- *Corresponding Author:
- S. D. Jagtap
Department of Herbal Medicine, Interactive Research School for Health Affairs (IRSHA), Bharati Vidyapeeth (Deemed to be University), Pune, Maharashtra 411043, India
E-mail: chiritatml@rediffmail.com
| Date of Received | 01 September 2022 |
| Date of Revision | 13 June 2024 |
| Date of Acceptance | 25 October 2024 |
| Indian J Pharm Sci 2024;86(5):1865-1871 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To assess the comparative potential, the immunomodulatory activity of Embelia ribes and Embelia tsjeriam-cottam species was evaluated by checking humoral and cell-mediated immune responses by sheep red blood cells induced antigen challenges, neutrophil adhesion tests on female Wistar rats were studied using ethanol extracts. Embelia ribes and Embelia tsjeriam-cottam were collected from village Kemse in the Koyna area of Sahyadri Hills of Maharashtra. All the experimental protocols for in vivo study were carried which were approved by the animal ethical committee. The animals were studied for their relative organ weight, haematological parameters, serum biochemical parameters, neutrophil adhesion test, delayed type of hypersensitivity response, humoral immune response, and histopathology analysis using ethanolic extracts. According to findings, Embelia species can enhance both cellular and humoral immunity, indicating that it has immunomodulatory abilities. At dosages 100 and 200 mg/kg of Embelia ribes and Embelia tsjeriam-cottam no significant change was observed in relative organ weight as compared to healthy control. In haematological studies, both extracts could maintain the range of white blood cells, lymphocytes, red blood cells, and haemoglobin as compared to the group of healthy control. Moreover, these extracts at a dosage of 200 mg/kg significantly decreased the levels of serum glutamic pyruvic transaminase while significantly maintaining serum glutamic oxaloacetic transaminase levels within the range when compared to the negative control group. The treatment with Embelia ribes and Embelia tsjeriam-cottam increased neutrophil adherence to nylon fibers. While appreciable immunomodulatory activity by compelling humoral and cellular immunity to the antigenic challenges with sheep red blood cells was observed by both extracts. Hence, it can be concluded that Embelia ribes and Embelia tsjeriam-cottam have exhibited significant immunomodulatory action by proving humoral and cellular immunity to antigenic challenges with sheep red blood cells and neutrophil adhesion tests on female Wistar rats. Therefore, both species of fruit extracts can be used as complementary therapeutic agents. Moreover, present investigations suggest that Embelia tsjeriam-cottam can be employed in place of Embelia ribes in immunomodulatory formulations.
Keywords
Vidanga Embelia ribes, Embelia tsjeriam-cottam, myrsinaceae, immunomodulation
The immune response is a highly complex defence mechanism forthe pathophysiology of many diseases. Aggressive and advanced techniques are required to develop new therapies to treat immunological diseases. These diseases include various asthma, autoimmune diseases, blood-related issues, exposure totuberculosis, inflammatory bowel diseases, immunodeficiency, neutrophil defects, rheumatoid arthritis, type I diabetes mellitus, ulcers, various allergic conditions[1].
Recently, plants have been studied for their immunomodulating properties to achieve the desired impact on disease prevention. Accordingly, herbal remedies have been utilized for centuries for safety, effectiveness, lesser side effects, easy affordability, and acceptance[2].
Previously studies have shown various medications derived from natural sources, either herbal or mineral, have been employed to influence human behaviour immune system[3]. Ayurvedic concepts of preventive health care with non-specific immune stimulation by medicinal herbs such as Sirisha, Agastya, Haridra, Haritaki Salaparni, Arjuna, Pushkaramula, Bakuchi, Vidanga, Shatavari, Guggulu, Bhallataka and Pippali, and many moreexample, help with nutrition by enhancing digestive and metabolic processes, protecting skin disease, keeping the respiratory and circulating system healthy, powerful immune promoting substance, etc[4].
Under the name ‘Vidanga’ two species viz. Embelia ribes (E. ribes) Burm., and Embelia tsjeriam-cottam (Roem. & Schult) A. DC., are of the family Myrsinaceae. The fruits, including as antioxidants, polysaccharides, terpenoids, and flavonoids, are known to influence immunological state and are employed for immunomodulation in Integrative and Ayurvedic Medicine, which has been the subject of current studies[5]. Recently Jagtap et al.[6] reported that E. ribes and Embelia tsjeriam-cottam exhibited the highest free radical scavenging potential[6]. Moreover, they have high demand due to their many applications and powerful Rasayana benefits for the healthy immune system.
As both species are marketed under the same name, the purpose of this study was to compare the immunomodulatory ability of these two species in ethanolic extracts on humoral and cellular immune responses to an antigenic challenging task by Sheep Red Blood Cells (SRBC), with efficacy measured using serological, hematological, and vital organs.
Materials and Methods
Plant material collection and extraction:
E. ribes and Embelia tsjeriam-cottam were collected from village Kemse from the Koyna area of Sahyadri Hills of Maharashtra with prior permission from Maharashtra State Biodiversity Board (No: MSBB/Research/Desh-5/811/2021-22). The plants were identified and authenticated by Dr. Suresh Jagtap, Taxonomist and Associate Professor at Bharati Vidyapeeth Deemed to be University (BVDU)-Interactive Research School for Health Affairs (IRSHA), Pune, India. The voucher specimen has been deposited at Herbaria of Medicinal Plants Conservation Centre, Pune (MPCC1526, MPCC2743). Fruits were shed dried and pulverized to a fine powder in a mechanical blender. These fine powders were utilized for further experimental purposes. Extracts were prepared by using the hot extraction method using a Soxhlet device (Rotamantal, Remi) and ethanol as a solvent, at 60°-80° for 24 h[7]. As a result, these extracts were evaporated using rotary evaporator at 45° under reduced pressure (IKA RV 10). All dried-out extracts were kept at 4° until they were needed.
Animals used in research:
Female Wistar rats were used in the current research experiment (150-250 g, 6 w old). The animals were kept in regular husbandry settings of 25°±2° and fed a standard pellet diet and ad libitum tap water. All the animals were obtained from Pavo Research Solution in Gujarat, India, and were cared for and used following international guidelines for the care and use of laboratory animals. The Organisation for Economic Co-operation and Development (OECD) Principles of Good Laboratory Practice (ENV/MC/CHEM (98)17 (as revised in 1997)) were followed in this work and were approved by the OECD Council on November 26th, 1997 (C (97)186/Final). The Animal Ethical Committee of PAVO Research Solutions, 78, Brushellz Industrial Park, Gujarat, India, accepted the study (PAVO/IAEC/2021/02/009).
Antigen preparation for SRBCs:
To avoid coagulation, sheep blood was taken aseptically from a city butcher and placed in sterile Alsever’s solution (1:1). To allow red blood cells to settle at the bottom of the test tube, Red Blood Cells (RBCs) were cleaned repeatedly by vigorously mixing and centrifuging at 2000 rpm for 10 min. The supernatant was decanted, and the centrifugation process was continued 4-5 times till the supernatant fluid became clear. The obtained SRBCs were resuspended in saline and adjusted to the concentration required for immunization and antigen challenge[8,9].
Treatment groups:
The animals (female Wistar rats) were separated into seven groups, with each plant extract having two effective concentrations[10-12], respectively 100 mg/kg and 200 mg/kg body weight, as well as healthy control, negative control, and positive control group, each with six (n=6) rats. After acclimatization, all animals were randomized depending on their body weight. A total of 42 animals were randomly assigned to seven groups; healthy control-normal saline; negative control-SRBC i.p (intraperitoneal); positive control (prednisolone) dose-10 mg/kg body weight; E. ribesdose-test drug 100 and 200 mg/kg body weight with 0.4 ml of 5×109 SRBC i.p and Embelia tsjeriam-cottam dose-test drug 100 and 200 mg/kg body weight with 0.4 ml of 5×109 SRBC i.p.
Haematological parameters:
Blood samples were taken by cardiac puncture in Ethylenediaminetetraacetic Acid (EDTA) coated heparinized vials to assess haematological parameters using an auto-analyser (Selectra PRO S). Total RBC count, total White Blood Cell (WBC) count, differential WBC count, and lymphocyte count were all evaluated as haematological parameters. Haemoglobin, which is measured in gm percent, was measured in whole blood.
Serum biochemical parameters:
The biuret method was used to assess serum total protein, albumin to globulin ratio, Serum Glutamic Pyruvic Transaminase (SGPT), and Serum Glutamic Oxaloacetic Transaminase (SGOT)[13]. Separation of the serum was carried out by centrifugation at 3000 rpm at 4° for 10 min. The levels of aminotransferase enzyme were determined using the references provided in the kit literature, which included the methodology used to conduct the tests.
Neutrophil adhesion test:
On the 18th d, blood samples were collected by heart puncture in EDTA-coated collecting vials after intraperitoneal delivery of SRBCs on 0 d. The Total Leukocyte Count (TLC) and Differential Leukocyte Count (DLC) of the blood samples were determined. Following the initial count, the tubes were incubated at 37o for 15 min with 80 mg/ml nylon fibers. TLC and DLC were determined once more on these incubated samples[14]. TLC of a blood sample yields the Neutrophil Index (NI), which is expressed as a percentage of neutrophils.
The formula was used to calculate the percent neutrophil adherence.
Percent neutrophil adhesion=[NIu-NIt/NIu]
Where;
NIu is the neutrophil index of a blood sample that has not been treated and NIt is the neutrophil index of a blood sample that has been incubated with nylon fibre.
Delayed Type of Hypersensitivity (DTH) response:
On the 7th d, treated rats were again sensitized subcutaneously in the right hind footpad with 0.1 ml of 1.25×109 cells (SRBC)/ml[15]. The healthy control group, on the other hand, was given the same amount of saline as the healthy group. A paleothermometer (Orchid Scientific, PLM 01 PLUS, India) was used to measure swelling edema in footpad thickness 24 h after sensitization. The difference in footpad thickness before and after the challenge was used to determine DTH response.
Humoral Immune (HI) response:
The humoral immune response was assessed by using aHemagglutination Antibody (HA) titre assay, which used the Ismail and Asad" with "Ismail and Asad (2009) technique to titrate serum against SRBCs. 0.1 ml sterile normal saline was used to fill the microtiter plate, and repeated two-fold dilutions of the serum in sterile saline solution were made. The titre value was determined by titrating each microtiter well with 0.1 ml of 1.25×109 cells of 3X saline-washed SRBCs. The plate was left at room temperature overnight until the control wells displayed a negative pattern (small button formation). As an antibody titre, the minimal volume of serum required to produce hemagglutination was calculated and transformed to log 2 values for simple comparison[8,16,17].
Histopathology analysis:
On 8 d, an overdose of Carbon dioxide (CO2) was used to euthanize all of the rats. All obtained rats were observed for external and internal gross pathology. The thymus, spleen, liver, and kidney were taken and histopathologically examined in 10 % Neutral phosphate-Buffered Formalin (NBF). All preserved important organs/tissue samples from all groups, including the liver, kidneys, spleen, and thymus, were processed and embedded in paraffin. Hematoxylin and eosin stain wereused to stain slices of 3-5 mm thickness. Histopathology examination of all the organs was carried out by a board-certified toxicopathologist. After that, slides were created for observation at a microscopic level.
Statistical analysis:
Graph Pad Prism was used to do the statistical analysis. The data on body weight, body weight change, hematological, clinical biochemistry, organ weight, and other variables were all subjected to a parametric one-way Analysis of Variance (ANOVA). Dunnett's test was performed to compare the test item treatment group to the control group if ANOVA revealed statistical significance (p<0.05).
Results and Discussion
Effect of Vidanga species i.e. E. ribes and Embelia tsjeriam-cottam at both concentrations show relative organ weight (Table 1). When E. ribes was administered at a dosage of 100 mg/kg, it was found that there was no change in the relative body weight, but that a dose of 200 mg/kg significantly increased the weight of the organs i.e., thymus, spleen, and kidney when compared to the negative control. Whereas, the weight of the liver, thymus, and spleen increased somewhat in response to Embelia tsjeriam-cottam at doses of 100 mg/kg and 200 mg/kg, respectively, compared to the negative control.
| Group | Weight of the organs (in grams) | |||
|---|---|---|---|---|
| Liver | Thymus | Spleen | Kidney | |
| HC | 4.48±0.14 | 0.19±0.02 | 0.41±0.03 | 1.33±0.02 |
| NC | 3.90±0.14 | 0.24±0.00 | 0.37±0.02 | 1.28±0.02 |
| Pred | 3.97±0.09 | 0.24±0.01 | 0.40±0.01 | 1.30±0.01 |
| Er100 | 4.07±0.06 | 0.24±0.02 | 0.41±0.00 | 1.33±0.03 |
| Er200 | 4.16±0.07 | 0.25±0.01** | 0.43±0.01** | 1.33±0.03** |
| Et100 | 4.20±0.04** | 0.25±0.02** | 0.44±0.01** | 1.31±0.02 |
| Et200 | 4.20±0.02** | 0.24±0.02 | 0.43±0.00** | 1.35±0.03** |
Note: Values are expressed as mean±SEM; n=6. *p<0.05; **p<0.01 and ***p<0.001 compared to NC Data were analysed using one-way ANOVA followed by Dunnett's multiple comparison test HC: Healthy control; NC: Negative control; Pred: Prednisolone; E. ribes 100 mg/kg, 200 mg/kg and Embelia tsjeriam-cottam 100 mg/kg, 200 mg/kg
Table 1: Relative Organ Weight
Immune variation can lower the count of WBCs, lymphocytes, RBC, and haemoglobin content when treated with prednisolone and a negative control group. While groups treated with E. ribes and Embelia tsjeriam-cottam 100 mg/kg, 200 mg/kg maintain the range of WBCs, lymphocytes, RBC, and haemoglobin as compared to the group of healthy controls (Table 2).
| Groups | Total leukocyte count | Differential WBC (%) | Hb (gm %) | ||||
|---|---|---|---|---|---|---|---|
| WBCs (×103/cmm) | Total RBCs (×106/cmm) | Neutrophil | Lymphocyte | Eosinophil | Monocyte | ||
| HC | 14.65±0.40 | 8.12±0.08 | 14.17±1.11 | 83.33± 1.15 | 1.67±0.21 | 0.83±0.17 | 15.28±0.26 |
| NC | 16.27±0.25 | 8.16±0.18 | 13.17±0.31 | 84.50±0.22 | 2.00±0.26 | 0.50±0.22 | 15.08±0.29 |
| Pred | 15.90±0.32 | 8.11±0.26 | 13.33±0.67 | 82.83±0.40 | 1.83±0.17 | 0.67±0.08 | 15.5±0.50 |
| Er100 | 16.60±0.52 | 7.90±0.26 | 12.67±0.21 | 83.23±0.44 | 1.84±0.08 | 0.80±0.05 | 16.75±0.20 |
| Er200 | 17.47±0.63 | 8.18±0.24 | 14.67±0.33 | 83.98±0.42 | 2.16±0.17 | 0.33±0.21 | 15.67±0.35 |
| Et100 | 15.80±0.48 | 8.97±0.17 | 14.23±0.41 | 84.83±0.48 | 1.67±0.21 | 0.67±0.21 | 15.68±0.25 |
| Et200 | 16.88±0.45 | 7.96±0.26 | 13.08±0.27 | 84.33±0.61 | 2.00±0.00 | 0.80±0.05 | 16.03±0.40 |
Note: Values are expressed as mean±SEM; n=6. p<0.05 compared to NC Data were analysed using one-way ANOVA followed by Dunnett's multiple comparison test HC: Healthy control; NC: Negative Control; Pred: Prednisolone; E. ribes 100 mg/kg, 200 mg/kg and Embelia tsjeriam-cottam 100 mg/kg, 200 mg/kg
Table 2: Effect of E. ribes and Embelia tsjerium-cottam with Vidanga Extracts on WBCS, RBCS, Lymphocytes and Hemoglobin
E. ribes and Embelia tsjeriam-cottam extract administration significantly reduced liver enzymes. These extracts at a dosage of 200 mg/kg significantly decreased the levels of SGPT and SGOT while significantly maintaining SGOT levels within the range when compared to the negative control group (Table 3). Total serum proteins, albumin, and globin ratio are shown in Table 3. In comparison to the SRBC-treated group, all the extracts show a non-significant rise in total serum protein, although the A/G ratio shows a non-significant increase but when compared to the negative group, is not statistically significant.
| Group | SGPT (U/ml) | SGOT (U/ml) | Total serum protein (g/dl) | A/G ratio |
|---|---|---|---|---|
| HC | 77.33±0.54 | 148.23±0.39 | 7.25±0.12 | 0.43±0.15 |
| NC | 86.27±2.66 | 177.25±0.68 | 7.48±0.14 | 7.47±0.35 |
| Pred | 70.77±1.16 | 147.45±1.34 | 7.24±0.07 | 4.75±0.13 |
| Er100 | 75.93±0.62*** | 148.95±1.17*** | 7.28±0.06 | 5.07±0.08* |
| Er200 | 67.23±0.47 | 145.12±0.29 | 7.62±0.18*** | 4.82±0.20 |
| Et100 | 74.32±0.39*** | 147.83±1.18*** | 7.35±0.08 | 4.80±0.14 |
| Et200 | 67.70±0.81 | 146.25±0.46 | 7.90±0.09* | 5.13±1.18*** |
Note: Values are expressed as mean±SEM; n=6. *p<0.05; **p<0.01 and ***p<0.001 compared to NC. Data were analysed using one-way ANOVA followed by Dunnett's multiple comparison test HC: Healthy Control; NC: Negative Control; Pred: Prednisolone; Embelia ribes 100 mg/kg, 200 mg/kg and Embelia tsjeriam-cottam 100 mg/kg, 200 mg/kg
Table 3: Effect of Vidanga Extracts on Aminotransferase Level, Total Serum Protein and A/G Ratio
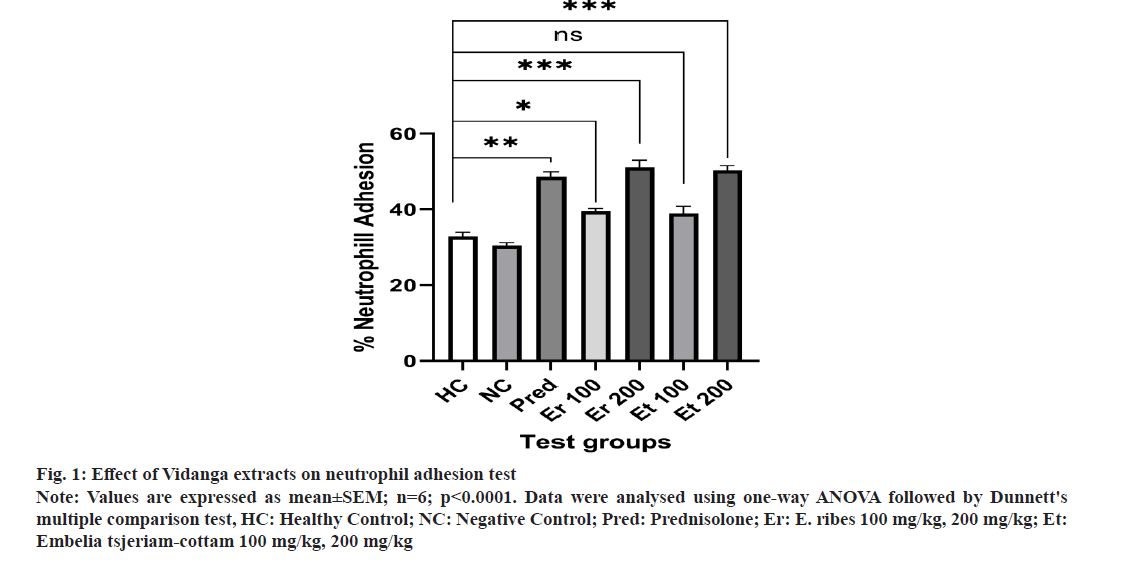
The percentage of neutrophils decreased when neutrophils were cultured with nylon fibers because they adhered to the fibers. When compared to the negative control group, using plant extracts at dosages of 100 mg/kg and 200 mg/kg significantly increased neutrophil adhesion. As a result, it was concluded that the treatment with E. ribes and Embelia tsjeriam-cottam increased neutrophil adherence to nylon fibers, which is related to the process by which cells in blood vessels marginate toward the site of inflammation (fig. 1).
Fig 1: Effect of Vidanga extracts on neutrophil adhesion test
Note: Values are expressed as mean±SEM; n=6; p<0.0001. Data were analysed using one-way ANOVA followed by Dunnett's multiple comparison test, HC: Healthy Control; NC: Negative Control; Pred: Prednisolone; Er: E. ribes 100 mg/kg, 200 mg/kg; Et: Embelia tsjeriam-cottam 100 mg/kg, 200 mg/kg
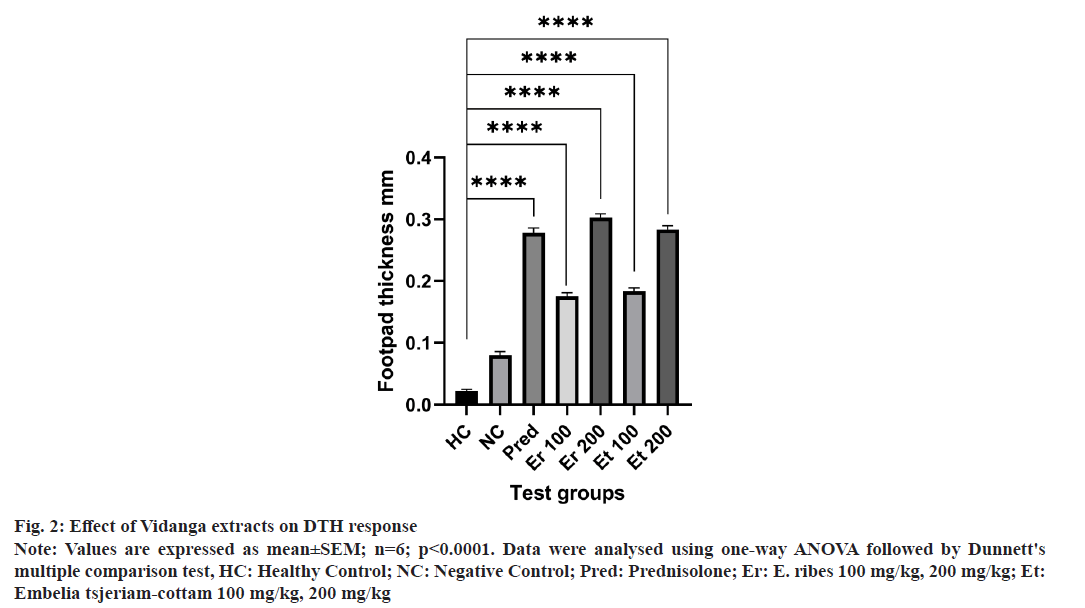
Increased DTH response is a sign that the extract has stimulated the lymphocytes and accessory cell types needed for the reaction’s manifestation. The fig. 2 illustrates that the DTH response for the negative and conventional drug-treated groups had a variable time course. Extracts of E. ribesand Embelia tsjeriam-cottam at the dosage of 200 mg/kg generated a considerable DTH response than the animals used in negative controls.
Fig 2: Effect of Vidanga extracts on DTH response
Note: Values are expressed as mean±SEM; n=6; p<0.0001. Data were analysed using one-way ANOVA followed by Dunnett's multiple comparison test, HC: Healthy Control; NC: Negative Control; Pred: Prednisolone; Er: E. ribes 100 mg/kg, 200 mg/kg; Et: Embelia tsjeriam-cottam 100 mg/kg, 200 mg/kg
Enhancing DHT reaction extracts of E. ribes and Embelia tsjeriam-cottam show the immunostimulatory effect that is observed by the thicker footpads in comparison to the negative control group.
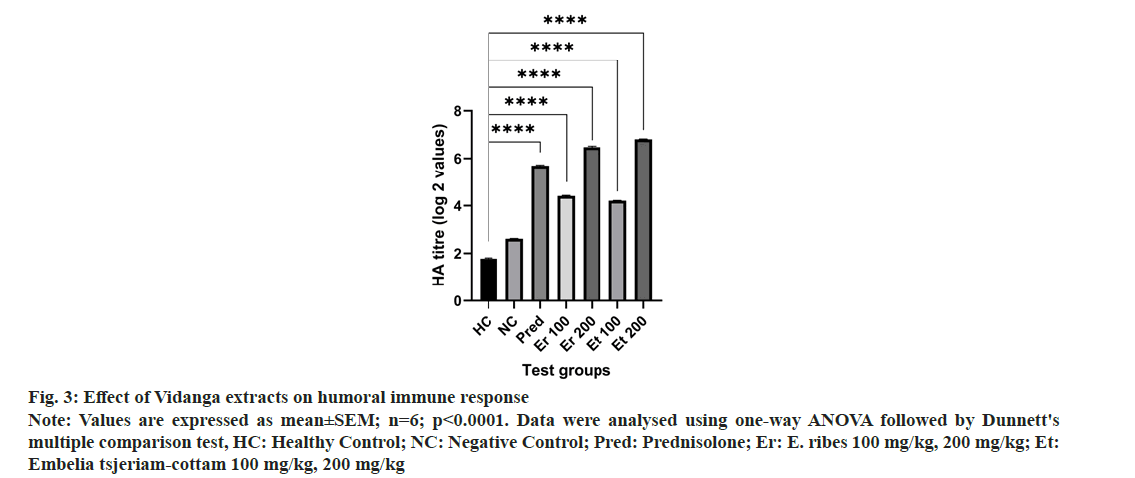
In the recent study, E. ribes and Embelia tsjeriam-cottam extract considerably increased the anti-SRBC antibody titer compared to the negative control group, indicating improved T and B lymphocyte subsets responsiveness. The indirect hemagglutination titer value was taken into consideration to confirm the impact of medication therapy on the humoral arm of the immune system. In fig. 3, treated with all dosages of E. ribes and Embelia tsjeriam-cottam, a significant rise in the titer values of circulating antibodies was observed. E. ribes and Embelia tsjeriam-cottam at 100 mg/kg, 200 mg/kg, and antibody production was considerably increased when compared to the negative control group.
Fig 3: Effect of Vidanga extracts on humoral immune response
Note: Values are expressed as mean±SEM; n=6; p<0.0001. Data were analysed using one-way ANOVA followed by Dunnett's multiple comparison test, HC: Healthy Control; NC: Negative Control; Pred: Prednisolone; Er: E. ribes 100 mg/kg, 200 mg/kg; Et: Embelia tsjeriam-cottam 100 mg/kg, 200 mg/kg
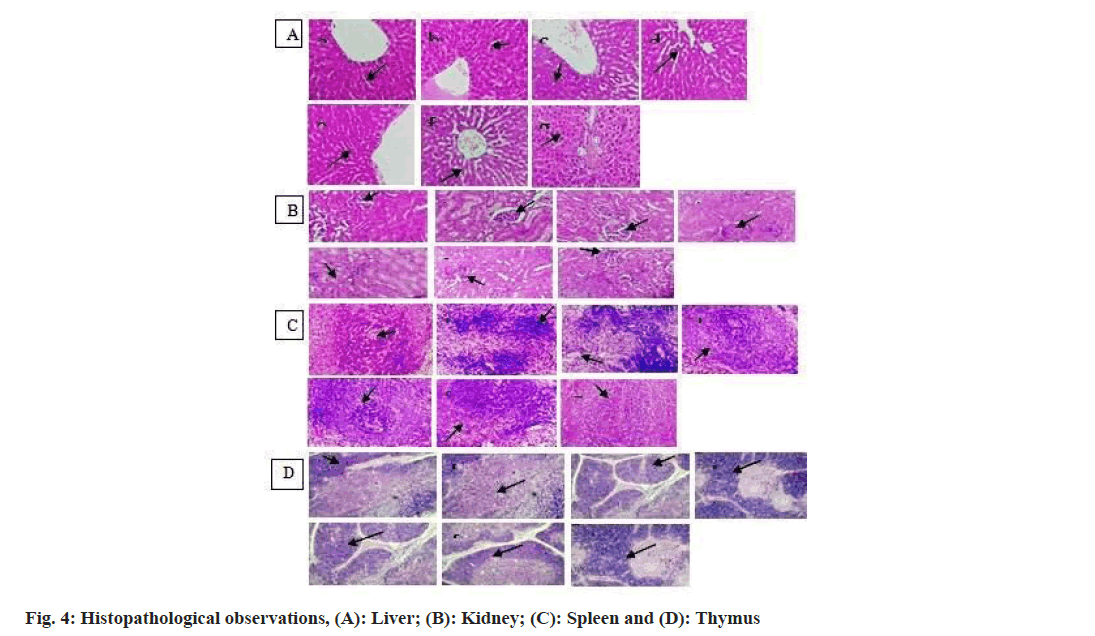
The various organs were taken for histopathological examination. The liver, spleen, kidney, and thymus tissues representative histopathology photographs have been provided (fig. 4A-fig. 4D). Histopathology can be used as a screening tool to identify any potential immune system effects that the treatment with drugs may have. It assists in locating the alleged target cell population that is affected. Results of healthy control, negative control, and treated groups were illustrated in fig. 4A-fig. 4D.
Microscopic examination of a negative control group (b) of the liver tissue section shows inflammatory infiltration in comparison to a group of healthy control (a). E. ribes at 100 mg/kg and 200 mg/kg(c and d) and Embelia tsjeriam-cottam at 100 mg/kg and 200 mg/kg(f and g) groups maintain the normal cytoarchitecture of the tissue in comparison to groups of the negative control (b).
In the histology examination of the kidney, the negative control group (b) showed a focal area of inflammation. The positive control group (a), E. ribes at 100 mg/kg and 200 mg/kg(c and d), and Embelia tsjeriam-cottam at 100 mg/kg and 200 mg/kg(f and g) groups compared to the negative control group (b), effectively maintained the normal cytoarchitecture.
In spleen tissue congestion of blood vessels was seen in the negative control group (b) in comparison to a healthy control group (a). In E. ribes at 100 mg/kg and 200 mg/kg(c and d) and Embelia tsjeriam-cottam at 100 mg/kg and 200 mg/kg(f and g) treated groups showed mild blood vessel congestion. There was no pathology change observed in the tissue.
In Wistar rats, the current study demonstrated the immunomodulatory potential of E. ribes and Embelia tsjeriam-cottam. Immunomodulators are the agents that change immunological responses either by activating or suppressing immune system activity. Immune system activation and immunomodulatory substances of plant origin improve an organism's immunity to various diseases[18]. Under the name Vidanga having three species viz. E. ribes Burm. f., Embelia drupacea (Dennst.) M. R. Almeida& S. M. Almeida, Embelia tsjeriam-cottam (Roem. & Schult) A. DC. and Maesa indica (Roxb.) A. DC., belongs to the family Myrsinacea[19].
The ayurvedic description given suggests the use of E. ribeshowever, a literature review indicates that Embelia tsjeriam-cottam is being used in place of E. ribes[6]. Embelia tsjeriam-cottam possesses morphologically and chemically close similarities with E. ribes and chemical similarity, in terms of the active compound Embelin. According to trade data, >95 % of the marketed species is Embelia tsjeriam-cottam[20]. Due to this confusion, the current research was conducted to compare the immunomodulatory capabilities of both Vidanga species.
The two ethanolic extracts were investigated in this study utilizing a rat model in which SRBCs were administered intraperitoneally. SRBCs were used for both sensitization and antibody titre determination[21]. Organ-to-bodyweight ratios are a helpful indicator for examining the toxic effects of drug treatment on critical organs and for assessing the severity and course of disease[22].
One of the most important secondary lymphoid organs is the thymus, whereas the liver serves as the body's main detoxification mechanism. The spleen is in charge of blood purification and they also store blood cells. Kidneys are concerned with foreign material excretion. The toxicities of medications can affect all of these organs[23]. In the liver and kidneys (fig. 4A and fig. 4B) all the groups when compared to the control group, did not exhibit any pathologically significant lesions. However, lymphoid depletion was seen in the spleen and thymus (fig. 4C and fig. 4D) of the animals treated with the negative control group as compared to the control group. The severity and incidence rate were decreased in both the thymus and spleen of standard control and Er 100 mg/kg and 200 mg/kg and Et 100 mg/kg, and 200 mg/kg treated animals suggestive of the protective effect of compounds.
An important part of the immune response is played by neutrophils, including WBC and lymphocytes. The first line of innate immunological defense and as effectors of adaptive immunity, they take a role in the defence of the host. The health of the body would be assured disease-freeby thesecellsnormally functioning[8]. In comparison to the control group, the E. ribes and Embelia tsjeriam-cottam treatments had no significant effect on the overall WBC count.
The enzymes SGPT and SGOT are generally found in the liver and heart. This enzyme activity can be measured in tissue or bodily fluids to determine liver injury[24]. In the present study, the administration of E. ribes and Embelia tsjeriam-cottam extracts significantly attenuated liver enzymes. Both extracts at the doses of 200 mg/kg have effectively reduced the levels of SGPT while the levels of SGOT have significantly maintained within the range as compared to the negative control group. In the immune system, circulatory proteins, particularly albumin and globulin, play a crucial role. They play a role in immunity or are synthesized as required. In comparison to the SRBC-treated group, all the extracts show a non-significant rise in total serum protein, although the Albumin/Globulin (A/G) ratio shows a non-significant increase but is not statistically significant when compared to the negative control group. Albumin and globulins help drugs and bioactive substances move through the bloodstream. While some immune-system globulins may help with infection management by transporting metal ions[25]. Ethanolic extracts of E. ribes and Embelia tsjeriam-cottam were utilized to cure cellular tissue in key organs including the liver (fig. 4A).
The DTH response is a type IV hypersensitivity reaction that is used to determine how a portion affects cell-mediated immunity[8]. T lymphocytes mediate the DTH response, a type IV allergic reaction. This increased DTH during the Cell-Mediated Immunity (CMI) response could be attributed to sensitized T-lymphocytes[24]. Therefore, the degree of local tissue turgor due to the inflammatory response reflects the cellular immune function[26]. As seen by the greater DTH response, the extract had a stimulatory effect on the lymphocytes and accessory cell types essential for the reaction's expression. E. ribes extract and Embelia tsjeriam-cottam at 200 mg/kg evoked a substantial DTH response as compared to negative control mice with increased footpad thickness compared to the negative control group.
Circulating antibodies mediate Humoral Immunity (HI). B cells are converted into plasma cells, which generate and secrete antigen-specific antibodies. Antibodies bind to antigens and neutralize or aid in the removal of antigens by allowing phagocytic cells to eat them more easily[27]. To confirm the impact of drug treatment on the humoral arm of the immune system, the indirect haemagglutination titer value was used. The circulating antibody titer values increased significantly in all dosages of E. ribes and Embelia tsjeriam-cottam treatment. E. ribes 100 mg/kg, 200 mg/kg and Embelia tsjeriam-cottam 100 mg/kg, 200 mg/kg produced significantly more antibodies than the negative control group.
Amongst the two ethanolic extracts of E. ribes and Embelia tsjeriam-cottam have an immunostimulatory effect on humoral response, maintain blood leucocytes haemoglobin content, beneficial effect on organ weight, increase neutrophil with the margination process of the cells, decrease aminotransferase, increase in delayed-type hypersensitivity response assisting cell-mediated immune response, normal cytoarchitecture of cells of vital organs which prove to be immune protective and effective as an immunomodulator. Hence, Embelia tsjeriam-cottamis utilized in place of E. ribes in immunomodulatory formulations.
It can be concluded that E. ribes and Embelia tsjeriam-cottam have shown appreciable immunomodulatory activity by compelling humoral and cellular immunity to the antigenic challenges with SRBCs and by neutrophil adhesion tests on female Wistar rats using ethanolic extracts of both the species. Therefore, both species of fruit extracts can be used as complementary therapeutic agents. Moreover, present investigations suggest that Embelia tsjeriam-cottamcan be used as a substitute for E. ribes in immunomodulatory formulations. Apart from this, additional studies are required to confirm the present results including comparative characterization of chemical compounds of each species to know the phytoconstituents responsible for immunomodulation and their proportionate combination, which will be useful for their use in different formulations.
Authors’ contributions:
Suresh Jagtap proposed the research concept, designed the experimental model, and corresponded to the manuscript. Kartikey Jagtap and Anuradha Mulik performed the experimental work, interpreted the data, and wrote the manuscript. Suresh Jagtap and Kartikey Jagtap collected plant samples. All authors read and approved the final manuscript.
Acknowledgements:
The authors are thankful to the authorities of Interactive Research School for Health Affairs (IRSHA) and Bharati Vidyapeeth (Deemed to be University) for their overall support.
Conflict of interests:
The authors declared no conflict of interests.
References
- Sethi J, Singh J. Role of medicinal plants as immunostimulants in health and disease. Ann Med Chem Res 2015;1(2):1009.
- El-Ashmawy NE, El-Zamarany EA, Salem ML, El-Bahrawy HA, Al-Ashmawy GM. In vitro and in vivo studies of the immunomodulatory effect of Echinacea purpurea on dendritic cells. J Genet Eng Biotechnol 2015;13(2):185-92.
[Crossref] [Google Scholar] [PubMed]
- Mukherjee PK, Nema NK, Bhadra S, Mukherjee D, Braga FC, Matsabisa MG. Immunomodulatory leads from medicinal plants. Indian J Tradit Knowl 2014;13:235-56.
- Chandresh R, Kaundal M, Srivastava R. Role of Rasayana herbs as an immunomodulator. Int Ayurvedic Med J 2017;5:3644-8.
- Peterson CT, Iablokov SN, Uchitel S, Chopra D, Perez-Santiago J, Rodionov DA, et al. Community metabolic interactions, vitamin production and prebiotic potential of medicinal herbs used for immunomodulation. Front Genet 2021;12:584197.
- Jagtap K, Mulik A, Nangare N, Pawar S, Chougule S, Jagtap S. Ambiguity in the authenticity of selected Vidanga market sample with respect to their biochemical and chromatographic evaluation. Int J Green Pharm 2021;15:385-93.
- Vargas RA, Guerrero RV, Petricevich VL. Evaluation of anti-arthritic potential of partitioned extracts of Bougainvillea buttiana (var. Rose) holttum and standl. Int J Pharm Pharm Sci 2018;10(3):117.
- Narkhede AN, Nirmal PS, Nagarkar BE, Singh EA, Harsulkar AM, Jagtap SD. Validation of the immunomodultory potential of Amarkand species. Indian J Pharm Sci 2017;79(6):965-73.
- Nfambi J, Bbosa GS, Sembajwe LF, Gakunga J, Kasolo JN. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in Wistar albino rats. J Basic Clin Physiol Pharmacol 2015;26(6):603-11.
[Crossref] [Google Scholar] [PubMed]
- Dwivedi S, Ghatuary SK, Prasad S, Jain PK, Parkhe G. Phytochemical screening and in vivo anti-inflammatory activity of hydroalcoholic extract of Embelia ribes Burm. F. J Drug Deliv Ther 2019;9(4):386-9.
- Ugwu CE, Suru MS. Traditional medicinal plants with significant protection against antitubercular drug-induced liver injury: A systematic review. Future Integr Med 2023;2(4):227-58.
- Sambrekar SN, Patil PA, Kangralkar VA. Protective activity of Mussaenda frondosa leaf extracts against paracetamol induced hepatic damage in Wistar rats. J Pharm Res 2010;3(4):711-3.
- Chawla R. Practical clinical biochemistry: Methods and interpretations. 3rd ed. New Delhi: Jaypee brothers Medical Publishers; 2003. p. 136-41.
- Fulzele SV, Satturwar PM, Joshi SB, Dorle AK. Study of the immunomodulatory activity of Haridradi ghrita in rats. Indian J Pharmacol 2003;35(1):51-4.
- Gaur K, Kori ML, Nema RK. Comparative screening of immunomodulatory activity of hydro-alcoholic extract of Hibiscus rosa-sinensis Linn. and ethanolic extract of Cleome gynandra Linn. Global J Pharmacol 2009;3(2):85-9.
- Ismail S, Asad M. Immunomodulatory activity of Acacia catechu. Indian J Physiol Pharmacol 2009;53(1):25-33.
[Google Scholar] [PubMed]
- Narkhede AN, Jagtap SD, Kasote DM, Kulkarni OP, Harsulkar AM. Comparative immunomodulation potential of Tinospora cordifolia (Willd.) Miers ex Hook. F., Tinospora sinensis (Lour.) Merrill and Tinospora cordifolia growing on Azadirachta indica A. Juss. Indian J Exp Biol 2014;52(8):808-13.
[Google Scholar] [PubMed]
- Thangakrishnakumari S, Nishanthini A, Muthukumarasamy S, Mohan VR. Immunomodulatory activity of ethanol extracts of Sarcostemma secamone (L.) bennet (Asclepiadaceae) in mice. J Harmonized Res Pharm 2013;2:84-90.
- Asadulla S, Rajasekharan. Pharmacognosy of Embelia ribes Burm F. Int J Curr Res Chem Pharm Sci 2011;1:1236-51.
- Patwardhan A, Ray S, Roy A. Molecular markers in phylogenetic studies-A review. J Phylogen Evolution Biol 2014;2(2):131.
- Manjuladevi K, Reddy G, Kothai A, Thenmozhi M, Dhanalakshmi M, Sarumathy S. Evaluation of immunomodulatory activity of aqueous extract of a polyherbal formulation by in vivo method. Asian J Pharm Clin Res 2013;6(2):129-33.
- Rajani J, Ashok BK, Patgiri BJ, Prajapati PK, Ravishankar B. Immunomodulatory activity of ?malaki Ras?yana: An experimental evaluation. Ancient Sci Life 2012;32(2):93-8.
[Crossref] [Google Scholar] [PubMed]
- Bigoniya P, Rana AC. Subacute effect of Euphorbia neriifolia Linn. on hematological, biochemical and antioxidant enzyme parameters of rat. Acad J Plant Sci 2009;2(4):252-9.
- Sharififar F, Pournourmohammadi S, Arabnejad M. Immunomodulatory activity of aqueous extract of Achillea wilhelmsii C. Koch in mice. Indian J Exp Biol 2009;47:668-671.
- Mishra P, Gupta S. Modulatory effect of Eclipta alba on biochemical parameters of catfish, Clarias batrachus. World J Pharm Res 2014;3(4):1223-33.
- Yu F, He K, Dong X, Zhang Z, Wang F, Tang Y, et al. Immunomodulatory activity of low molecular-weight peptides from Nibea japonica skin in cyclophosphamide-induced immunosuppressed mice. J Funct Foods 2020;68:103888.
- Dhumal JS, Yele SU, Ghodekar SN. Evaluation of immunomodulatory activity of Vigna mungo (L) Hepper. J Pharm Phytother 2013;2:9-14.