24:157-8.
- Rao PS, Jacob CM, Ramsastri BV. The nutritive value of bamboo seeds. Indian J Nutr Dietetics1969;6:192-5.
- Jarapala SR, Shivudu G, Mangathya K, Rathod A, Panda H, Reddy PK. A new approach for identifying potentially effective indigenous plants consumed by Chenchu tribes and their nutritional composition-India. Am J Plant Sci 2021;12(8):1180-96.
- Sebastian J, Longvah T, Subhash K, Chary PM, Loukrakpam B, Aaliya B, et al. Morphology, comprehensive physico-chemical and cooking characteristics of Bamboo rice (Bambusa arundinacea Wild.). Measure Food 2023;10:100089.
- Manohari RG, Saravanamoorthy MD, Vijayakumar TP, Vijayan B, Gowri Manohari R, Poongodi Vijayakumar T, et al. Preliminary phytochemical analysis of bamboo seed. World J Pharm Pharm Sci 2016;5(4):1336-42.
- O'Fallon JV, Busboom JR, Nelson ML, Gaskins CT. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J Animal Sci 2007;85(6):1511-21.
[Crossref] [Google Scholar] [PubMed]
- Darragh AJ, Moughan PJ. The effect of hydrolysis time on amino acid analysis. J AOAC Int 2005;88(3):888-93.
[Crossref] [Google Scholar] [PubMed]
- Moore S. On the determination of cystine as cysteic acid. J Biol Chem 1963;238:235-7.
- Sorenson WR, Sullivan D. Determination of campesterol, stigmasterol, and beta-sitosterol in saw palmetto rawmaterials and dietary supplements by gas chromatography: Single-laboratory validation. J AOAC Int 2006;89(1):22-34.
- Toivo J, Phillips K, Lampi AM, Piironen V. Determination of sterols in foods: Recovery of free, esterified, and glycosidic sterols. J Food Compos Anal 2001;14(6):631-43.
- Tachakittirungrod S, Okonogi S, Chowwanapoonpohn S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem 2007;103(2):381-8.
- Giusti F, Caprioli G, Ricciutelli M, Torregiani E, Vittori S, Sagratini G. Analysis of 17 polyphenolic compounds in organic and conventional legumes by high-performance liquid chromatography-diode array detection (HPLC-DAD) and evaluation of their antioxidant activity. Int J Food Sci Nutr 2018;69(5):557-65.
[Crossref] [Google Scholar] [PubMed]
- Charmforoshan E, Karimi E, Oskoueian E, Es-Haghi A, Iranshahi M. Inhibition of human breast cancer cells (MCF-7 cell line) growth via cell proliferation, migration, and angiogenesis by auraptene of Ferula szowitsiana root extract. Food Measure 2019;13:2644-53.
- Chow CK, editor. Fatty acids in foods and their health implications. CRC press; 2007.
- O’Keefe SF. Nomenclature and classification of lipids. In: Food Lipids; CRC Press 2002:20-59.
- Kim MH, Ahn SI, Lim CM, Jhoo JW, Kim GY. Effects of germinated brown rice addition on the flavor and functionality of yogurt. Korean J Food Sci Animal Resour 2016;36(4):508-15.
[Crossref] [Google Scholar] [PubMed]
- Taira H, Nakagahra M, Nagamine T. Fatty acid composition of Indica, Sinica, Javanica, Japonica groups of nonglutinous brown rice. J Agri Food Chem 1988;36(1):45-7.
- Longvah T, Mangthya K, Subhash K, Sen S, Rathi S. Comprehensive nutritional evaluation of popular rice varieties of Assam, Northeast India. J Food Composition Anal 2021;101:103952.
- Chen Z, Pan X, Hu L, Ji H, Yu X, Shao JF. A comparative evaluation of chemical composition and nutritional value of bamboo rice and major cereals reveals the potential utility of bamboo rice as functional food. Food Chem X 2023;18:100723.
- Kitta K, Ebihara M, Iizuka T, Yoshikawa R, Isshiki K, Kawamoto S. Variations in lipid content and fatty acid composition of major non-glutinous rice cultivars in Japan. J Food Compos Anal 2005;18(4):269-78.
- Ren W, Li Y, Yin Y, Blachier F. Structure, metabolism and functions of amino acids: An overview. Nutritional and physiological functions of amino acids in pigs; 2013:91-108.
- Zhang H, Hu CA, Kovacs-Nolan J, Mine Y. Bioactive dietary peptides and amino acids in inflammatory bowel disease. Amino Acids 2015;47:2127-41.
[Crossref] [Google Scholar] [PubMed]
- Nanda JS, Coffman WR. IRRI's efforts to improve the protein content of rice. Los Banos (Philippines): IRRI; 1979.
- Cagampang GB, Cruz LJ, Espiritu SG, Santiago RG, Juliano BO. Studies on the extraction and composition of rice proteins. 1966.
- Hegsted DM. Nutritional value of cereal proteins in relation to human needs. Protein-enriched cereal foods for world needs; 1969:38-48.
- Gopalan C, Sastri BR, Balasubramanian S. Nutritive value of Indian foods; 1971.
- Moongngarm A, Saetung N. Comparison of chemical compositions and bioactive compounds of germinated rough rice and brown rice. Food Chem 2010;122(3):782-8.
- Young VR, Yu YM, Borgonha S. Proteins, peptides and amino acids in enteral nutrition: overview and some research challenges. Proteins, Peptides and Amino Acids in Enteral Nutrition 2000;3:1-23.
[Crossref] [Google Scholar] [PubMed]
- Longvah T. Indian Food Composition Tables. National Institute of Nutrition: xxxi. 2017.
- Liu RH. Whole grain phytochemicals and health. J Cereal Sci 2007;46(3):207-19.
- Rezig L, Abdelkrim YZ. Phytosterols: Potential therapeutic effects and challenges in food industry. In: Implication of Oxysterols and Phytosterols in Aging and Human Diseases; 2023:453-62.
- Piironen V, Lampi A-M. Occurrence and levels of phytosterols in foods. Phytosterols as functional food components and nutraceuticals2004:1-32.
- Piironen V, Toivo J, Lampi AM. Plant sterols in cereals and cereal products. Cereal Chem 2002;79(1):148-54.
- Nyström L, Paasonen A, Lampi AM, Piironen V. Total plant sterols, steryl ferulates and steryl glycosides in milling fractions of wheat and rye. J Cereal Sci 2007;45(1):106-15.
- Gani A, Wani SM, Masoodi FA, Hameed G. Whole-grain cereal bioactive compounds and their health benefits: A review. J Food Process Technol 2012;3(3):146-56.
- Okarter N, Liu RH. Health benefits of whole grain phytochemicals. Critic Rev Food Sci Nutr 2010;50(3):193-208.
[Crossref] [Google Scholar] [PubMed]
- Pérez-Jiménez J, Saura-Calixto F. Literature data may underestimate the actual antioxidant capacity of cereals. J Agric Food Chem 2005;53(12):5036-40.
[Crossref] [Google Scholar] [PubMed]
- Zhou Z, Robards K, Helliwell S, Blanchard C. The distribution of phenolic acids in rice. Food Chem 2004;87(3):401-6.
- Gong ES, Luo SJ, Li T, Liu CM, Zhang GW, Chen J, et al. Phytochemical profiles and antioxidant activity of brown rice varieties. Food Chem 2017;227:432-43.
[Crossref] [Google Scholar] [PubMed]
- Zhou K, Su L, Yu L. Phytochemicals and antioxidant properties in wheat bran. J Agri Food Chem 2004;52(20):6108-14.
[Crossref] [Google Scholar] [PubMed]
- Tananuwong K, Tangsrianugul N. Effects of storage conditions and cooking on colour and antioxidant activities of organic pigmented rice. Int J Food Sci Technol 2013;48(1):67-73.
- Liyana-Pathirana CM, Shahidi F. Antioxidant and free radical scavenging activities of whole wheat and milling fractions. Food Chem 2007;101(3):1151-7.
- Andreasen MF, Christensen LP, Meyer AS, Hansen Å. Content of phenolic acids and ferulic acid dehydrodimers in 17 Rye (Secale cereale L.) Varieties. J Agric Food Chem 2000;48(7):2837-42.
[Crossref] [Google Scholar] [PubMed]
- Huang SH, Ng LT. Quantification of polyphenolic content and bioactive constituents of some commercial rice varieties in Taiwan. J Food Compos Anal 2012;26(1-2):122-7.
- Tian S, Nakamura K, Kayahara H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J Agri Food Chem 2004;52(15):4808-13.
[Crossref] [Google Scholar] [PubMed]
- Vichapong J, Sookserm M, Srijesdaruk V, Swatsitang P, Srijaranai S. High performance liquid chromatographic analysis of phenolic compounds and their antioxidant activities in rice varieties. LWT Food Sci Technol 2010;43(9):1325-30.
- Van Hung P. Phenolic compounds of cereals and their antioxidant capacity. Crit Rev Food Sci Nutr 2016;56(1):25-35.
[Crossref] [Google Scholar] [PubMed]
- Zhang MW, Zhang RF, Zhang FX, Liu RH. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J Agric Food Chem 2010;58(13):7580-7.
[Crossref] [Google Scholar] [PubMed]
- Yodmanee S, Karrila TT, Pakdeechanuan P. Physical, chemical and antioxidant properties of pigmented rice grown in Southern Thailand. Int Food Res J 2011;18(3):1-8.
- Galanakis CM. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci Technol 2018;79:98-105.