- *Corresponding Author:
- E. James
Department of Pharmacy Practice, Amrita School of Pharmacy,India
E-mail: emmanuelj@aims.amrita.edu
| Date of Submission | 23 May 2016 |
| Date of Revision | 29 August 2016 |
| Date of Acceptance | 05 September 2016 |
| Indian J Pharm Sci 2016;78(5):566-574 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Deterioration of kidney function is the most worrisome long term complication following liver transplantation. This study was performed to identify the risk factors of renal dysfunction following living donor liver transplant and the effect of immunosuppressant dosage adjustment on the recovery of renal function. A retrospective observational study was conducted on 133 consecutive adult living donor liver transplant recipients over an 8 year period from 2006 onwards. An increase in serum creatinine of >1.4 mg/dl was taken as a marker for renal insufficiency. The incidence of post living donor liver transplant renal dysfunction was 35% and maximum incidence occurred at one year of liver transplantation. Male gender, pretransplant diabetes, hepatorenal syndrome, posttransplant hypertension, bilirubin >1.2 mg/dl, albumin <3.5 g/dl, pretransplant serum creatinine>1.4 mg/dl and tacrolimus trough level >7mcg/l at 3rd month of liver transplantation were significant risk factors for the development of renal dysfunction. Reduction of tacrolimus dosage was the most effective intervention to restore renal function. This however resulted in abnormal liver function tests secondary to rejection and necessitated the addition of alternative non-nephrotoxic immunosuppressants. Two and four year survival rates were 98.9% and 96.6% for patients without renal dysfunction compared to 97.4% and 91.6% for those complicated by renal dysfunction after living donor liver transplant. Maintaining lower levels of tacrolimus along with addition of non-nephrotoxic immunosuppressants may be a worthwhile strategy to protect the kidneys and preserve long term graft function in patients at high risk of post living donor liver transplant renal dysfunction.
Keywords
Immunosuppressants, living donor liver transfplantation, renal dysfunction, risk factors
Liver transplantation outcomes have improved greatly with advances in surgical techniques since the 1960s. With 1 year liver transplant (LT) survival rates now beyond 85% [1], increased attention is being paid to improve long term morbidity and mortality in LT recipients. Renal dysfunction is the most worrisome long term complication following LT. It was estimated that 18% of the recipients would develop chronic renal failure within 5 year of LT which may in turn decrease patient survival [2-4]. Several factors have been implicated for the occurrence of chronic renal impairment in LT recipients [2,4,5]. Among these, high level exposure to calcineurin inhibitors, namely cyclosporine A and tacrolimus, is a welldocumented risk factor [2,6,7]. Chronic calcineurin-induced nephrotoxicity is associated with structural changes in the kidney [8-10].
After India’s first successful living donor liver transplant (LDLT) in the year 1998, nearly 7500 LTs have been performed across the country at the various LT centers which stand up to 30 as of 2015 [11]. Though a considerable number of research and review articles have been published [12-19] since then on the various aspects of liver transplantation in India there is paucity of data from India regarding the incidence and risk factors of renal dysfunction and the effect of immunosuppressant therapy modification on renal function in post LDLT recipients. A better understanding of these facts can improve patient outcomes in post LDLT patients.
Materials and Methods
This retrospective and observational study was carried out in the Department of Gastrointestinal Surgery at Amrita Institute of Medical Sciences and Research Centre (AIMS), a 1250 bedded tertiary care teaching and super specialty referral hospital in Kochi, Kerala. Since 2006, 407 LTs were done in AIMS, majority (363) of which was LDLTs making this one of the largest LDLT program in the country. Approximately 50-80 liver transplant recipients are followed up monthly in this department. Retrospective data of post LT patients were collected for a period of 8 years (from 1st June 2006- 30th June 2014) and the patients were followed up during a period of 9 mo from 17th September 2014 to 17th June 2015. Patients older than 18 years of age who had undergone LDLT at AIMS and who had at least one year follow up following LT were included. Patients who died within 3 mo of LT or LT recipients from deceased donors or patients unwilling to provide informed signed consent were excluded.
Patient data relevant to the study were collected by reviewing the digital medical records of the patients in the hospital information system and by direct interview of the patients who came for follow up. The study got approval of the Hospital Research and Ethics Committee. Using a predesigned data collection form, information regarding the demographic data, pre-transplant details including diagnosis of liver disease, comorbidities and post-transplant details like dose, frequency and duration of immunosuppressant administration and rejection episodes were collected. Laboratory data including haematological parameters, liver function tests and kidney function tests were monitored regularly. Serum TAC levels were monitored by chemiluminescent immunoassay and the immunosuppressant dosage adjustments were recorded.
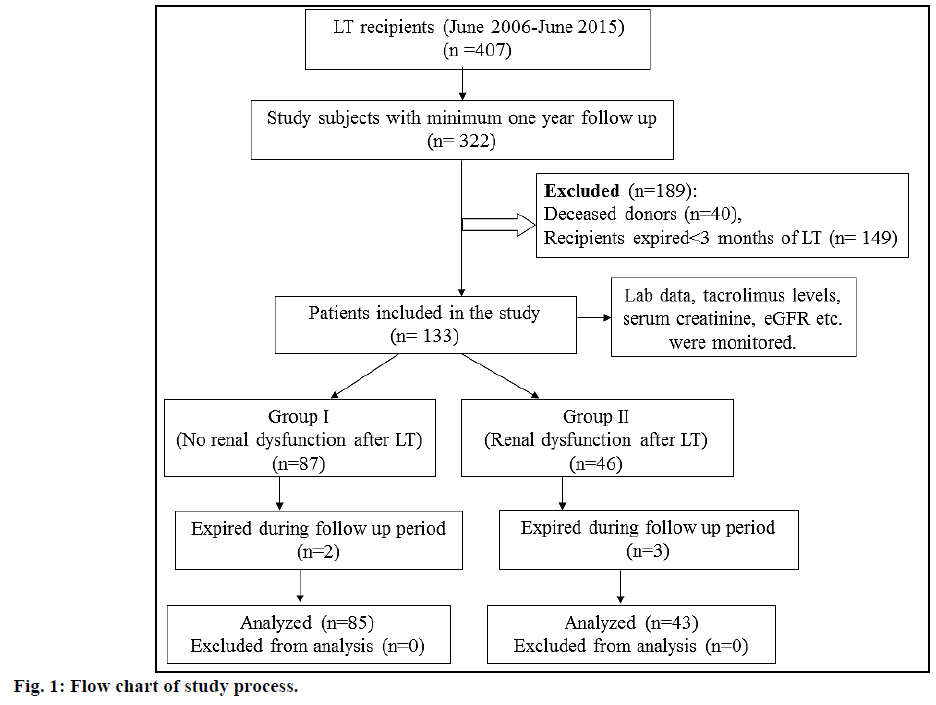
A rise in serum creatinine to >1.4 mg/dl as estimated by Jaffe method [20] was considered as a surrogate marker for renal dysfunction in post LT patients. A value of more than 43 mg/dl of serum urea (estimated by kinetic UV method) indicated elevated blood urea nitrogen [21]. Persistent rejection was defined as more than 2 episodes of rejection despite adequate levels of immunosuppression and pulse methylprednisolone therapy [22]. The criteria for acute kidney injury (AKI) was an increase in serum creatinine by 0.3 mg/ dl or ≥1.5 times the baseline value within 48 h, or an increase of ≥1.5 times the baseline value within seven days [23]. Estimated glomerular filtration rate (eGFR) was calculated using Modification of Diet in Renal Disease (MDRD) study equation [24] and stages of kidney disease were categorized using Kidney Disease: Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guidelines [25]. Chronic kidney disease (CKD) was considered if eGFR <60 ml/min per 1.73 m² or evidence of kidney damage such as albuminuria or abnormal findings on renal imaging were present for ≥3 mo. The study patients were stratified into group I (no renal dysfunction 3 mo post LT) and group II (renal dysfunction 3 mo post LT). The flow chart of the study process is shown in fig. 1.
The collected data were compiled using Microsoft Excel and analysis was carried out using Statistical Package for Social Sciences (SPSS), version 20. Quantitative variables were expressed as mean±standard deviation (SD) while qualitative variables were expressed as number (percentage). Pearson’s chi-square test was used to compare the two groups of patients for qualitative variables and independent Student’s t-test for quantitative variables. Mann-Whitney test was used for comparing measurable continuous variable like duration of follow up. Immunosuppressant dosage adjustment on renal function was correlated using McNemar test. Kaplan Meier survival analysis was performed to evaluate the survival data and log rank test (Mantel-Cox test) was performed to compare survival rates of group I and II patients. A P-value<0.05 was considered significant.
Results and Discussion
Men (113, 85%) outnumbered the women (20, 15%). The highest number of patients (36, 27%) was in the age group 41-47 y, followed by 55-61 y and the lowest (6, 4.5%) in the age group of 18-25 y and 62-68 y. The median age of the study patients was 46 y, with a range of 47 (65-18) y and a coefficient of variation of 0.2. The baseline characteristics of study patients are shown in Table 1. Indications for liver transplantation in these patients included chronic alcoholic-cirrhosis (59, 44.3%), cryptogenic cirrhosis (35, 26.3%), seronegative liver failure (9, 6.8%), hepatocellular carcinoma (8, 6%), hepatitis B (7, 5.3%), hepatitis C (6, 4.4%), hepatitis A (3, 2.3%), yellow phosphorous poisoning (3, 2.3%), autoimmune hepatitis (2, 1.5%) and drug induced (1, 0.7%) liver failure.
| Characteristics | Group I (n=87) |
Group II (n=46) |
Total (n=133) |
P-value* |
|---|---|---|---|---|
| Age (y±SD) | 44±1.1 | 48.8±8.4 | 45.7±10.5 | 0.013* |
| Duration of follow up (y±SD) | 2.87±1.72 | 3.26±1.73 | 3.01±1.7 | 0.15 |
| Social history No. (%) | ||||
| Ex-alcoholic | 39 (44.8) | 20 (43.4) | 59 (67.8) | 0.88 |
| Ex-smoker | 8 (9.2) | 5 (10.9) | 13 (14.9) | 0.75 |
| Past medical history No. (%) | ||||
| Asthma | 3 (3.4) | 3 (6.5) | 6 (4.5) | 0.41 |
| Cancer | 5 (5.8) | 1 (2.2) | 6 (4.5) | 0.35 |
| Heart failure | 1 (1.1) | 1 (2.2) | 2 (1.5) | 0.64 |
| Diabetes | 30 (34.5) | 25 (54.3) | 55 (41.4) | 0.027* |
| Dyslipidemia | 1 (1.1) | 1 (2.2) | 2 (1.6) | 0.64 |
| Encephalopathy | 20 (23) | 9 (19.6) | 29 (22) | 0.65 |
| Hepatitis | 2 (2.3) | 6 (13) | 8 (6) | 0.64 |
| Hepatopulmonary syndrome | 1 (1.1) | 1 (2.2) | 2 (1.5) | 0.64 |
| Hepatorenal syndrome | 0 | 5 (10.9) | 5 (3.8) | 0.002* |
| Hypertension | 13 (15) | 8 (17.3) | 21 (15.8) | 0.67 |
| Jaundice | 7 (8) | 8 (17.4) | 15 (11.2) | 0.76 |
| Kidney diseases | 0 | 3 (2.2) | 3 (2.2) | 0.04* |
| Lower limb cellulitis | 3 (3.5) | 2 (4.3) | 5 (3.7) | 0.79 |
| Variceal bleeding | 8 (9.2) | 4 (8.7) | 12 (9) | 0.92 |
| Donor type No. (%) | ||||
| Non blood related | 48 (55.1) | 32 (69.5) | 80 (91.9) | 0.10 |
| First degree relative | 39 (44.8) | 14 (30.4) | 53 (60.9) | 0.08 |
| MELD score | 23.1±5.5 | 23.1±5.2 | 23.1±5.4 | 0.25 |
| MELD >17 No. (%) | 73 (65.2) | 39 (34.8) | 112 (84.2) | 0.89 |
Group I: no renal dysfunction after LT, Group II: renal dysfunction after LT; MELD: Model for End-stage Liver disease; *P-value <0.05 is considered significant
Table 1: Baseline Characteristics of Post Liver Transplant Recipients
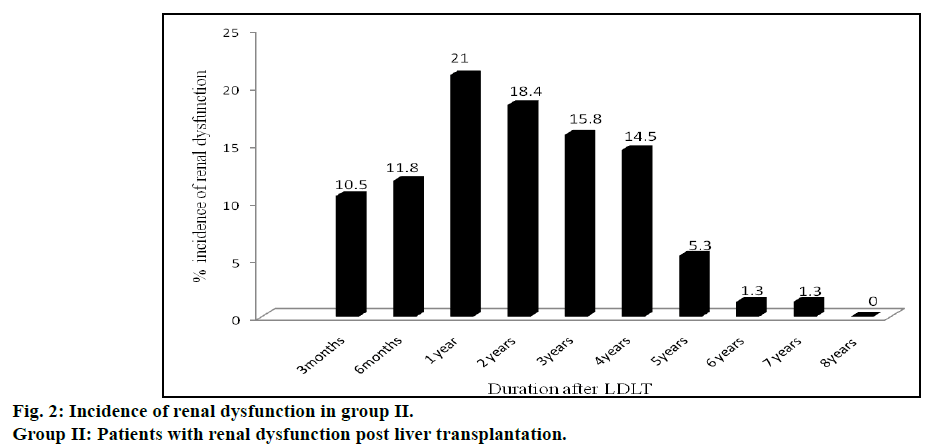
Incidence of renal dysfunction in post LT recipients is represented in fig. 2. Seventy six incidences of renal dysfunction were observed in 46 patients of group II. The incidence of renal dysfunction during the posttransplant period of 8 y was estimated to be 35%. Some of these patients experienced renal dysfunction on multiple occasions during this time period (12 patients experienced renal dysfunction 2 times, 5 patients experienced 3 times, 1 patient experienced 4 times and another 1 patient experienced more than 5 times. Incidences of renal dysfunction were found to be maximum (16, 21%) at one year after LT followed by two, three and four years. Incidence of renal dysfunction was very rare after the 5th y of LT. Comparison of various risk factors for development of renal dysfunction between group I and II patients are shown in Table 2.
| Risk factors | Group I (n=87) | Group II (n=46) | χ2 | P-value* |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Age (≥50 years) | 29 (60.4) | 19 (39.5) | 0.8 | 0.363 |
| Male recipient | 68 (60.2) | 45 (39.8) | 9.1 | 0.003* |
| Ex-smoking | 8 (61.5) | 5 (38.5) | 0.01 | 0.757 |
| Ex-alcoholism | 39 (66.1) | 20 (33.9) | 0.02 | 0.882 |
| Pretransplant diabetes | 30 (54.5) | 25 (45.5) | 4.9 | 0.027* |
| Post-transplant diabetes | 25 (64.1) | 14 (35.8) | 0.04 | 0.838 |
| Pretransplant hypertension | 13 (62) | 8 (38) | 0.14 | 0.713 |
| Post-transplant hypertension | 6(30) | 14 (70) | 13.0 | <0.001* |
| Pretransplant hepatorenal syndrome | 0 (0) | 5 (100) | 9.8 | 0.002* |
| Pretransplant dyslipidemia | 1 (50) | 1 (50) | 0.2 | 0.642 |
| Post-transplant dyslipidemia | 33 (56.9) | 25 (43.1) | 3.3 | 0.069 |
| Pretransplant bilirubin (>1.2 mg/dl) | 84 (70.6) | 35 (29.4) | 13.4 | <0.001* |
| Pretransplant albumin(<3.5 g/dl) | 74 (72.5) | 28 (27.5) | 9.8 | 0.002* |
| Pretransplant serum creatinine (≥1.4 mg/dl) | 9 (42.9) | 12 (57.1) | 5.6 | 0.018* |
| Elevated blood urea nitrogen (>43 mg/dl) | 9 (25) | 27 (75) | 35.6 | <0.001* |
| Tacrolimus level at 3rd month (≥7 μ/l) | 15 (47) | 17 (53) | 7.2 | 0.007* |
| MELD score(≥20) | 60 (63.2) | 35 (36.8) | 0.75 | 0.387 |
| HCV recurrence | 3 (60) | 2 (40) | 0.07 | 0.795 |
| Pre-transplant sodium level (<135 mmol/l) | 64 (66.7) | 32 (33.3) | 0.24 | 0.625 |
| Graft rejections | 41 (61.2) | 26 (38.8) | 1.06 | 0.303 |
Group I: no renal dysfunction after LT, Group II: renal dysfunction after LT; *P-value <0.05 is considered significant
Table 2: Risk Factors for Development of Renal Dysfunction in Post Liver Transplant Recipients
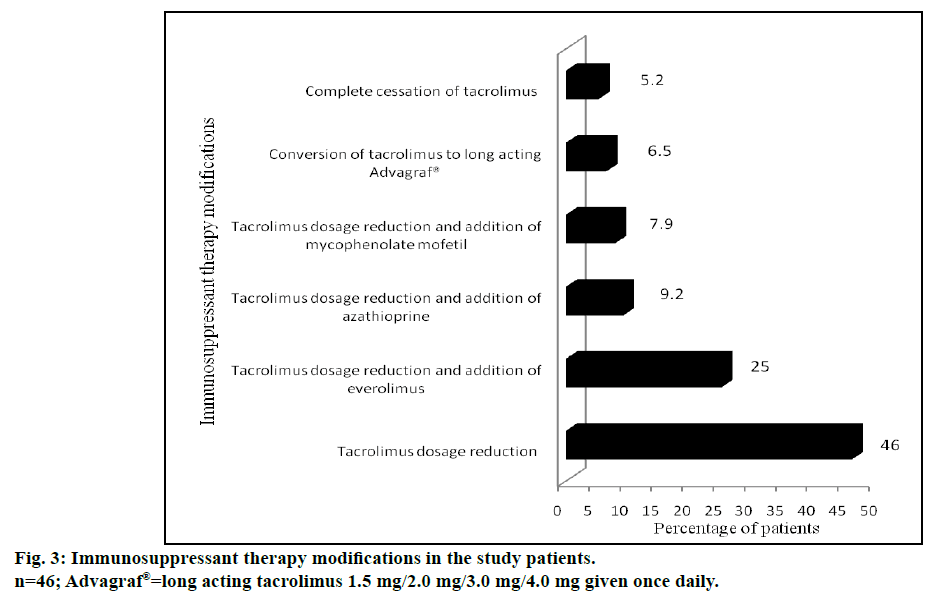
Male gender, pretransplant diabetes, post-transplant hypertension, pretransplant bilirubin concentrations (>1.2 mg/dl), pretransplant albumin levels (<3.5 g/dl), pretransplant serum creatinine (≥1.4 mg/dl), elevated blood urea nitrogen (>43 mg/dl), pretransplant hepatorenal syndrome and TAC concentrations in the blood sample at 3rd mo (≥7 μ/l) were found to be significant risk factors for development of renal dysfunction following LDLT. The immunosuppressant therapy modifications in post LT recipients with renal dysfunction are represented in fig. 3.
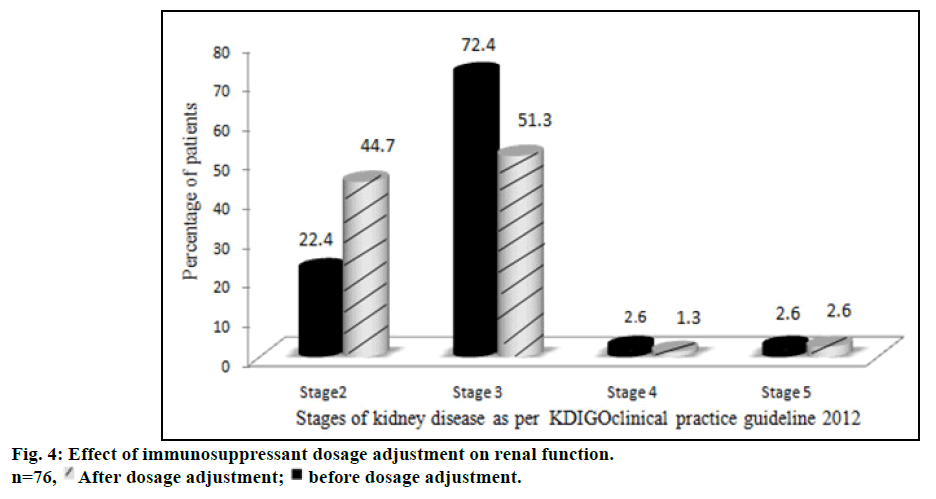
The proportion of patients with various stages of kidney disease before and after immunosuppressant dosage adjustment is represented in fig. 4. The number of patients with stage 3 and stage 4 kidney diseases decreased while the number of stage 2 patients increased after dosage adjustment. Stage 5 kidney disease, necessitating hemodialysis, developed in 1 patient (1.3%). The dosage adjustment of immunosuppressants significantly improved the renal function of stage 3 patients (P<0.05).
Two patients from group I and three patients from group II expired after a year of LT. The median survival time of group I and group II patients were 6.8 and 7.4 y, respectively. The overall survival rates of group I and group II patients are shown in Table 3. The one, three, five year survival rates were 98.9, 96.6 and 96.6%, respectively for group I, but for group II were 100, 97.4 and 83.3%, respectively. The comparison of survival rates with follow up years for group I and II are shown in fig. 5. The log rank test (Mantel-Cox test) suggested that there was no association with renal dysfunction and the mortality of these patients (P=0.336).
| Survival time | Survival rates (%) | Cause of death and number of patients* | |
|---|---|---|---|
| Group I (n=87) |
Group II (n=46) |
||
| First year | 98.9* | 100 | MODS, MI, Sepsis, ARDS, Metabolic acidosis (n=1) |
| Second year | 98.9 | 97.4* | Sepsis, MODS (n=1) |
| Third year | 96.6* | 97.4 | Acute cellular rejection (n=1) |
| Fourth year | 96.6 | 91.6* | MI, sepsis, MODS (n=1) |
| Fifth year | 96.6 | 83.3* | Severe coagulopathy, acute liver failure (n=1) |
MODS: Multiple organ dysfunctions; MI: myocardial infarction; ARDS: acute respiratory distress syndrome
Table 3: Survival Rates of Study Patients After Liver Transplantation
In this study, renal dysfunction occurred in considerable number of patients (46, 35%) following LDLT over 8 y; nevertheless the renal dysfunction did not appear to increase the 5 y mortality. The significant correlation between renal dysfunction and factors like male gender, pre-transplant diabetes, post-transplant hypertension, pre-transplant bilirubin concentrations (>1.2 mg/ dl), pre-transplant albumin levels (<3.5 g/dl), pretransplant serum creatinine(≥1.4 mg/dl), elevated blood urea nitrogen (>43 mg/dl), pretransplant hepatorenal syndrome and tacrolimus concentrations in blood sample at 3rd month (≥7 μ/l) may be of help in better understanding of various predictors of renal dysfunction following LT and as much as possible, preventing its development. Manipulation of immunosuppression to avoid or limit calcineurin-nephrotoxicity is one of the major tools to overcome this problem [26]. Calcineurin minimization and conversion to other non-nephrotoxic drugs seems to be a safe approach that results in significant improvement in renal function.
This study, to our knowledge, is the largest work from India to analyze the occurrence of renal dysfunction following LDLT. An earlier Indian study [27] on 70 patients who had undergone LT during 2005-09 was primarily on DDLT patients as there were only 18 (25.7%) LDLT patients in the cohort. The patients were grouped into those with both liver and renal failure (n=29) and those with hepatic failure but with normal renal function (n=41) prior to LT. The authors evaluated the development of acute renal failure on the 5th and 30th day of LT. In contrast, our study looked into the development renal dysfunction at various intervals beginning three months after LT (fig. 2) in patients who survived. We excluded the patients who died within the first three months of LDLT as our objective was on assessing factors responsible for chronic renal dysfunction after LDLT. Death occurred in some of LT recipients within the first three months of LT due to a variety of reasons like sepsis, liver dysfunction and technical reasons like vascular block or biliary leaks.
The incidence of post LT renal dysfunction in our study was lower (34.6%) compared to another study conducted in the Netherlands [28] where chronic renal failure developed in 168 (43%) patients despite the fact that they had considered only CKD stage III patients for estimation of incidence of renal dysfunction and we considered all patients from stage II and above. Another study from Ireland by O’Riordan et al. [29] found that 10 y cumulative incidence of renal dysfunction for stages 0/1, 2, 3, 4 and 5 of CKD as 9.61, 53.7, 56.77, 6.11 and 2.62%, respectively. The incidence of AKI was 60.5% at an average tacrolimus trough level of 10.3±0.51 (μ/l) in a study conducted by Utsumi et al. [30] from Japan. The lower incidence of renal dysfunction in our study may be due to the relatively low trough levels (<7 mcg/l) of tacrolimus maintained for our patients. Calcineurin inhibitors like tacrolimus is often cited as the primary reason for the occurrence of post LT renal impairment [31,32]. Tacrolimus trough level at 3rd month of transplantation was <7 μ/l in the majority of our patients (101, 76%). In most of the western series, deceased donors form the major share of the liver donors where the whole liver is transplanted unlike partial liver used as graft in LDLT. Usually in deceased donor liver transplantation, tacrolimus levels are targeted at 8 to 12 μ/l. It is known that LDLT recipients require lower trough levels compared to DDLT due to the lower hepatocyte mass needed [33]. Ours’ being a predominantly LDLT programme, the dosage of tacrolimus was decisively aimed to keep a trough level around 5 μ/l. We feel that the lower tacrolimus level might have prevented renal dysfunction in some of the high risk patients in our study. A previous study also advocated the benefit of maintaining tacrolimus levels at 5-7 μ/l [34]. In contrast, a study from Spain by Gallardo et al. [3] estimated a low incidence (16.7%) of late onset renal dysfunction (LD). This may be because they defined LD as serum creatinine >2 mg/dl beyond 3 mo post LT.
We found that male gender, pretransplant diabetes, posttransplant hypertension, pretransplant bilirubin >1.2 mg/dl, pre-transplant albumin <3.5 g/dl, pretransplant serum creatinine >1.4 mg/dl, blood urea nitrogen >43 mg/dl, pretransplant hepatorenal syndrome and tacrolimus trough level at 3rd mo >7 μ/l as significant risk factors for development of post LT renal dysfunction. This was comparable to other studies; for instance in a study by Utsumi et al. [20] from Japan, preoperative diabetes and over exposure to calcineurin inhibitors were risk factors for post-transplant renal dysfunction. Likewise, in the study by Brandao et al. [35] from Brazil, male gender and median tacrolimus levels (11.3 μ/l) in the first 3 mo of post LT were significant risk factors whilst pretransplant serum creatinine (>1.2 mg/dl) was cited as the important determinant of post LDLT renal dysfunction in the study by Ling et al. [36] from China and Pawarode et al. [4] from USA.
Another salient finding of our study was that the maximum incidence of renal dysfunction occurred at a time period of one year following transplantation. Interestingly at 2, 3 and 4 y, the incidence of renal dysfunction appeared to be decreasing. This is probably due to change in immunosuppression that was carried out at the index episode of renal dysfunction. Among different immunosuppressant/immunosuppressant combination approaches, reduction of tacrolimus dose was found to be the most common initial therapy modification in majority of group II patients. This alone improved the renal function in 35 patients (46%). Needless to mention, reduction of tacrolimus dosage often resulted in abnormal liver function tests secondary to rejection, which necessitated the addition of other non-nephrotoxic immunosuppressant drugs. These included everolimus (19 patients, 25%), azathioprine (7 patients, 9.2%) and mycophenolate mofetil (6 patients, 7.9%). Complete cessation of tacrolimus was required only in 4 patients (5.2%). This immunosuppressant therapy modification was comparable to a study conducted in UK by Neuberger et al. [37] Tweaking of immunosuppressive medication probably had a beneficial effect in improving the renal function in our patients as well. The number of patients with stage 3 renal dysfunction decreased from 72.4% to 51.3%, during follow up study period. This “stage migration” was reflected as an increase in the number of patients with stage 2 renal dysfunction from 22.4 to 44.7%. McNemar test provided strong evidence that the dosage adjustment of immunosuppressants improved the renal function particularly for patients of stage 3 (P<0.05) kidney disease.
It is reassuring that stage 5 kidney disease, necessitating dialysis occurred only in 1 patient of our study (1.3%). It is likely that longer follow up may result in detection of more number of patients developing end stage renal disease following LDLT. In any case, in our study, there was no significant correlation between renal dysfunction and mortality. The 2 and 4 y survival rates were found to be 98.9 and 96.6% for patients without renal dysfunction compared to 97.4 and 91.6% for those complicated by renal impairment. This finding contrasts with other research studies where renal dysfunction had a definite negative impact on survival. In a study conducted in Japan, 1, 5 and 10 y survival rates were 96.7, 90.6 and 88.1%, respectively for patients without renal dysfunction and 71.1, 65.9 and 59.3% for those with renal dysfunction [29]. This difference in survival may be due to variation in selection criteria of our study subjects, which excluded mortalities occurring in the first three months of transplantation.
During the follow up period, worsening as well as improvement of renal function was observed in some patients. Being a retrospective study, precise aggravating or relieving factors affecting variation in renal function could not be clearly ascertained. Nevertheless factors such as infections, alteration of immunosuppressant dosage and glycaemia control could be possible explanations.
Our study does have limitations, due to its retrospective and observational nature. Recently, cystatin C has been reported as a better representative marker of renal function [38] than creatinine used in our study. Additionally, the histological diagnosis of renal dysfunction by renal biopsy in appropriate cases might have given more valuable information.
Renal dysfunction occurred in 46 (34.6%) patients following LDLT. Various risk factors for occurrence of renal dysfunction following LDLT were identified. Patients with such risk factors for development of renal impairment should be counseled regarding this complication, which may occur after successful transplant surgery. Additionally our study has stressed the importance of maintaining lower levels of tacrolimus to protect the kidneys. Nevertheless, physicians should be cautious that whilst maintaining lower levels of tacrolimus, there could be concomitant increase in rejection. Therefore for patients who are deemed to be at high risk of post-transplant renal dysfunction, maintaining low tacrolimus levels along with addition of non-nephrotoxic immunosuppressants like everolimus, azathioprine and mycophenolate mofetil may be a worthwhile strategy for preserving long term graft function. However properly conducted randomized trials are necessary to substantiate this conjecture.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Port FK, Merion RM, Goodrich NP, Wolfe RA. Recent trends and results for organ donation and transplantation in the United States, 2005. Am J Transplant 2006;6:1095-100.
- Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a non-renal organ. N Engl J Med 2003;349:931-40.
- Gallardo ML, Gutierrez MH, Perez GS, Balsera EC, Ortega JF, Garcia GQ. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transpl 2004;10:1379-85.
- Pawarode A, Fine DM, Thulumath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl 2003;9:741-47.
- Moreno JM, Cuervas-Mons V, Rubio E, Pons F, Herreros de TA, Turrion VS, et al. Chronic renal dysfunction after liver transplantation in adult patients: prevelance, risk factors and impact on mortality. Transplant Proc 2003;35:1907-8.
- Campbell KM, Yazigi N, Ryckman FC, Alonso M, Tiao G, Balistreri WF, et al. High prevalence of renal dysfunction in long term survivors after pediatric liver transplantation. J Pediatr 2006;148:475-80.
- Cantarovich M. Renal dysfunction in liver transplantation: the problem and preventive strategies. Can J Gastroenterol 2004;18:27C- 40C.
- Johnson DW, Saunders HJ, Johnson FJ, Huq SO, Field MJ, Pollock CA. Fibrogenic effects of cyclosporin A on the tubulointerstitium: role of cytokines and growth factors. Exp Nephrol 1999;7:470-8.
- Johnson DW, Saunders HJ, Johnson FJ, Huq SO, Field MJ , Pollock CA. Cyclosporin exerts a direct fibrogenic effect on human tubulointerstitial cells: role of insulin-like growth factors I, transforming growth factor beta 1 and platelet-derived growth factor. J Pharmacol Exp Ther 1999;289:535-42.
- Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine associated chronic nephropathy. N Engl J Med 1984;311:699-705.
- Narasimhan G, Kota V, Rela M. Liver transplantation in India. Liver Transpl 2016;22:1019-24.
- Narasimhan G, Safwan F, Kota V, Reddy MS, Bharathan A, Dabora A, et al. Donor outcomes in living donor liver transplantation-analysis of 275 donors from a single centre in India. Transplantation 2016;100:1251-6.
- Varghese J, Sachan D, Reddy MS, Cherian T, Jothimani D, Venugopal K, et al. Hepatitis B immunoglobulin prophylaxis after liver transplantation: experience in a tertiary transplant centre. J Clin Exp Hepatol 2014;4:209-13.
- Varghese J, Reddy MS, Venugopal K, Perumalla R, Narasimhan G, Arikichenin O, et al. Tacrolimus-related adverse effects in liver transplant recipients: its association with trough concentrations. Indian J Gastroenterol 2014;33:219-25.
- Varghese J, Gomathy N, Rajashekhar P, Venugopal K, Olithselvan A, Vivekanandan S, et al. Perioperative bacterial infections in deceased donor and living donor liver transplant recipients. J Clin Exp Hepatol 2012;2:35-41.
- Acharya SK. Liver transplant in India: miles to go. Trop Gastroenterol 1992;13:127-28.
- Soin AS, Thiagarajan S. Liver transplant scene in India. MAMC J Med Science 2016;2:6-11.
- Narasimhan G. Living donor liver transplantation in India. Hepatobiliary Surg Nutr 2016;5:127-32.
- Kelly D, Sibal A. Current status of liver transplantation. Indian J Pediatr 2003;70:731-36.
- Peake M, Whiting M. Measurement of serum creatinine-current status and future goals. Clin Biochem Rev 2006;27:173-84.
- Contreras G, Garces G, Quartin AA, Cely C, LaGatta MA, Barreto GA, et al. An epidemiologic study of early renal replacement therapy after orthotopic liver transplantation 2001;13:228-33.
- Locke JE, Singer AL. Evolving concepts in the selection of immunosuppression regimen for liver transplant recipients. Hepat Med 2011;3:53-62.
- Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 2008;20:672-79.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 1999;130:461-70.
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: Improving Global Outcomes [KDIGO]. Kidney Int 2005;67:2089-100.
- Duvoux C, Pageaux GP. Immunosuppression in liver transplant recipient with renal impairment. J Hepatol 2011;54:1041-54.
- Naik P, Premsagar B, Mallikarjuna M. Acute renal failure in liver transplant patients: Indian study. Ind J Clin Biochem 2015;30:94-98.
- Azimpoura M, Kazemier G, Hansen B, De man RA, Zietse R, Metselar HJ. Renal failure incidence and risk factors after orthotopic liver transplantation. Erasmus MC 2010;1:9-13.
- O’Riordan A, Wong V, McCormick PA, Hegarty JE, Watson AJ. Chronic kidney disease post liver transplantation. Nephro Dial Transplant 2006;21:2630-36.
- Utsumi M, Umeda Y, Sadamori H, Nagasaka T, Takaki A, Matsuda H, et al. Risk factors for acute renal injury in living donor liver transplantation: evaluation of the RIFLE criteria. Transpl Int 2013;26:842-52.
- Bennett WM. Insights into chronic cyclosporine nephrotoxicity. Int J Clin Pharmacol Ther 1996;34:515-19.
- Bennett WM, DeMattos A, Meyer MM, Andoh T, Barry JM. Chronic cyclosporine nephropathy: the Achilles’ heel of immunosuppressive therapy. Kidney Int 1996;50:1089-100.
- Liu F, Li Y, Lan X, Wei YG, Li B, Yan LN, et al. Tacrolimus dosage requirements in living donor liver transplant recipients with small for size grafts. World J Gastroenterol 2009;15:3931-36.
- Sudhindran S, Aboobacker S, Menon RN, Unnikrishnan G, Sudheer OV, Dhar P. Cost and efficacy of immunosuppression using generic products following living donor liver transplantation in India. Indian J Gastroenterol 2012;31:20-23.
- Brandao VBA, Faria LC, Bicalho DM, Pereira FH, Lima AS, Ferrari TC. Early serum tacrolimus levels predict long-term chronic kidney disease after liver transplantation. Medical Express 2014;1:143-49.
- Ling Q, Wang K, Lu D, Guo HJ, Jiang WS, He XX, et al. Major influence of renal function on hyperlipidemia after living donor liver transplantation. World J Gastroenterol 2012;18:7033-39.
- Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H, et al. Delayed introduction of reduced dose tacrolimus and renal function in liver transplantation. The respect study. Am J Transplant 2009;9:327-36.
- Shilpasree AS, Prakash S, Itagappa M. Cystatin C: A better indicator than creatinine to assess the renal functions. Int J Pharm Bio Sci 2013;3:372-77.
 After dosage adjustment; ■ before dosage adjustment.
After dosage adjustment; ■ before dosage adjustment.
 - Group I,-
- Group I,-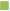 -group II,
-group II,  renal,
renal,  non-renal,
non-renal,  renal-censored,
renal-censored,  non-renal-censored
non-renal-censored