- *Corresponding Author:
- S. Paulsamy
Department of Botany, Kongunadu Arts and Science College, Coimbatore-641 029, India
E-mail: paulsami@yahoo.com
| Date of Submission | 14 May 2015 |
| Date of Revision | 15 December 2015 |
| Date of Acceptance | 21 February 2016 |
| Indian J Pharm Sci, 2016;78(1):103‑110 |
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
To evaluate the traditional use, the mosquito repellent property of Thalictrum javanicumand to confirm the predicted larvicidal activity of the isolated compound, oleic acid, eicosyl ester from its aerial parts by PASS software, the present study was carried out using 4th instar stage larvae of the mosquitoes, Aedes aegypti(dengue vector) and Culex quinquefasciatus(filarial vector). Insecticidal susceptibility tests were conducted and the mortality rate was observed after 24 h exposure. The chitinase activity of isolated compound was assessed by using purified β-N-acetyl glucosaminidase (chitinase). Ecdysone 20-monooxygenase assay (radioimmuno assay) was made using the same larval stage of A. aegyptiand C. quinquefasciatus. The results were compared with the crude methanol extract of the whole plant. The isolated compound, oleic acid, eicosyl ester was found to be the most effective larvicide against A. aegypti (LC50/24 h -8.51 ppm) and C. quinquefasciatus (LC50/24 h - 12.5 ppm) than the crude methanol extract (LC50/24 h - 257.03 ppm and LC50/24 h - 281.83 ppm, respectively). The impact of oleic acid, eicosyl ester on reducing the activity of chitinase and ecdysone 20-monooxygenase was most prominent in both the target species, A. aegyptiand C. quinquefasciatusthan the control. The results therefore suggest that the compound, oleic acid, eicosyl ester from Thalictrum javanicummay be considered as a potent source of mosquito larvicidal property.
Keywords
Thalictrum javanicum, aerial parts, oleic acid, eicosyl ester, larvicidal activity
Mosquitoes are the major vectors for the transmission of various tropical and subtropical diseases which cause devastating effects to human [1]. The most common dreadful diseases associated with mosquitoes are malaria, yellow fever, filariasis, schistosomiasis, japanese encephalitis (JE) [2] and the worst, dengue hemorrhagic fever, caused by Aedes aegypti [3]. Filariasis is carried by the mosquito, Culex quinquefasciatus which is a pantropical pest and urban vector of Wuchereria bancrofti [4]. Interest has been focused to control of Aedes aegypti and Culex quinquefasciatus, lies in the fact that they act as a vector of dengue and filarial fever, respectively, which is a serious public health problem in countries like India. Therefore, the studies in search of novel entities from plants to prevent proliferation of mosquito borne diseases and to protect environment from the application of chemical pesticide, the mosquito control is essential.
Thalictrum javanicum Blume (Ranunculaceae) is a perennial herbaceous plant. In India, it is predominantly distributed in temperate Himalayas and high hills of Kodaikanal and Nilgiris of Western Ghats, Tamil Nadu. The whole plant is used as herbal spray to encourage the control of insect vectors by Thoda tribal communites of Nilgiris, the Western Ghats, India [5]. Venkatachalapathi et al. [6] reported on basis of use/reports and informant consensus factor that this is a most prescribed species for mosquito repellency by the Thoda tribal and other local healers in Nilgiris, the Western Ghats. The aerial parts of the plant are perceived as germicidal in the field of veterinary medicine [7]. A phytochemical investigation of this genus, Thalictrum is afforded with fatty acids [8]. Literature data validates that oleic acid isolated from different species of the genus, Thalictrum has larvicidal activity [8-10]. Despite, data on the larvicidal activity of the isolated compound, oleic acid, eicosyl ester from aerial parts of Thalictrum javanicum is still inadequate. To fulfill this lacuna, an attempt was made to evaluate larvicidal activity of this compound in comparison to that of the crude methanol extract of aerial parts of T. javanicum.
Materials and Methods
The aerial parts of the species, Thalictrum javanicum were collected from Thottapetta, Nilgiris, the Western Ghats, Tamil Nadu, India and they were cleaned and shade dried. The dried material was further crushed and coarsely powdered in a Willy mill to 60 mesh size (Nippon Electricals, Chennai).
Preparation of plant extract
One hundred grams of aerial parts were extracted with methanol (500 ml) in soxhlet apparatus for a period of 25 h. The obtained extract was filtered and concentrated under vacuum which gave a semisolid mass with respect to the dried powder (extraction yield 13 g). The crude extract thus obtained was stored and maintained at 4º in refrigerator before the commencement of the experiment.
Compound isolation
The methanol extract was purified by column chromatography (silica gel 60-120 mesh) [11] and eluted with step-wise gradient of petroleum ether: ethyl acetate (100:0, 95:5, 90:10, 85:15 and so on). Fourteen column fractions (100 ml each) were eluted and analysed by TLC. The fractions 7 to 10 (100% pure petroleum ether) exhibited similar TLC pattern (Rf-0.78) and were combined to provide a pure compound (400 mg).
GC-MS analysis
Crude methanol extract and the purified compound were subjected to GC-MS analysis. Chromatographic separation was carried out with CE GC 8000 top MSMD 8000 Fyson instrument with Db 35 mr column (10 m×0.5 mm, 0.25 μm film thickness). Heating programmes were executed at 100-250° for 3 min using helium as carrier gas with a flow rate of 1 ml/min in the split mode (1:50). An aliquot (2 μl) of oil was injected into the column with the injector heater at 250°. Injection temperature at 250°, interface temperature at 200°, quadruple temperature at 150° and ion source temperature at 230° were maintained. Injection was performed in split less mode. The mass spectra of compounds in samples were obtained by electron ionization (EI) at 70 eV, and the detector operated in scan mode was from 20 to 600 atomic mass units (amu). Identifications were based on the molecular structure and mass and calculated fragmentations. Resolved spectra were identified for phytochemicals by using the standard mass spectral database of WILEY and NIST [12,13].
PASS prediction
PASS estimates the probabilities of a particular substance belonging to the active and inactive sub-sets from the SAR (structure-activity relationships) base [14]. The result of prediction contains the list of biological activity with the appropriate probability values (i.e) the values defining the likelihood for a given activity type are either revealed (Pa) or not revealed (Pi) for each activity type from the predicted biological activity spectrum. Their values vary from 0.000 to 1.000. Only those activity types for which Pa>Pi are considered possible [15].
Larvicidal activity
Larvae (4th instar stage) of filarial vector, Aedes aegypti and dengue vector, Culex quinquefasciatus were procured from National Centre for Disease Control Field Station at Mettupalayam, Tamil Nadu, India. They were kept free from exposure to pathogens, insecticides or repellents and maintained in laboratory condition at 25-30°. The larvae were fed on a powdered mixture of biscuits and dried yeast powder (3:1). They became pupae and emerged as adults. The adult female colony was provided with blood of chick (alternate days) and both the male and female were supplied with 10% sucrose solution on wicks. The eggs/rafts laid by the adult mosquitoes were allowed to hatch in separate containers and the larvae were grown with fish food. The larvae at 4th instar stage obtained from this culture were used for this experiment.
Bioassay test
Standard method of assessing larvicidal activity were determined according to the WHO manual [16] with slight modifications. Bioassay was carried out in five replicates using twenty larvae of the two mosquito species and they were introduced into tray separately. Various concentrations of crude methanol extracts of Thalictrum javanicum (50, 100, 150, 200, 250, 300, 350, 400, 450, 500 and 550 ppm) and the isolated compound, oleic acid, eicosyl ester (2, 4, 6, 8, 10, 12, 14, 16, 18, 20 and 22 ppm) were prepared. The neemarin (natural product) in different concentrations (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0 and 1.1 ppm) was used as standard. The per cent mortality of larvae in each experimental tray was recorded at 24 h after introduction. They were subjected to Finney’s probit analysis [17] in order to compute median lethal concentration (LC50/24 h).
Chitinase (β-N-acetyl glucosaminidase) activity
β-N-acetyl glucosaminidase activity was measured according to Dziadik-Turner et al. [18] with some modifications. Briefly, 100 μl of the sample was mixed with 1 ml of the substrate and P-nitro phenyl- 2-deoxy-β-D-glucopyranoside (1.2×10-4 m) and dissolved in 0.5 M of sodium phosphate buffer, pH 8.0 and bovine serum albumin to prevent loss of activity [19]. The reaction mixture was incubated for 10-15 min at 26±1°. After incubation, 0.01 N sodium hydroxide (1 ml) was added to arrest the reaction and the production of β- Nitrophenol was measured at 410 nm.
Preparation of buffer
Buffer was prepared by dissolving 20% sucrose, 1 mM disodiumphenyl phosphofluridate (DPF) and trace amount of phenylthio urea (PTU) in 50 mM of phosphate buffer (pH 6.8) [18].
Source and partial purification of β-N-acetyl glucosaminidase
Newly emerged 4th instar stage larvae were sacrificed and the cuticle was homogenized using buffer. The homogenate was centrifuged at 10 000 rpm for 5 min and the clear supernatant was freeze dried using speedvac concentrator (Savanl, USA) and stored. The samples were reconstituted in a known volume of buffer and was partially purified by gel filtration using sephadex G-100. The column was diluted with 0.2 M citrate phosphate buffer (pH 6.5). The collected fractions with activity were pooled and freeze dried until further use.
Radioimmunoassay for abdomen ecdysteroid
The ecdysteroid harmone titrate in abdomen region with an interval of 0, 12 and 24 h of A. aegypti and C. quinquefasciatus was estimated according to the method of Brost and O’Connor [20].
Preparation of sample for the determination of ecdysone 20-monooxygenase
The abdomen regions were homogenized separately in a known volume of 75% methanol and the samples were diluted again with the same solvent and stored at least overnight at -20° to precipitate proteins. This mixture was vortexed vigorously and centrifuged at 3000 rpm for 15 min. The supernatant obtained was evaporated to (6×50 mm tubes) dryness using speedvac concentrator and evaporator (Savant, USA) and the samples were stored at -20°. Ecdysteroid was quantified by radioimmuno assay (RIA) using ecdysteroid antiserum obtained from Manian Laboratories, Coimbatore [21].
RIA of E20M
Ten microlitres of the sample was taken in 6×50 mm tubes to which 100 μl 3H-ecdysone was added and vortexed followed by the addition of 100 μl of antiserum and vortexed immediately. The serum along with sample H3 ecdysone served as control. The mixture was vortexed thoroughly and incubated at 40° for 8-12 h. The assay was terminated by adding 200 μl of 100% saturated ammonium sulphate. Then the mixture was allowed to stand for 20 min in the refrigerator. After incubation period, it was centrifuged at 3000 rpm for 10 min. The supernatant was aspirated off carefully without disturbing the pellet, which was resuspended in 0.4 ml of 50% saturated ammonium sulphate and incubated for 20 min. The precipitate was centrifuged as before and aspirated off the supernatant. The pellet was added with 25 μl of water and vortexed to resolubilize at room temperature. 300 μl of scintillation cocktail was added and vortexed thoroughly. Tubes were kept in the scintillation vials and counted at 5000 cpm for 10 min which were came first (usually by 10 min/sample) using the scintillation counter.
Results
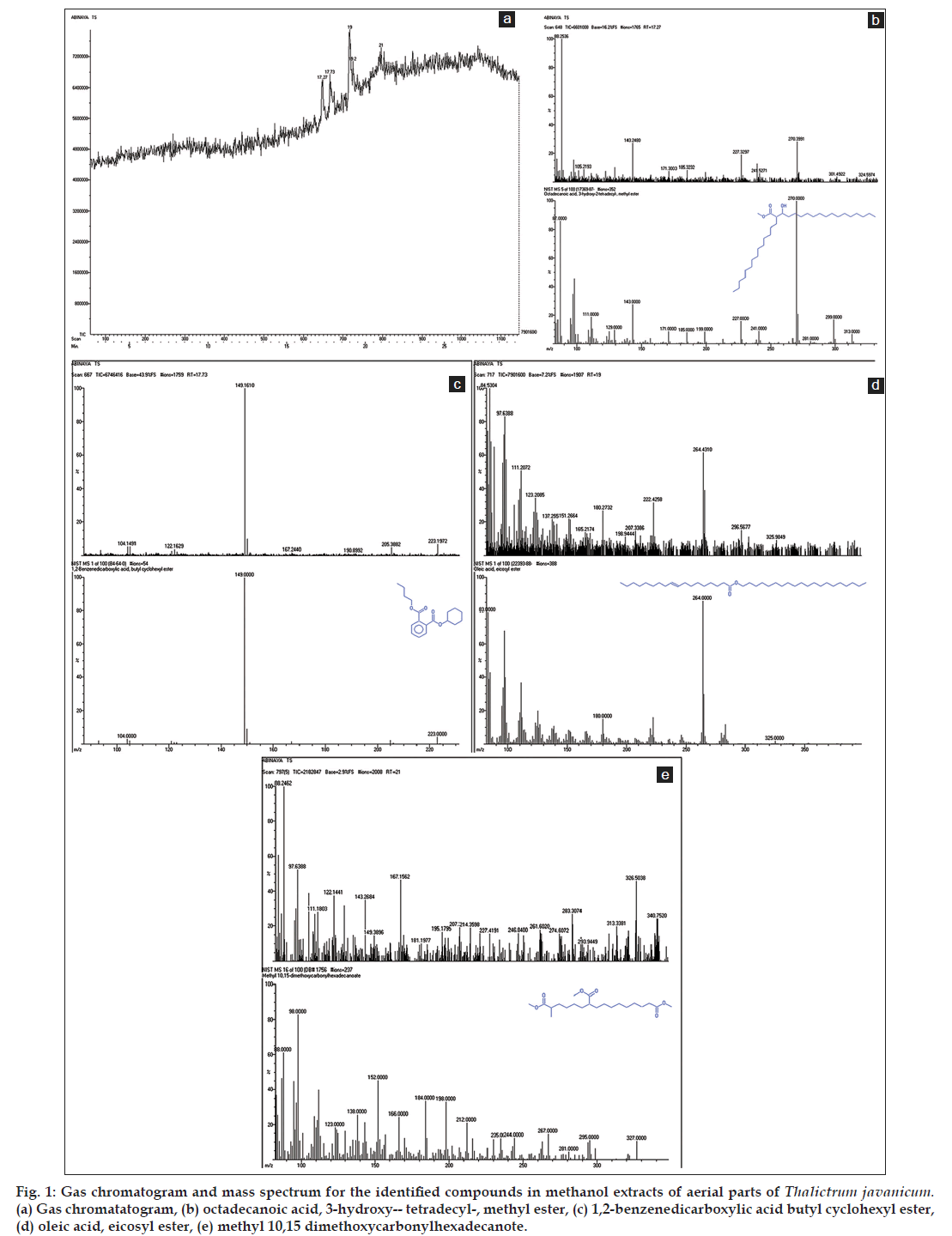
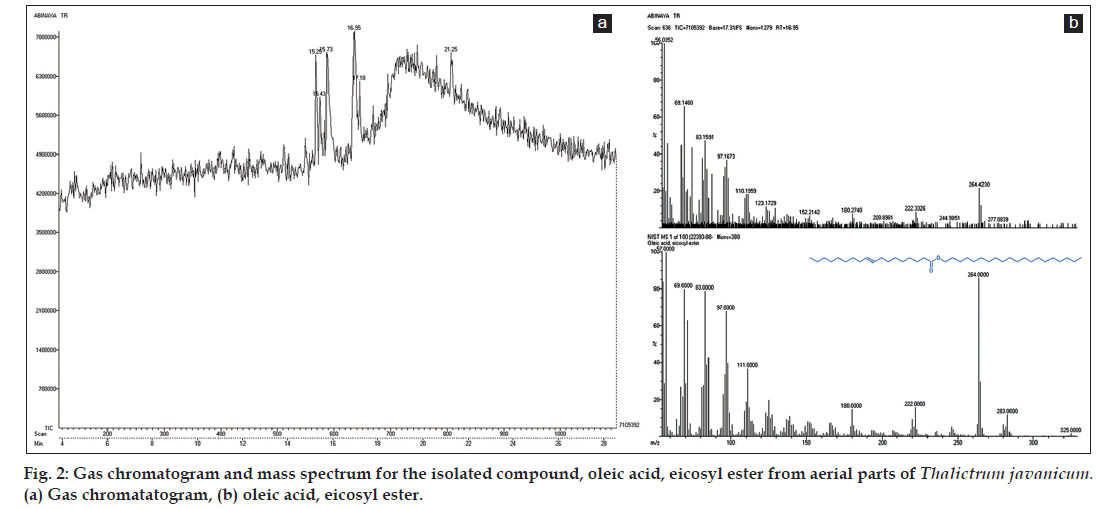
The results by GC-MS analysis lead to the identification of compounds in the crude methanol aerial parts extract and its purified column fractions of T. javanicum. The identified compounds with their retention time, molecular formulae and molecular weight are depicted in Table 1. The prevailing compounds in crude methanol extract were octadecanoic acid, 3-hydroxy-2-tetradecyl-, methyl ester (17.27 min), 1,2-benzenedicarboxylic acid butyl cyclohexyl ester (17.73 min), oleic acid, eicosyl ester (19.00 min) and methyl 10,15-dimethoxycarbonylhexadecanote (21.00 min, fig. 1a-e). The underivatized petroleum ether fraction displayed the presence of single peak in GC (fig. 2a) and it was confirmed as oleic acid, eicosyl ester with the retention time 16.95 min (fig. 2b).
| Name of the compound | Retention time (min) | Molecular formulae | Molecular weight (Dalton) | Nature of the compound | Activity |
|---|---|---|---|---|---|
| Aerial parts Octadecanoic acid, 3‑hydroxy‑ | 17.27 | C33H66O3 | 510.50 | Ester derivatives | Not available |
| 2‑tetradecyl‑, methyl ester | of fatty acids | ||||
| 1,2‑Benzenedicarboxylic acid | 17.73 | C18H24O4 | 304.17 | Ester derivatives | Antimicrobial and antifouling activity |
| butyl cyclohexyl ester | of fatty acids | ||||
| Oleic acid, eicosyl ester | 19.00 | C38H74O2 | 562.57 | Ester derivatives of fatty acids | Insectifuge, antiinflammatory, cancer preventive and hypocholesterolemic |
| Methyl 10,15‑ dimethoxycarbonylhexadecanote Fraction | 21.00 | C21H38O6 | 386.27 | Ester derivatives of fatty acids | Not available |
| Oleic acid, eicosyl ester | 16.95 | C38H74O2 | 562.57 | Ester derivatives of fatty acids | Insectifuge, antiinflammatory, cancer preventive and hypocholesterolemic |
Table 1: Gas Chromatography Mass Spectrometry Analysis Of Extract And Isolated Compound Of Thalictrum Javanicum
PASS prediction
Of the various biological activities predicted by PASS for oleic acid, eicosyl ester, those related with larvicidal property are presented in Table 2. The revealed Pa and Pi values were in the range of 0.969 and 0.701, and 0.001 and 0.022, respectively. The highest larvicidal activity predicted for this compound was related to the inhibition of chitinase (0.900Pa-0.003Pi) and ecdysone 20 monooxygenase (0.803Pa-0.003Pi).
| Pa | Pi | Activity |
|---|---|---|
| 0.900 | 0.003 | Chitinase inhibitor |
| 0.803 | 0.003 | Ecdysone 20‑monooxygenase inhibitor |
Pa: Probability of active, Pi: probability of inactive
Table 2: Best Predicted Insecticidal Activity of Oleic Acid, Eicosyl ester Isolated from Thalictrum Javanicum by Pass Software
Larvicidal activity
Apparently, the isolated compound, oleic acid, eicosyl ester unveiled prominent mortality rate against both the targets viz., Aedes aegypti and Culex quinquefasciatus (LC50 /24 h – 8.51 and 12.50 ppm, respectively) and it was markedly effective than that of the crude methanol extract of aerial parts of Thalictrum javanicum (LC50 /24 h – 257.0 and 281.83 ppm, respectively, Table 3).
| Test samples | Mosquitoes* | LogLC50 | LC50 (ppm) | Fiducidal limit (95%) | Variance | χ2 | |
| LL | UL | ||||||
| Crude methanol extract of aerial | A | 2.410 | 257.030 | 1.536 | 2.743 | 0.094 | 00.75 |
| parts of Thalictrumjavanicm | C | 2.450 | 281.83 | 2.065 | 2.834 | 0.038 | 03.65 |
| Oleic acid, eicosyl ester | A | 0.930 | 8.510 | 0.789 | 1.071 | 0.005 | 06.60 |
| C | 1.100 | 12.50 | 0.690 | 1.510 | 0.044 | 19.80 | |
| Standard, Neemarin | A | 0.016 | 0.470 | 0.070 | 0.112 | 0.002 | 03.69 |
| C | 0.018 | 0.700 | 0.003 | 0.033 | 0.007 | 04.08 | |
*A: Aedes aegypti, C: culex quinquefasciatus, LL: lower limit, UP: upper limit
Table 3: Larvicidal Activity
Chitinase (β-N-acetyl glucosaminidase) activity
Inhibition of β-N-acetyl glucosaminidase activity by oleic acid, eicosyl ester was determined to be more prominent and significantly greater (A. aegypti - 0.179 OD/mg protein/min and C. quinquefasciatus - 0.184 OD/mg protein/min) than the control (Table 4). Further, the inhibition level by this compound was comparable to that of the standard, neemarin (A. aegypti - 0.141 OD/mg protein/min and C. quinquefasciatus - 0.162 OD/mg protein/min).
| Biochemical parameters | Untreated Control | Treated | |||
|---|---|---|---|---|---|
| Aedes aegypti | Culex quinquefasciatus | ||||
| Standard | Oleic acid, eicosyl ester | Standard | Oleic acid, eicosyl ester | ||
| Sample concentration in ppm | - | 0.5 | 18 | 0.8 | 20 |
| β‑N‑acetyl glucosaminidase activity | 0.365 ± 0.002 | 0.141 ± 0.001a | 0.179 ± 0.009a | 0.162 ± 0.005a | 0.184 ± 0.001a |
Values are performed in mean±SD, n=5. Mean values followed by different superscripts in a row are significantly different. P<0.05 as compared with control. SD: Standard deviation
Table 4: Chitinase Activity
Radioimmnuno assay of E20M
The data of RIA exhibited that the ecdysteroidal level after 24 h of incubation was found to be significantly lower (ap<0.05) in the 4th instar larvae of both the mosquitoes Ades aegypti and Culex quinquefasciatus than the control. Further, the levels of this enzyme in both mosquitoe larvae were not varied significantly with that of standard, neemarin (Table 5).
| Time (h) | Quantity of ecdysone (pg/20 HE/min/abdomen equivalent) | ||||
|---|---|---|---|---|---|
| Untreated Control | Treated | ||||
| Aedes aegypti | Culex quinquefasciatus | ||||
| Standard | Oleic acid, eicosyl ester | Standard | Oleic acid, eicosyl ester | ||
| 0 | 2.0 ± 0.07 | 1.0 ± 0.06* | 0.92 ± 0.05* | 1.3 ± 0.02* | 0.85 ± 0.03* |
| 12 | 16.5 ± 0.02 | 10.3 ± 0.01* | 11.4 ± 0.02* | 10.7 ± 0.003* | 12.1 ± 0.02* |
| 24 | 29.3 ± 0.06 | 17.2 ± 0.02* | 18.4 ± 0.004* | 18.8 ± 0.00* | 19.6 ± 0.01* |
Values are performed in mean±SD, n=5. Mean values followed by different superscripts in a row are significantly different. P<0.05 as compared with control. SD: Standard deviation
Table 5: Radioimmuno Assasy
Discussion
Chemical profiling of medicinal plants through various techniques is employed to isolate and identifying several bioactive compounds responsible for curing many dreadful diseases. In the present study, GC-MS confirmed the occurrence of four compounds in crude methanol extract of aerial parts, and one compound, oleic acid, eicosyl ester in column isolated fraction of the study species, Thalictrum javanicum (Table 1 and fig. 2a-b). Among the four compounds in the crude methanol extract, 1,2-benzenedicarboxylic acid butyl cyclohexyl ester is reported to have antimicrobial and antifouling activities [22] and oleic acid, eicosyl ester (also exists in fraction) has the property of insectifuge, antiinflammatory, cancer preventive and hypocholesterolemic [23].
In order to accelerate the search for potent bioactive property, computer aided drug discovery program i.e., Prediction Activity Spectra for Substance (PASS) was used to predict the biological activity of isolated compounds in recent periods. PASS tools are established using 20 000 principle compounds [24] and about 4000 kinds of biological activities based on the structural formula with mean accuracy about 90% [25]. The predicted biological activities with Pa>Pi are considered as possible for a particular compound and if Pa>0.7, the chance to find the activity experimentally will be high. A total number of 110 biological activities were predicted by PASS software (Pa- 0.969 to 0.701 and Pi- 0.001 to 0.022) and for the sake of brevity, only the insecticidal activity are presented (Table 2). Interestingly, the predicted biological activity of oleic acid, eicosyl ester unveiled the plausible larvicidal activity by the presence of certain inhibitors of chitinase and ecdysone-20 monooxygenase. The sugar phosphotase regulates the methyl erythritol phosphate (MEP) pathway in malarial parasite, Plasmodium falciparum [26]. Chitinase enzyme is essential for insect growth and morphogenesis and it is found in molting fluid that digest the main constituent of the endocuticle [27]. Ecdysone-20 monooxygenase is found to be critical to all stages of insect development [28]. Thus the traditional usage of this species Thalictrum javanicum by Thoda tribal community in Nilgiris of Western Ghats, India for mosquitosidal property is proved by PASS software. The experimental work undertaken in this study also confirmed the mosquito larvicidal activity of crude extract and isolated the compound, oleic acid, eicosyl ester from methanol extract of T. javanicum as detailed under.
Mosquito control at the larval stage is an effective practice for controlling the mosquito born diseases [29] as the larval stage has low mobility. Environmental safety by using the insecticides is of first and foremost criterian for mosquito control programmes [30]. Plant extracts have promising larvicidal efficacies owing to the vast repository of bioactive organic chemicals present in them which posses more beneficial effects over synthetic insecticides and less toxic to environment [31]. The compound, oleic acid, ecicosyl ester from T. javanicum acts as good control agent than the crude methanol extract against the dengue vector, Aedes aegypti (4th instar) (LC50/24h- 8.51 ppm). This bioefficacy against the larvae may be attributed to the dechitinizing effect of body wall and inhibition of ecdysone 20 monooxygenase, an enzyme required to promote cell membrane development in insects. Further, in comparision to the mosquito, Culex quinquefasciatus, the oleic acid, eicosyl ester has promising larvicidal activity against the 4th instar larvae of Aedes aegypti.
Insect chitin is found in the exoskeleton, respiratory tracheal system and peritrophic matrix that can be potential target substrate for intestinal pathogens. It was demonstrated that degradation of chitin in the peritophic matrix (PM) by a pathogen-encoded chitinolytic allowed an avian malaria parasite to overcome its mosquito vector intestinal PM barrier. The chitin is the target substrate for the mosquitocidal toxin and leads to the degradation of peritrophic membrane and thereby supports the proposed mode of action for mosquitocidal metabolites [32]. In the present study, the mosquitocidal toxin oleic acid, eicosyl ester sufficiently hydrolyze the cuticular protein, chitin evidenced by significantly low levels of chitinase (β-N-acetyl glucosaminidase) in both mosquito species (Table 4) as well as the peritrophic membrane which is a protective sleeve for the midgut epithelium of mosquito species and binds to the gut regions of larvae of Aedes aegypti and Culex quinquefasciatus. Subsequently, oleic acid, eicosyl ester ingested larvae of both the mosquitoes were died due to swelling of mitochondria, endoplasmic reticulum and enlargement of vacuoles, followed by lysis of epithelial cells and midgut perforation [32].
Growth and development in insects, which are punctuated by periods of molting are regulated by the steroid, 20 hydroxy ecdysone [33]. In the adult stage, this hormone also involves in the regulation of reproduction maturation [34]. The molting process in insect is initiated by an increase in the titer of 20 E (20-hydroxyecdysone). In larval stage, it undergoes a larval molt and stops feeding. In that stage, digestion of the old cuticle is increasing with the increase in 20E titer [35]. In the present study, the compound, oleic acid, eicosyl ester showed significant reduction in quantity of ecdysone on treated mosquitoes of both Aedes aegypti and Culex quinquefasciatus (ap<0.05, Table 5). This minimal effect obviously unveiled the toxic nature of the compound by arresting digestion of cuticle which is more important for successful completion of molt.
Based on the above results, the isolated compound, oleic acid, eicosyl ester from the aerial parts Thalictrum javanicum has shown greater larvicidal activity against 4th instar larvae of both the mosquito species, Aedes aegypti and Culex quinquefasciatus than the crude methanol extract of the species. Findings from chitinase and E20M assay evinced that the compound, oleic acid, eicosyl ester plays a significant role in the degaradation of cuticular region of both A. aegypti and C. quinquefasciatus. Therefore, the use of this compound from Thalictrum javanicum for larvicidal property offers a safer alternative method against synthetic chemical insecticides. However, conducting toxicological assessment of this compound to ascertain its safety on human is most needed before going for commercial preparations of drug.
Acknowledgements
The authors are thankful to Directorate of Collegiate Education, Government of Tamil Nadu, Chennai for providing financial support to carry out this research (RC. No. 8760/K2/2014 dt. 13.09.2014).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Service MW. Management of vector. In: Youdeowei A, Service N, editors. Pest and Vector Management in the Tropics. England: Longman Ltd.; 1983. p. 7-20.
- Das MK, Ansari MA. Evaluation of repellent action of Cymbopoganmartinii martinii Stapfvarsofia oil against Anopheles sundaicus intribal villages of Car Nicobar Island, Andaman and Nicobar Islands, India. J Vector Borne Dis 2003;40:100-4.
- Udonsi JK. The status of human filariasis in relation to clinical signs in endemic areas of the Niger Delta. Ann Trop Med Parasitol 1986;80:425-32.
- Samuel T, Jayakumar M, William SJ. Culex mosquito: An overview. In: William SJ, editors. Defeating the Public Enemy, the Mosquito: A Real Challenge. Chennai: Loyola Publications; 2007. p. 95-116.
- Abraham Z. Ethonobotany of todas, the kotas and irulars of the Nilgiris. In: Jain SK, editor. Glimpses of Indian Ethnobotany. New Delhi: Oxford and IBH Publications; 1981. p. 308-10.
- Venkatachalapathi A, Sangeeth T, Paulsamy S. Ethnobotanicalinformations on the species of selected areas in Nilgiri biosphere reserve, the Western Ghats, India. J Res Bio 2015;5:43-57.
- Umberto QFLS. CRC world dictionary of medicinal and poisonous plants: Common names, scientific names, eponyms, synonyms and tymology. Vol. 5. Boca Raton, Florida: Taylor and Francis Group; 2012.
- Bagby MO, Smith CR Jr., Mikolajczak KL, Wolff IA. Thalictrumpolycarpumfatty acids – A new class of fatty acids from vegetableseed oils. Biochemistry 1962;1:632-9.
- Markman AL, Freiman RE. The oil of Thalictrum simplex.Chem Nat Comp 1965;1:96-9.
- Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K, Bagavan A. Mosquito larvicidal activity of isolated compounds from the rhizome of Zingiberofficinale. Phytother Res 2008;22:1035-9.
- Reid RG, Sarker SD. Isolation of natural products by low-pressure column chromatography. Methods MolBiol 2012;864:155-87.
- Suo MR, Yang JS. Survey in studies on chemical constituents of sesquiterpene and their physic ological activities in plants of Helianthus L. Chin Tradit Herb Drugs 2006;37:135-40.
- Guido F, Pier LC, Ivano M, Ammar B. Essential oils of the aerial parts of three Salvia species from Jordan: Salvia lanigera, S. spinosa and S.syriaca. Food Chem 2007;100:732-5.
- Gloriozova TA, Filimonov DA, Lagunin A, Poroikov V. Evaluation of computer system for prediction of biological activity PASS on the set of new chemical compounds. Pharm Chem J 1998;32:8-17.
- Poroikov V, Akimov D, Shabelnikova E, Filimonov D. Top 200 medicines: Can new actions be discovered through computer-aided prediction? SAR QSAR Environ Res 2001;12:327-44.
- WHO. Guidelines for Laboratory and Field testing of Mosquito Larvicides. Geneva: WHO; 2005. p. 10-12.
- Finney DJ. In Probit Analysis. London: Cambridge University Press; 1971. p. 68-78.
- Dziadik-Turner C, Koga D, Mai MS, Kramer KJ. Purification and characteristics of two β-N-acetylhexosaminidases from the tobacco hornworm, Manducasexta(L.) (Lepidoptera: Sphingidae). Arch Biochem Biophys 1981;212:546-60.
- Nagamatsu Y, Yanagisawa I, Kimoto M, Okamoto E, Koga D. Purification of a chitooligosaccharidolytic β-N-acetylglucosaminidasefrom Bombyxmori larvae during metamorphosis and the nucleotide sequence of its cDNA.Biosci Biotechnol Biochem 1995;59:219-25.
- Borst DW, O’connor JD. Arthropod molting hormone: Radioimmune assay. Science 1972;178:418-9.
- Borovsky D, Thomas BR, Carlson DA, Whilsenton LR, Fuchs MS. Juvenile harmone and 20 hydroxyecdysone as primary and secondary stimuli to vitellogenesis in Aedesaegypti. Arch Insect BiochemPhysiol 1985;2:75-90.
- Gnanavel V, SaralAM. GC-MS analysis of petroleum ether and ethanol leaf extracts from Abrusprecatorius Linn. Int J Pharm Bio Sci 2013;4:37-44.
- Sheela V, Uthayakumari F. GC-MS analysis of bioactive constituents from coastal sand dune taxon – Sesuviumportulacastrum(L.). BiosciDiscov 2013;4:47-53.
- Lagunin A, Stepanchikova A, Filimonov D, Poroikov V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000;16:747-8.
- Av P. Computer assisted mechanism of action analysis of large data bases, including 250, 000 open NCI database compounds. Plant Resour 1998;34:61-4.
- Guggisberg AM, Park J, Edwards RL, Kelly ML, Hodge DM, Tolia NH, et al. A sugar phosphatase regulates the methylerythritolphosphate (MEP) pathway in malaria parasites. Nat Commun 2014;5:4467.
- Reynolds SE, Samuels RI. Physiology and biochemistry of insect moulting fluid. Adv Insect Physiol 1996;26:157-232.
- Drummond C, Smith S. Ecdysone 20-monooxygenase activity during embryogenesis of the tobacco hornworm, Man- ducasexta. Curr Top Steroid Res 2012;9:67-72.
- Nandita C, Subrata L, Goutam C. Mosquito larvicidal and antimicrobial activity of protein of Solanumvillosum leaves. BMC Complement Altern Med 2008;8:62.
- Ghnimi W, Dicko A, Khouja ML, El FO. Larvicidal activity, phytochemical composition, and antioxidant properties of different parts of five populations of Ricinus communis. Ind Crops Prod 2014;56:43-51.
- Anupam G, Nandita C, Goutam C. Plant extracts as potential mosquito larvicides. Indian J Med Res 2012;135:581-98.
- Usharani B, Kummankottil P. Chitinase like activity of metabolites of Pseudomonas fluorescens Migula on immature stages of the mosquito, Culex quinquefasciatus(Diptera:Culicidae). Afr J Microbiol Res 2012;6:2718-26.
- Boudjelida H, Bouaziz A, Soin T, Smagghe G, Soltani N. Effect of ecdysone agonist halofenozide against Culexpipiens. Pestic Biochem Physiol 2005;83:115-23.
- Pak MD, Gilbert LI. A developmental analysis of ecdysteroids during the metamorphosis of Drosophila melanogaster. J LiqChromatogr 1987;10:2591-611.
- Dhadialla TS, Carlson GR, Le DP. New insecticides with ecdysteroidal and juvenile hormone.Annu Rev Entomol 1998;43:545-69.