- *Corresponding Author:
- S. Francis Kevin Raj
Department of Pharmacology, C. L. Baid Metha College of Pharmacy, Thoraipakkam, Chennai 600097, India
E-mail: franciskvn03@gmail.com
| Date of Received | 26 August 2023 |
| Date of Revision | 21 March 2024 |
| Date of Acceptance | 22 October 2024 |
| Indian J Pharm Sci 2024;86(5):1774-1780 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To evaluate the effect of hydroalcoholic extract of Withania somnifera on cuprizone induced multiple sclerosis models on Swiss albino mice. Multiple sclerosis was induced in mice by using cuprizone as the inducing agent which caused demyelination of neuron and ultimately death of nerve cells. Glatiramer acetate was used as the standard drug and extract was used as the study drug. Neuronal damage was assessed by in vivo parameters (assessment of learning and memory, locomotor activity, motor coordination and assessment of sensation). The study showed that orally administered extract had ameliorative effect on cuprizone induced oxidative and neurodegenerative stress. From the findings it is evident that extract enhanced the learning, memory, motor coordination and sensory perception in demyelinated mice. Treatment with Withania somnifera prevents the neuronal death, causes remyelination of damaged neurons and also enhanced the sensory perception in mice.
Keywords
Multiple sclerosis, neurodegeneration, Withania somnifera, remyelination, oxidative stress
Multiple Sclerosis (MS) is the commonest non- traumatic disabling disease to affect young adults[1]. There is increasing incidence and prevalence of MS in both developed and developing countries[2], the underlying cause of which remains uncertain. MS is a complex disease; many genes modestly increase disease susceptibility in addition to several important environmental factors, in particular vitamin D or Ultraviolet B (UVB) light exposure, Epstein-Barr Virus (EBV) infection, obesity and smoking[3]. MS has historically been classified as an organ specific T-cell mediated autoimmune disease. However, the success of B-cell targeted therapies challenges the standard T-cell autoimmune dogma[4]. It is traditionally viewed as a two-stage disease, with early inflammation responsible for relapsing-remitting disease and delayed neurodegeneration causing non- relapsing progression, i.e., secondary and primary progressive MS[5,6]. The emergence of increasingly effective biological therapies and an active approach to treating MS, in particular treating to a target of No Evident Disease Activity (NEDA), are changing the long-term outcome for people with MS (pwMS). More aggressive Immune Reconstitution Therapies (IRTs), that result in a proportion of pwMS entering long-term remission, offer a small number of pwMS a potential cure[7]. Recent positive trials of Disease- Modifying Therapies (DMTs) in progressive MS offer those with more advanced MS the hope of slowing their disease progression, with preservation of residual function[8]. The fact that treatments appear to work at multiple stages in the disease course significantly challenges the traditional 2-stage view of the natural history of MS[9].
Materials and Methods
Collection and authentication of plant material:
The roots of Withania somnifera (W. somnifera) were collected randomly from local sources, Tirunelveli district, Tamilnadu. The plant material was taxonomically identified and authenticated by Siddha Central Research Institute, Arumbakkam, Chennai-600106.
Hydroalcoholic extraction:
The collected roots were washed with running water and air dried in shade at room temperature. The dried roots were powdered by means of mechanical grinder as coarse powder and the resulting dried plant material is extracted with ethanol and water at ratio of 70:30 using Soxhlet apparatus for 12 h. The extract was collected and allowed to concentrate to obtain solid mass. After concentration, the dried extract was scraped, collected and the percentage yield of extract were calculated. Extract was stored at 4° until use for further analysis.
Experimental animals:
The female Swiss albino mice which weighed at (20- 25) g, (6-8) w old were used for experiments. The animals were procured from Mass Biotech Pvt. Ltd., Chennai and then housed in C. L. Baid Metha College of Pharmacy, Thoraipakkam, Chennai.
Preliminary phytochemical analysis:
The Hydroalcoholic Extract of W. somnifera (HEWS) roots was subjected to preliminary phytochemical screening to identify the presence or absence of important phytoconstituents.
Grouping:
Animals were numbered and randomly divided into 5 equal groups using Microsoft excel randomisation; each group consists of 6 mice (n=6) as follows and placed into respective cages.
Group I has control (untreated); group-II has Cuprizone (CPZ) model group 200 mg/kg (post operation (p.o.)) (negative control); group-III has CPZ 200 mg/kg (p.o)+glatiramer acetate 100 mcg/kg (subcutaneous (s.c)) (standard); group-IV has CPZ 200 mg/kg (p.o)+HEWS 200 mg/kg (p.o) (low dose) and group-V has CPZ 200 mg/kg (p.o)+HEWS 400 mg/kg (p.o) (high dose). The study is an open trial with no allocation concealment or blinding.
Experimental design:
To induce MS, Swiss albino mice at (6-8) w of age were given CPZ through oral route. CPZ was suspended in 0.5 % Methyl Cellulose (MC) in water and given by oral gavage at a daily dose of 200 mg/kg body weight. Stock solution of 0.5 % MC was prepared weekly and stored at 4°. CPZ and MC suspension were freshly prepared and administered daily. CPZ did not react chemically with MC and was just present in the suspension mixture. The control group received only 1 % MC without CPZ by oral gavage. The cortex of all mice except control group was completely demyelinated after 5 w of CPZ feeding. CPZ treatment allows the study of demyelination[10].
Assessment of learning and memory:
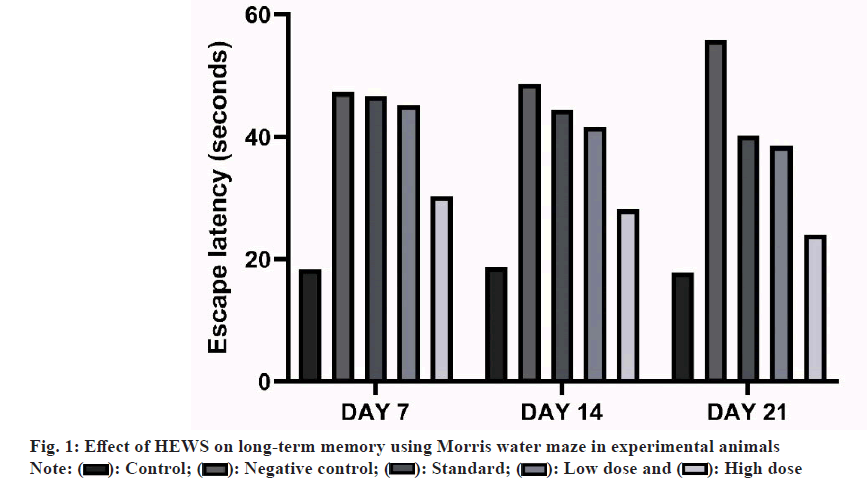
Morris water maze: The Morris water maze method was performed to evaluate spatial working and reference memory. The experimental apparatus consists of a circular tank (120 cm in diameter, 45 cm in height). An invisible platform (15 cm in diameter, 35 cm height) was placed 1.5 cm below the surface of the water. Water was kept opaque by dissolving small quantity of milk at a temperature of 21°-23°. The pool was located in a test room and many cues external to the maze were visible from the pool, which could be used by the mice for spatial orientation. The position of the cues was kept constant through the task. The training trials were carried out. The platform is located in a constant position throughout the test period in the middle of one quadrant, equidistant from the centre and edge of the pool. In each training session, the latency to escape to the hidden platform was recorded. On the day of experiment, the platform was removed and the animals were tested for its memory where the time spent by each animal in target quadrant searching for the hidden platform is noted as an index of retrieval. The escape latency was measured in each group on 7, 14 and 21 d following the study drug administration[11].
Y-maze test: This working is not based on spontaneous exploration and alteration between arms-water nor food restrictions were required. Three identical arms were mounted symmetrically on an equilateral triangular centre. Y-shaped maze with three opaque arms spaced 120° apart. Large Y-maze-three 40×8×15 cm arms and small Y-maze-two 15.24×7.62×12.7 cm arms, one 20.32×7.62×12.7 cm arm. The percentage alterations were measured in each group during a test period of 5 min. Trial sessions were conducted and the values were measured on 7, 14 and 21 d following the administration of study drug[12].
Assessment of locomotor activity:
Actophotometer: Locomotor activity (horizontal activity) was measured using actophotometer orchid scientific model, actophotometer operates on photoelectric cells connected with a counter. When a beam of light falling on the photocell is cut off by the animal, a count is recorded and displayed digitally. Each mouse was placed individually in the activity cage floor for 10 min and the locomotion count was observed from the digital reading displayed in the actophotometer. Trial sessions were conducted and the values were measured on 7, 14 and 21 d following the administration of study drug[13].
Assessment of motor co-ordination:
Rotarod activity: Motor coordination and balance was assessed using a rotarod apparatus. In total, four training trials per day with an interval trial time of 1 h were performed. Mice falling off during a training trial were put back on the rotating rod. Following the training days, 1 d test of three trials was performed using two speed levels (10 and 20 revolutions per minute (rpm)) mode of the apparatus over 5 min. Mice are placed individually in separate lanes on rod rotating at 5 rpm such that animals may walk forward to keep balance. The rotating rod is allowed to rotate at 10 rpm and mice from each group were placed individually on the rod and the fall off time was recorded for each mouse. Similar procedure was followed by increasing the speed to 20 rpm. The readings were taken on 7, 14 and 21 d after the administration of study drug[14].
Assessment of sensation:
Test for touch sense (modified method): Mice of 6 w of age were selected first and tested for touch sense using glass rod. Glass rod is rolled on the tail of the mice and the time taken to react (turning of the head) is noted. Minimum time taken must be 1 s-2 s. Such animals are selected and grouped. The grouped animals are further used for the study. Then the test drug is administrated and the reaction time is noted. The readings were taken on 7, 14 and 21 d after the administration of study drug.
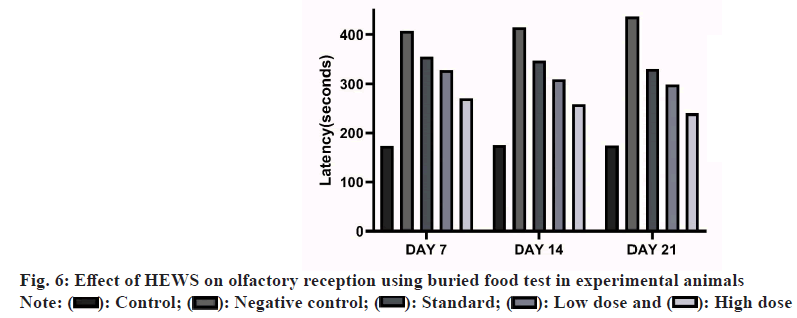
Buried food test[15]: The buried food test measures how quickly an overnight-fasted animals can find a small piece of familiar palatable food, such as cookies, cereal, chocolate chips, food pellets, that is hidden underneath a layer of bedding. The assumption is that food-restricted mice which fail to use odour cues to locate the food within a 15 min period are likely to have deficits in olfactory abilities. Mice at the age of 6 w were chosen. Plastic cages of 46 cm length×23.5 cm width×20 cm height, or similar are used. Place cage bedding to create a 3 cm layer in each cage. For each subject, a clean cage is washed and dried. The test begins by placing a subject mouse in a clean cage containing 3 cm deep of clean bedding. The subject is allowed to acclimate to the cage for 5 min. Transfer the subject to an empty clean cage. In the cage containing bedding, bury the food stimulus approximately 1 cm beneath the surface, in a random corner of the cage. Smooth out the surface. Trials were conducted and the readings were taken on 7, 14 and 21 d after the administration of study drug.
Statistical analysis:
Appropriate descriptive and analytical statistics were used. The analysis was done using Graphpad Prism 9 software. Q-Q plot and Shapiro-Wilk test ensured normal distribution of data. Group III animals were compared with groups IV and V. The values were expressed as mean±Standard Error of the Mean (SEM) from all 6 animals in each group. The difference between groups were determined using one-way Analysis of Variance (ANOVA) followed by Dunnet’s t-test after ensuring normal distribution, independence of observations and homogeneity of variance. p<0.05 was considered as significant.
Results and Discussion
Extraction yield including the hydroalcoholic extract of the roots of W. somnifera was dark brown in colour and was stored at 4°-8° for further use. The percentage yield of the extract was 5.69 % w/w.
Preliminary phytochemical study including the HEWS showed presence of various phytochemical constituents such as alkaloids, flavonoids, phenols, steroids, glycosides and terpenoids are shown in Table 1.
| S. No | Constituents | Remarks |
|---|---|---|
| 1 | Alkaloids | Present |
| 2 | Tannins | Absent |
| 3 | Glycosides | Present |
| 4 | Saponins | Absent |
| 5 | Flavanoids | Present |
| 6 | Phenols | Present |
| 7 | Steroids | Present |
| 8 | Terpenoids | Present |
| 9 | Proteins | Absent |
| 10 | Carbohydrates | Present |
Note: The table represents the phytochemicals present/absent in the HEWS
Table 1: Preliminary Phytochemical Screening
Effect of HEWS on Morris water maze including the group V animals showed significant decrease (p<0.001) in the escape latency on to the hidden platform when compared with group III animals. The group IV animals showed no significant increase in the escape latency when compared with group III animals as shown in fig. 1.
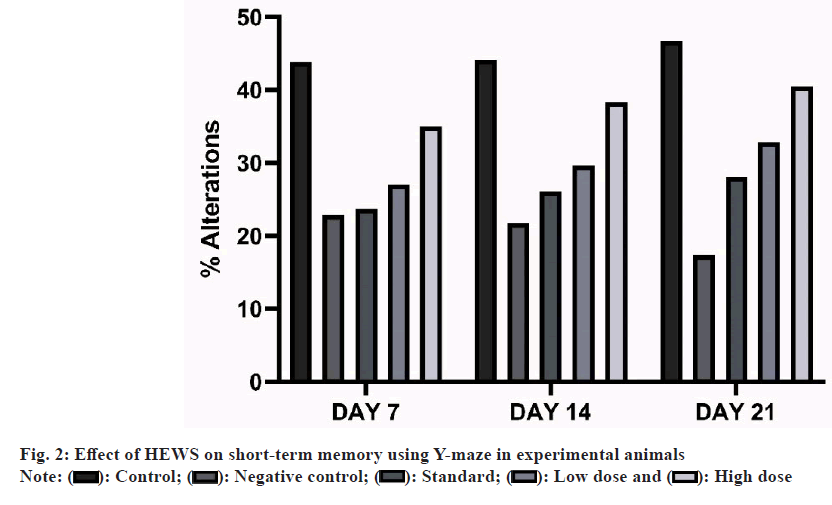
Effect of HEWS on Y-maze including the group V animals showed significant increase (p<0.01) in the percentage alterations when compared with group III animals, whereas group IV animals showed no significant increase in the percentage alterations when compared with group III animals as shown in fig. 2.
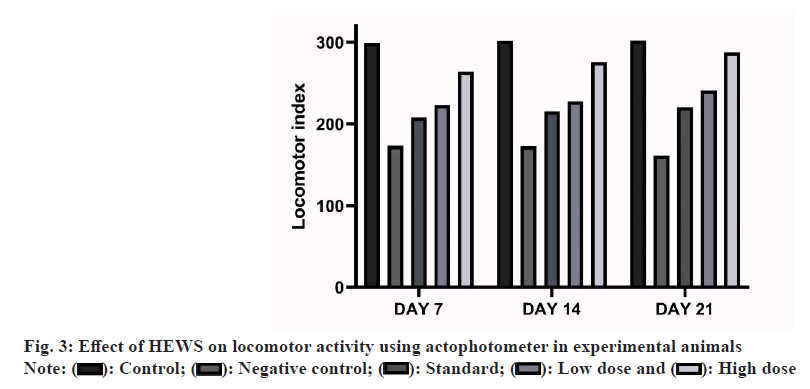
Effect of HEWS on actophotometer including the group V animals showed significant increase (p<0.001) in the locomotor activity when compared with group III animals, whereas group IV animals showed no significant increase in the locomotor activity when compared with group III animals as shown in fig. 3.
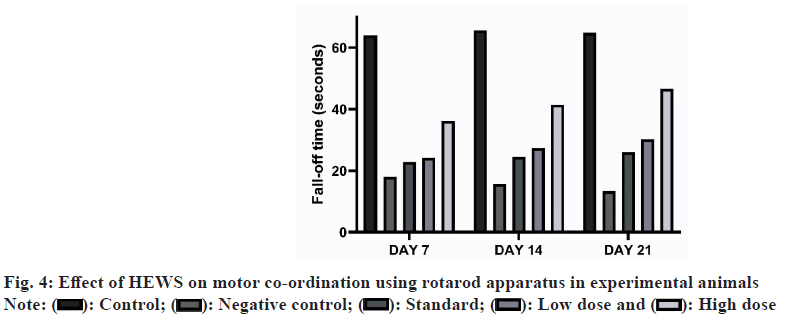
Effect of HEWS on rotarod including the group V animals showed significant increase (p<0.01) in the fall-off time when compared with group III animals, whereas group IV animals showed slight increase in the fall-off time when compared with group III animals as shown in fig. 4.
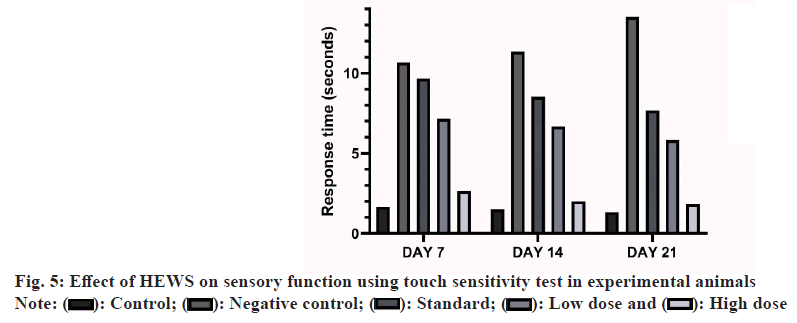
Effect of HEWS in touch sensitivity test including the group V animals showed significant decrease (p<0.001) in the response time when compared with the group III animals. The group IV animals also showed significant decrease (p<0.05) in the response time upon comparing with group III as shown in fig. 5.
Effect of HEWS in buried food test including the group V animals showed significant decrease (p<0.01) in the latency to find buried food when compared with group III animals. The group IV animals also showed significant decrease (p<0.05) in the latency to find buried food upon comparing with group III animals as shown in fig. 6.
The present study investigated the potential ameliorative effects of orally administered HEWS on CPZ-induced oxidative and neurodegenerative stress and accompanying memory decline. The study findings indicated that administration of HEWS effectively restored the oxidative balance and neuronal integrity in the cerebellum, a region crucial for both long-term and short-term memory in the experimental animals. The neurobehavioural tests done in this study revealed that animals treated with HEWS had decreased escape latency period when compared with standard group animals. CPZ treatment significantly depleted long and short-term memory in animals[16].
As a measure of long-term memory, animals were subjected to Morris water maze which is the stress induced behavioural test. Animals treated with CPZ had increased escape latency period which is the marker for the long-term memory loss and HEWS treated animals had decreased escape latency. Spontaneous alteration (Y-maze) was used to assess the short-term memory. The animals showed decrease in the percentage of alteration when treated with CPZ and an increase in the percentage of alteration when subsequently treated with HEWS.
Previous study on MS patients has linked hippocampal demyelination to progressive cognitive and memory dysfunction[17]. On treatment of experimental animals, HEWS has restored the long-term and short-term memory and it is consistent with the report that HEWS improve memory impairment in Swiss albino mice.
CPZ treatment has well established effects on the locomotor activity and muscle coordination of experimental animals. Locomotor activity was assessed using actophotometer. The animals treated with CPZ showed low locomotor index and when treated with HEWS the locomotor index significantly increases. This proves that the extract has effects on the movement of the animals. Muscle coordination was assessed using rotarod apparatus. CPZ treated animals tend to fall quickly in the rotarod apparatus while the animals treated with HEWS showed significant increase in the fall-off time.
MS patients have sensory impairments and these are caused by the damage to the various sensory receptors in the body. CPZ also causes the damage to the olfactory receptors and various other sensory receptors in the body[10]. Animals treated with HEWS showed a gradual improvement in the sensation owing to the repair of the damaged sensory receptors. In the buried food test, animals treated with HEWS had lesser latency than the animals treated with CPZ alone. Our findings also show that the animals treated with HEWS (400 mg/kg) showed a significant decrease in the latency period when compared with the standard group. In touch sense test, animals treated with HEWS had lesser response time to touch than the CPZ treated animals. Our findings also show that the animals treated with HEWS (400 mg/ kg) showed a significant decrease in the response time when compared with the standard group.
HEWS has high neuroprotective effect and therapeutic potential as observed in the works done above and also enhance the remyelination process. It reversed the action of CPZ that had extensively damaged the neurons, destroyed the myelin sheath and induced demyelination.
The pathology of MS is complex including inflammation and neurodegeneration, which the CPZ model of demyelination may not fully replicate. This remains as a major limitation of this study.
This study shows positive evidence that HEWS has a potential remyelination and neuroprotective activity. HEWS prevents cell death and fast regeneration of neuronal cells in the brain. The exact molecular mechanism for this phenomenon is unclear. Further work has to be carried out to explore the full potential of the study drug and its mechanism of action. Clinical trials in the future to ensure the relevance in clinical MS patients are recommended in the long run.
Ethical approval:
All experiments were carried out according to the guidelines for care and use of experimental animals and approved by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The study was approved by Institutional Animal Ethic Committee (IAEC). IAEC Reference No: 01/321/PO/ Re/S/01/CPCSEA.
Acknowledgements:
The author would like to acknowledge the Tamil Nadu Chemists and Druggists Educational Trust (TNCDE) and C. L. Baid Metha College of Pharmacy for the extended support.
Conflict of interest:
The authors declared no conflict of interests.
References
- Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 2017;23(8):1123-36.
[Crossref] [Google Scholar] [PubMed]
- Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014;83(11):1022-4.
[Crossref] [Google Scholar] [PubMed]
- Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol 2008;7(3):268-77.
[Crossref] [Google Scholar] [PubMed]
- Greenfield AL, Hauser SL. B-cell therapy for multiple sclerosis: Entering an era. Ann Neurol 2018;83(1):13-26.
[Crossref] [Google Scholar] [PubMed]
- Leray E, Yaouanq J, Le Page E, Coustans M, Laplaud D, Oger J, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010;133(7):1900-13.
[Crossref] [Google Scholar] [PubMed]
- Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: Evidence from monoclonal antibody therapy. J Neurol 2006;253:98-108.
[Crossref] [Google Scholar] [PubMed]
- Muraro PA, Pasquini M, Atkins HL, Bowen JD, Farge D, Fassas A, et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol 2017;74(4):459-69.
[Crossref] [Google Scholar] [PubMed]
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab vs. placebo in primary progressive multiple sclerosis. N Engl Med 2017;376(3):209-20.
[Crossref] [Google Scholar] [PubMed]
- Giovannoni G, Cutter G, Sormani MP, Belachew S, Hyde R, Koendgen H, et al. Is multiple sclerosis a length-dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult Scler Relat Disord 2017;12:70-8.
[Crossref] [Google Scholar] [PubMed]
- Kopanitsa MV, Lehtimaki KK, Forsman M, Suhonen A, Koponen J, Piiponniemi TO, et al. Cognitive disturbances in the cuprizone model of multiple sclerosis. Genes Brain Behav 2021;20(1):e12663.
[Crossref] [Google Scholar] [PubMed]
- Tomás Pereira I, Burwell RD. Using the spatial learning index to evaluate performance on the water maze. Behav Neurosci 2015;129(4):533.
[Crossref] [Google Scholar] [PubMed]
- Kraeuter AK, Guest PC, Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol 2019:1916:105-111.
[Crossref] [Google Scholar] [PubMed]
- Standard Operating Procedure. Standard Behavioural Functional Neuroscience Laboratory. Behavioural Assay.
- Deacon RM. Measuring motor coordination in mice. J Vis Exp 2013;29(75):e2609.
[Crossref] [Google Scholar] [PubMed]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci 2009;48(1):8-24.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, Adilijiang A, Wang W, You P, Lin D, Li X, et al. Arecoline attenuates memory impairment and demyelination in a cuprizone-induced mouse model of schizophrenia. Neuroreport 2019;30(2):134-8.
[Crossref] [Google Scholar] [PubMed]
- Praet J, Guglielmetti C, Berneman Z, van der Linden A, Ponsaerts P. Cellular and molecular neuropathology of the cuprizone mouse model: Clinical relevance for multiple sclerosis. Neurosci Biobehav Rev 2014;47:485-505.
[Crossref] [Google Scholar] [PubMed]
 Standard;
Standard;  High dose
High dose