- *Corresponding Author:
- Bishnupada Roy
Department of Zoology, Parasitology and Toxicology Laboratory, North Eastern Hill University, Shillong, Meghalaya 793022, India
E-mail: bishnuroy12@rediffmail.com
| Date of Received | 17 March 2021 |
| Date of Revision | 18 April 2022 |
| Date of Acceptance | 27 January 2023 |
| Indian J Pharm Sci 2023;85(1):199-206 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The process of delayed wound-healing and unhealed wounds can cause serious problems to the patients. In this context it is revealed that the scientists are looking for herbal treatment of wounds, as the remedy is known for having less or minimum undesired effects. Schima wallichii is a lesser known traditional medicinal plant used by certain section of people in Assam, India as a healing agent; however, record on scientific validation of its wound-healing potential is not available. Therefore, an in vivo study was undertaken to evaluate the wound-healing efficacy of the leaves of the plant using excision wound model in Swiss albino mice. The animals were divided into three groups after creating the wounds, namely control, positive control and treated groups. On application of methanolic crude extract of the leaves of Schima wallichii, it was observed that the epithelialization period was lesser and the rate of closure of wounds was faster in the extract treated group when compared with the control group indicating that the application of the extract has accelerated the healing process in the extract treated group. The concentration of protein, Schima wallichii deoxyribonucleic acid and hydroxyproline were also found to be augmented in the treated group than the control suggesting faster rate of healing as deoxyribonucleic acid and protein content in the healing tissues serve as indicator of protein synthesis and cellular proliferation. The above results were supported by the histological and ultra-structural observations as scanning electron microscopy revealed smoother surface topography, H and E staining revealed thicker epithelial layer in the treated animals. Van Gieson′s staining showed higher level of collagen in the extract treated group validating biochemical observation where higher concentration of hydroxyproline was found in treated group compared to the control. Thus, going by the results obtained in the study it can be concluded that the leaves of the plant possess wound-healing potential.
Keywords
Wound-healing, Schima wallichii, crude extract, mice, protein, deoxyribonucleic acid, hydroxyproline, ultrastructure
Wounds are physical injuries which cause breakage of the skin that disturbs its normal functioning. Wound healing is a complex process, the four phases of this process are hemostasis, inflammation, proliferation and tissue remodeling[1]. Hemostasis starts as soon as the wound is formed that leads to vascular constriction and formation of clot. The inflammatory phase is characterized by the inflammation of neutrophils, macrophages and lymphocytes[2]. The proliferative phase is marked by epithelial proliferation and in the last phase i.e. tissue remodeling, regression of newly formed capillaries takes place so that the wound′s vascular density reaches normal level[3]. The process of impaired healing or non-healing wounds is affecting millions of people worldwide. It can be noted that unhealed wounds continuously produce inflammatory mediators which cause pain and swelling at the injury site and sometimes this may lead to organ failure in severe conditions in the patients[4]. Although, there are many drugs available commercially for the treatment of wounds but most of them have undesired effects[5]. Therefore, the search for new healing sources are still in progress which can minimize the time required for healing and has minimal side effects.
In developing countries, for majority of the population the traditional medicinal plants are still playing a dominant role in the healthcare system[6]. At present, about half million medicinal plants are known to us, among which most are yet to be investigated for their efficacies. So, studies which deal with evaluation of medicinal potential of these plants will be of great medico-veterinary significance[7]. Developed countries such as United Kingdom (UK) and United States of America (USA) have also accepted the importance of herbal remedies, as a result of which both in developing as well as developed countries the demand for the herbal products have increased many folds[8]. Therefore, these medicinal plants are not only important in the present healthcare system but also an indispensable source of future medicine[9].
The north-eastern region of India is blessed with huge number of known and unknown medicinal plants. The region is inhabited by different ethnic groups, each of which has its unique culture and tradition. These people have been using many traditional medicinal plants, most of which are lesser known for curing different types of parametric and non-parametric ailments locally[10,11]. Schima wallichii (S. wallichii) (Korth.) Choisy is one such plant, belonging to the family ‘Theaceae’ and is widely distributed all over the north-east region of India. Review of literature and field surveys have revealed that the leaves of S. wallichii are used by certain section of people in the remote areas of Assam for healing small scale wounds. The people generally crush the leaves and the aqueous paste is applied on the fresh cuts, wounds and burns. The plant is also known to possess anti-malarial, anti-cancer and antiinflammatory activities[12-14]. However, scientific data is not available which can prove the efficacy of the plant as wound-healing agent. Therefore, an in vivo experiment was designed to study the wound-healing potential of the plant using Swiss albino mice.
Materials and Methods
Collection of plant material and preparation of extract:
The fresh leaves of S. wallichii were collected from Kokrajhar district of Assam, brought to Shillong, Meghalaya and submitted to Botanical Survey of India for identification. For preparation of crude extract the leaves were washed with water and then dried under shade. The dried leaves were grinded to fine powder with the help of an electric blender. The powder was then soaked in methanol for 10 d with constant stirring regularly for first few days. Later, the solution was filtered using filter paper and the solvent was evaporated out from the solution using Rota evaporator. The obtained dry crude extract of the leaves of S. wallichii was finally stored at 4° for future use[15].
Experimental animals:
Adult Swiss albino mice weighing between 25-30 g and of age 8-12 w were purchased from Pasteur Institute, Shillong, Meghalaya. The animals were maintained as per the guidelines of the Organisation of Economic Co-operation and Development (OECD). They were kept in standard cages in the animal house having uniform temperature of 25° with 12 h light and 12 h dark periodicity. Standard food (Pranav Argo Feed Ltd., New Delhi) and water ad libitum were provided to the animals. Before proceeding with the experiment, mice were acclimatized for 2 w. The Institutional Ethics Committee (IEC), North-Eastern Hill University, Shillong granted approval for the experimental procedures.
Acute dermal toxicity test:
The toxicity test was carried out following the OECD (2002)[16] guidelines to see whether the topical application of the methanolic crude extract of leaves of S. wallichii possess any toxic effects on the animals or not. During the test 3 animals of same sex were exposed to the test substance at a fixed dose i.e., 2000 mg/kg body weight. After anaesthetizing, the furs of the mice were shaved, cleaned with alcohol. The extract was applied on the pre-determined (400 mm2) area and the substance was held in contact with skin for 24 h. The animals were then observed continuously for 14 d to check whether the topical application causes any effects on the animals or not.
Infliction of wound:
By following the protocol of Morton and Malone (1972)[17] the ‘excision wound’ was created on the mice. Briefly, the animals were anaesthetized first and then the dorsal furs were removed with the help of an electric clipper. After that, the shaved area was cleaned with 70 % ethanol and the area where wound to be inflicted was outlined. Excision wound around the outlined area was made by removing circular piece of skin of full thickness with the help of toothed forceps, scalpel and scissors. After infliction, the wounds were again cleaned with cotton swab soaked in the normal saline.
Treatment protocol:
After the infliction of the excision wound, the mice were divided randomly into 3 groups each having 6 animals (3 Males and 3 Females). The first group animals were considered as ‘control group’ where the treatment was done with Carboxymethylcellulose (CMC). The second group received reference drug (Neosporin) thus, serving as ‘positive control’ and the animals of third group were treated with methanolic crude extract of the leaves of S. wallichii (300 mg/kg body weight). CMC, drug and extract were applied topically once daily for 14 d to the respective groups, on the 15th d the animals were sacrificed and wound tissues (skin) were collected for performing various studies.
Measurement of wound area and calculation of wound contraction:
On 4,7,11 and 14 d the wound areas of the animals in the respective groups were measured with the help of graph paper and noted down. Wound contraction, which indicates the reduction in the wound areas was calculated and expressed as percentage reduction of the wounds.
Percentage wound contraction=(Healed area/Total area)×100
Where, Healed area=Original wound area-Present wound area
Calculation of epithelialization period and morphometric analysis:
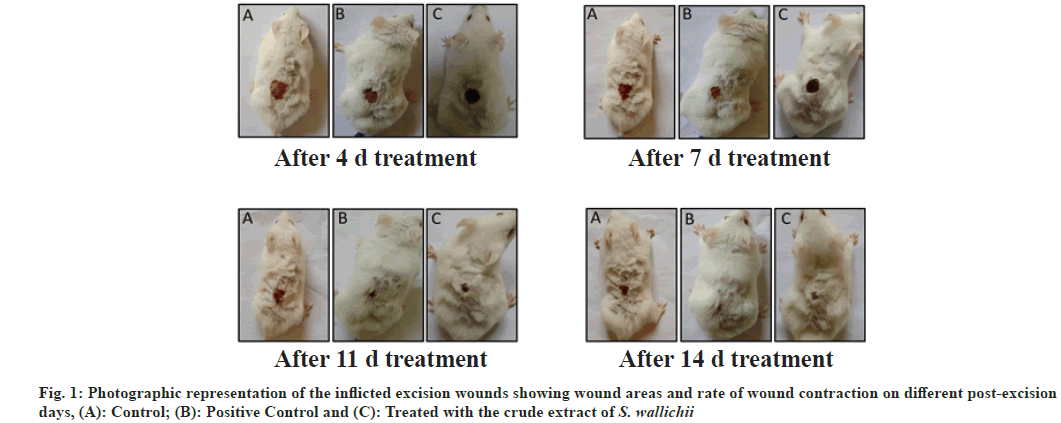
The epithelialization period denotes the time period (days) needed for healing of wounds where, no raw wound is left behind after the falling of scab or Escher, were also noted down for the three groups. To show the closure/contraction of the wounds morphometrically, photographs of the wounds of the three experimental groups were taken on 4, 7, 11 and 14 d; and were analyzed.
Estimation of protein, Deoxyribonucleic Acid (DNA) and hydroxyproline content:
The wound tissues of the three experimental groups were collected after sacrificing the animals and stored at -20° for performing the biochemical estimations. The protein content of the skin tissues was determined following the protocol of Lowry et al.[18] where, ‘Folinciocalteau’ reagent was used and the readings were taken at 750 nm. For estimation of DNA content of tissues standard ‘phenol-chloroform’ precipitation technique given by Sambrook and Russel et al.[19] was performed for isolation of DNA followed by its quantification. Measurement of hydroxyproline content of the wound tissues was done using ‘toluene extraction’ method suggested by Switzer and Summer[20].
Histological and micro topographical studies:
To perform histopathological analysis the wound tissues were collected after completion of the treatment period and preserved in the 10 % formalin solution. Later, the tissues were sectioned at thickness of 6-8 nm and temperature of -20° in a cryostat. The tissue sections were then passed through different ethanol grades finally stained with haematoxylin and eosin to observe epithelialization and Van Gieson′s staining was performed to observe collagenases. For micro topographical observation scanning electron microscopic study was carried out following the modified protocol[21] of Dey et al.[22]. Here, fixation of the tissues (skin) was done in neutral buffer formalin, followed by dehydration using different grades of acetone, after which using tetramethylsilane the samples were air dried. Finally, the gold coating of the samples was done and viewed using JOEL JSM 6360 electron microscope at 20 kV.
Statistical analysis:
All the numerical results have been expressed as mean±Standard Error of Mean (SEM). Using oneway Analysis of Variance (ANOVA) and students’ t-test statistical analysis was performed and the differences were considered as statistically significant at the value of p≤0.05.
Results and Discussion
During the 14 d of observation the animals did not show any signs of toxic effects. Briefly, there was no change in the color of skin and fur, intake of food and water was normal, no change in the body weight and also no changes were observed in any other behavior of the extract exposed animals.
The wound areas measured on different days have been shown in the Table 1. It was observed that after the completion of 14 d of treatment the control group had a small portion of wound (7.47±1.15 mm2) yet to be healed whereas in the remaining two treated groups the wounds were completely healed. Similarly, higher rate of wound contraction was observed in the drug and extract treated groups compared to the control group.
| Groups | D 4 | D 7 | D 11 | D 14 |
|---|---|---|---|---|
| Control | 57.55±3.28 | 46.59±2.09 | 20.24±1.88 | 7.47±1.15 |
| Positive control | 37.78±1.91* | 24.98±1.80* | 3.17±0.81* | 0 |
| Treated | 45.85±1.05* | 38.71±1.73 | 5.95±1.06* | 0 |
Note: The values have been expressed as mean±SEM, *p≤0.05 as compared to control
Table 1: Effects of Methanolic Crude Extract of S. wallichii and Reference Drug On Wound Areas (mm2) in Three Groups of Experimental Mice Calculated After Different Days of Treatment
A significant increase in contraction rate was observed after 11 d of treatment i.e., (92.42±1.35) % and (95.96±1.04) % for extract treated and positive control groups, respectively as shown in Table 2.
| Groups | D 4 | D 7 | D 11 | D 14 |
|---|---|---|---|---|
| Control | 26.69±4.18 | 40.65±2.67 | 74.22±2.39 | 90.48±1.46 |
| Positive control | 51.87±2.43* | 68.25±2.30* | 95.96±1.04* | 100 |
| Treated | 41.59±1.33* | 50.68±2.21 | 92.42±1.35* | 100 |
Note: Values are expressed as mean±SEM, *p≤0.05 as compared to the control
Table 2: Effects of Methanolic Crude Extract and Reference Drug on The Percentage of Wound Closure of Excision Wound Model In Mice
The time required for complete epithelialization (days) in case of positive control and extract treated groups were significantly lesser than the control group (Table 3), indicating that the application of the extract has fastened the process of healing. The photographs of the wounds taken on d 4, 7, 11 and 14 (fig. 1) also showed that the rate of healing of wound is slowest in case of the control group.
| Groups | Epithelialization period (days) | Protein content (µg/mg tissue) | DNA content (µg/mg tissue) | Hydroxyproline content (µg/mg tissue) |
|---|---|---|---|---|
| Control | 15.97±0.12 | 17.97±1.24 | 1.86±0.13 | 2.15±0.21 |
| Positive control | 11.68±0.03* | 30.74±0.71* | 3.29±0.09* | 4.38±0.10* |
| Treated | 12.73±0.05* | 25.34±0.47* | 2.72±0.25* | 3.96±0.18* |
Note: The values are expressed in the form of mean±SEM, *p≤0.05 as compared to control
Table 3: Epithelialization Period and Level of Different Biochemical Parameters in Three Different Groups of The Mice After 14 D of Treatment
Quantitative estimation of protein, DNA and hydroxyproline after the completion of 14 d of treatment period has revealed that the topical application of methanolic crude extract of leaves of S. wallichii has significantly increased the concentration of protein, DNA and hydroxyproline in the treated group when compared with the control. However, it was also found that the maximum concentration of the three components was observed in the drug treated animals as shown in Table 3.
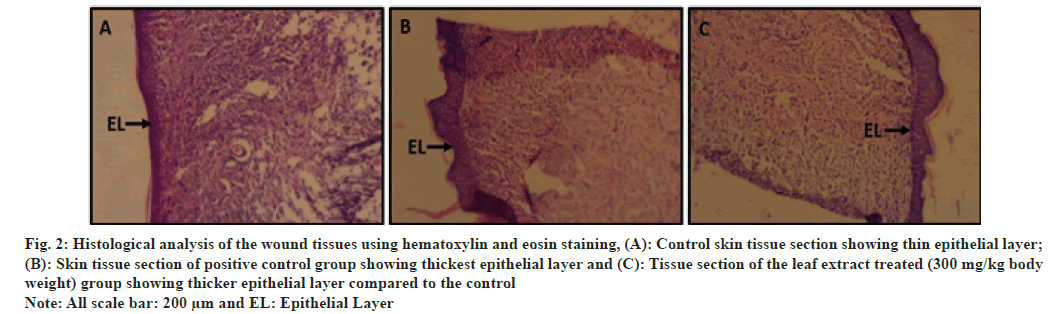
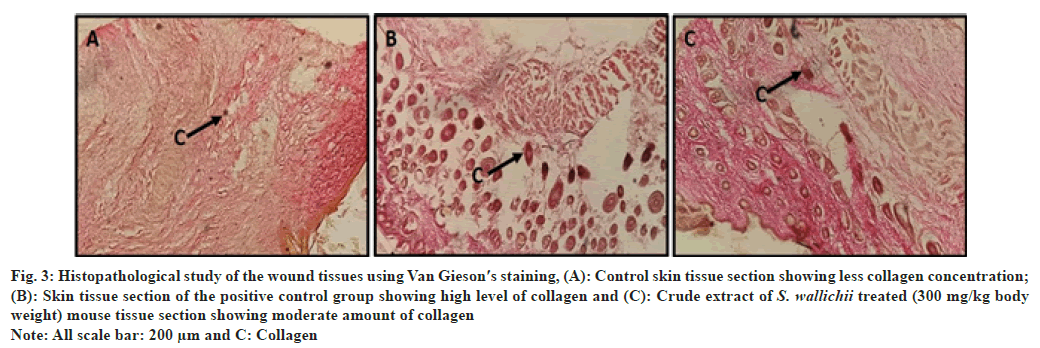
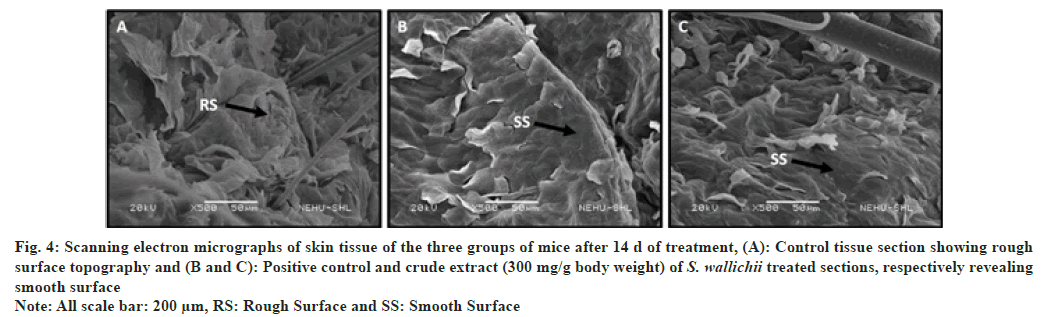
Hematoxylin and eosin stained skin tissues revealed that in case of the extract treated and drug treated groups complete epithelialization of epidermis was clearly visible (fig. 2A-fig. 2C), whereas in the control group the presence of thinner epidermal layer compared to the two treated groups suggests incomplete healing (fig. 2A). Van Gieson′s staining revealed the presence of lesser collagen in control tissue section compared to the tissue section of treated group (fig. 3A). The two treated groups had more reddish colored bundle like components than control section which marks the presence of more collagen in the tissue sections (fig. 3B and fig. 3C). Similar to the histological observation the scanning electron microscopic study showed that control skin had rough surface topography i.e., full of flakes (fig. 4A) and the treated animals had smoother skin surfaces (fig. 4B and fig. 4C) when compared to the control.
Fig. 2: Histological analysis of the wound tissues using hematoxylin and eosin staining, (A): Control skin tissue section showing thin epithelial layer;
(B): Skin tissue section of positive control group showing thickest epithelial layer and (C): Tissue section of the leaf extract treated (300 mg/kg body
weight) group showing thicker epithelial layer compared to the control
Note: All scale bar: 200 μm and EL: Epithelial Layer
Fig. 3: Histopathological study of the wound tissues using Van Gieson′s staining, (A): Control skin tissue section showing less collagen concentration;
(B): Skin tissue section of the positive control group showing high level of collagen and (C): Crude extract of S. wallichii treated (300 mg/kg body
weight) mouse tissue section showing moderate amount of collagen
Note: All scale bar: 200 μm and C: Collagen
Fig. 4: Scanning electron micrographs of skin tissue of the three groups of mice after 14 d of treatment, (A): Control tissue section showing rough
surface topography and (B and C): Positive control and crude extract (300 mg/g body weight) of S. wallichii treated sections, respectively revealing
smooth surface
Note: All scale bar: 200 μm, RS: Rough Surface and SS: Smooth Surface
In terms of mortality and morbidity wounds represent a major health crisis globally. The process of woundhealing is a complex one where many factors work in a coordinated manner to restore the normal functioning of the damaged tissue. The process is promoted by continuous tissue proliferation along with angiogenesis[23,24].
In our present study we have found that the application of the crude extract of the leaves of S. wallichii has significantly enhanced the wound-healing process in the excision wound model. Getie et al.[25] have suggested that when the reduction in the rate of wound contraction is higher, then the efficacy of the therapy used is also higher which supports our observation that the extract has enhanced healing. The application of the extract has caused higher rate of wound area contraction from 7 d onwards compared to the control group. The ability of the extract to fasten the healing process may be due to stimulation of proliferation of the macrophage cells[26]. Similar observations were also recorded by Pather et al.[27], when they studied the in vivo effects of extracts of Bulbine frutescens and Bulbine natatensis on cutaneous wound healing.
According to Allahtavakoli and Khaskar[28] the reduction of wound area is due to wound contraction and connective tissue deposition. The fibroblast cells which possess contracting properties help in the deduction of surface area of the wounds. Beldon et al.[29] also stated that during the healing process migration of fibroblasts to the wound tissue takes place. After reaching the site, the fibroblasts produce collagen which increases the elasticity of the wound as a result of which contraction of the wound takes place. The process of wound contraction reduces the healing time with the help of synthesis of granulation tissue which ultimately repairs the damaged tissue. During the healing process the phenomena of re-epithelialization is very essential as it helps in regeneration of damaged cells and closure of wound surface area[30]. The leaf extract of S. wallichii also has significantly shortened the period of epithelialization compared to the control animals. It may be due to plant extract′s ability to stimulate the rise in the level of synthesis of collagen in the wound granulation tissue[31].
The hematoxylin and eosin staining of the treated skin tissues after 14 d of treatment revealed to have normal epithelialization while the control skin was found to be present in the early stage of epithelialization. So, the faster healing of the wound in the treated groups may be due to the inflammatory cytokine stimulation leading to the deposition of the fibroblasts and keratinocytes, which ultimately may have caused rapid epithelialization by quicker maturation of the granulation tissue[32]. Similarly, incomplete epithelialization in control and complete epithelialization in the treated group as observed through scanning electron microscope where the control epidermis revealed rugged surface and irregular scale like projections compared to polished surface in the extract treated animals support the histological result in the present study. Therefore, it can be said that the application of the extract might have interfered with the various processes of woundhealing like inflammation, collagen synthesis and cell proliferation which ultimately hastened the process of healing compared to the control group[33].
The topical application of the crude extract has enhanced the rate of wound contraction and epithelialization period, these two along with collagenation are crucial phases of wound-healing. Collagenation is interlinked with inflammation, macroplasia and fibroplasia[34]. So, the extract might have interacted with one of the phases leading to the augmentation of collagen in the wound tissue. During wound-healing and tissue repair the process of metabolism and regulation of collagen is very crucial, as the healing of the damaged tissue needs the modular expression of collagen[35]. The synthesis of collagen increases immediately in the wound area after the injury occurs and it is the main constituent present in the granulation tissue. Hydroxyproline is produced from the breakdown of collagen, so the hydroxyproline content in the healing wound is considered as the biomarker for assessment of collagen synthesis[36]. In the present investigation, the data for collagen estimation revealed higher hydroxyproline content in the extract and drug treated groups has been well supported by Van Gieson′s staining, where the control tissue sections revealed to have lesser collagen content than the treated groups. Thus, further suggesting the pro-healing potential of the plant extract.
Steiling et al.[37] stated that DNA and protein content in the healing tissues serve as indicator for protein synthesis and cellular proliferation and according to Subalskshmi et al. higher amount of DNA and protein in the wound tissues is directly linked to the augmentation of collagen level in the concerned tissue. In the present study it is observed that the application of the leaf extract has significantly increased the concentration of DNA and protein in the wound tissues[38]. Higher level of DNA and protein in the extract treated group compared to the control group is an indication that the treatment has enhanced the process of healing[39].
Finally, as the leaves of the plants are applied topically by the traditional healers so it is very essential to see whether the extract is safe or not for topical use. The dermal toxicity test carried out revealed that the methanolic crude extract of leaves of S. wallichii is safe for topical application.
The present preliminary investigation carried out revealed that the crude leaf extract of S. wallichii contains certain compounds having potential to initiate cellular events essential for healing of external wounds. Thus, the in vivo experiment conducted confirms wound-healing properties and also justifies the traditional use of the leaves of the plant as woundhealing agent. However, further detailed clinical study involving active principles of the plant needs to be carried out for human welfare.
Author’s contributions:
Bishnupada Roy framed the work. Deepjyoti Dev and Ashish Sarkar performed the experiment, wrote the manuscript.
Acknowledgements:
The authors would like to acknowledge the University Grants Commission (UGC), New Delhi for providing research fellowship (NEHU NON-NET Fellowship) to Deepjyoti Dev and Ashish Sarkar. Infrastructural support provided by the Department of Zoology and Sophisticated Analytical Instrumental Facility (SAIF), North-Eastern Hill University, Shillong are also gratefully acknowledged.
Conflict of interests:
The authors declared no conflict of interests.
References
- Gosain A, DiPietro LA. Aging and wound healing. World J Surg 2004;28:321-6.
[Crossref] [Google Scholar] [PubMed]
- George Broughton II, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg 2006;117(7S):12S-34S.
[Crossref] [Google Scholar] [PubMed]
- Campos AC, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care 2008;11(3):281-8.
[Crossref] [Google Scholar] [PubMed]
- Roberts PR, LATG KW, Santamauro JT, Zaloga GP. Dietary peptides improve wound healing following surgery. Nutrition 1998;14(3):266-9.
[Crossref] [Google Scholar] [PubMed]
- Alfalah M, Zargham H, Moreau L, Stanciu M, Sasseville D. Contact allergy to polymyxin B among patients referred for patch testing. Dermatitis 2016;27(3):119-22.
[Crossref] [Google Scholar] [PubMed]
- World Health Organisation. Regulatory situation of herbal medicines: A review. Geneva, Switzerland; 1998. p. 1-5.
- Singh R. Medicinal plants: A review. J Plant Sci 2015;3(1):50-5.
- Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med 2013;10(5):210-29.
[Crossref] [Google Scholar] [PubMed]
- Hamburger M, Hostettmann K. Bioactivity in plants: The link between phytochemistry and medicine. Phytochemistry 1991;30(12):3864-74.
- Das P, Sinhababu SP, Dam T. Screening of antihelminthic effects of Indian plant extracts: A preliminary report. J Alternat Complement Med 2006;12(3):299-301.
[Crossref] [Google Scholar] [PubMed]
- Roy B, Giri BR. Carex baccans Nees, an anthelmintic medicinal plant in northeast India. Medicinal plants and its therapeutic uses. New York: OMICS Grp Int; 2017. p. 60-81.
- Dewanjee S, Mandal V, Sahu R, Dua TK, Manna A, Mandal SC. Anti-inflammatory activity of a polyphenolic enriched extract of Schima wallichii bark. Nat Prod Res 2011;25(7):696-703.
[Crossref] [Google Scholar] [PubMed]
- Diantini A, Subarnas A, Lestari K, Halimah EL, Susilawati Y, Supriyatna S, et al. Kaempferol-3-O-rhamnoside isolated from the leaves of Schima wallichii Korth. inhibits MCF-7 breast cancer cell proliferation through activation of the caspase cascade pathway. Oncol Lett 2012;3(5):1069-72.
[Crossref] [Google Scholar] [PubMed]
- Barliana MI, Suradji EW, Abdulah R, Diantini A, Hatabu T, Nakajima-Shimada J, et al. Antiplasmodial properties of kaempferol-3-O-rhamnoside isolated from the leaves of Schima wallichii against chloroquine-resistant Plasmodium falciparum. Biomed Rep 2014;2(4):579-83.
[Crossref] [Google Scholar] [PubMed]
- Simon MK, Nafanda WD, Obeta SS. In vivo evaluation for anthelmintic effect of alkaloids extracted from the stem bark of Afzelia africana in rats. J Adv Sci Res 2012;3(1):100-4.
- Organization of Economic Co-operation and Development. The OECD test guidelines 404: Acute dermal irritation and corrosion. Paris, France; 2002. p. 1-13.
- Morton JJ. Evaluation of vulnerary activity by open wound procedure in rats. Arch Int Pharmacodyn Ther 1972;196:117-20.
[Google Scholar] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75.
[Google Scholar] [PubMed]
- Sambrook J, Russell DW. Precipitation and analyses of eukaryotic genomic DNA in molecular cloning: A laboratory manual. 3rd ed. New York: Cold Spring Harbour Laboratory Press; 2011. p. 51-4.
- Switzer BR, Summer GK. Improved method for hydroxyproline analysis in tissue hydrolyzates. Anal Biochem 1971;39(2):487-91.
[Crossref] [Google Scholar] [PubMed]
- Roy B, Tandon V. Usefulness of tetramethylsilane in the preparation of helminths parasites for scanning electron microscopy. Riv Parassitol 1991;52(3):207-15.
- Dey S, Baul TB, Roy B, Dey D. A new rapid method of air-drying for scanning electron microscopy using tetramethylsilane. J Microscop 1989;156(2):259-61.
[Crossref] [Google Scholar] [PubMed]
- Mathieu D, Linke JC, Wattel F. Non-healing wounds. In: Handbook on Hyperabic Medicine. Springer; 2006. p. 401-28.
- Agarwal PK, Singh A, Gaurav K, Goel S, Khanna HD, Goel RK. Evaluation of wound healing activity of extracts of plantain banana (Musa sapientum var. Paradisiaca) in rats. Indian J Exp Biol 2009;47(1):32-40.
[Google Scholar] [PubMed]
- Getie M, Gebre-Mariam T, Rietz R, Höhne C, Huschka C, Schmidtke M, et al. Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoterapia 2003;74(1-2):139-43.
[Crossref] [Google Scholar] [PubMed]
- Murti K, Kumar U. Enhancement of wound healing with roots of Ficus racemosa L. in albino rats. Asian Pac J Trop Biomed 2012;2(4):276-80.
[Crossref] [Google Scholar] [PubMed]
- Pather N, Viljoen AM, Kramer B. A biochemical comparison of the in vivo effects of Bulbine frutescens and Bulbine natalensis on cutaneous wound healing. J Ethnopharmacol 2011;133(2):364-70.
[Crossref] [Google Scholar] [PubMed]
- Allahtavakoli M, Khaksar M. Assar Sh. Comparison the effect of Mummify and Phenitoin ointment on skin wound healing. J Babol Med Sci Univ 1993;18(5):7-13.
- Beldon P. Basic science of wound healing. Surgery (Oxford) 2010;28(9):409-12.
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3(5):349-63.
[Crossref] [Google Scholar] [PubMed]
- Wang JP, Ruan JL, Cai YL, Luo Q, Xu HX, Wu YX. In vitro and in vivo evaluation of the wound healing properties of Siegesbeckia pubescens. J Ethnopharmacol 2011;134(3):1033-8.
[Crossref] [Google Scholar] [PubMed]
- Moyer KE, Saggers GC, Allison GM, Mackay DR, Ehrlich HP. Effects of interleukin-8 on granulation tissue maturation. J Cell Physiol 2002;193(2):173-9.
[Crossref] [Google Scholar] [PubMed]
- Biswas TK, Mukherjee B. Plant medicines of Indian origin for wound healing activity: A review. Int J Low Extrem Wound 2003;2(1):25-39.
[Crossref] [Google Scholar] [PubMed]
- Devi M, Kumar B. Evaluation of wound healing activity of polyherbal Siddha formulation. J Pharm Biomed Sci 2011;5:6-8.
- Liu RM, Liu Y, Forman HJ, Olman M, Tarpey MM. Glutathione regulates transforming growth factor-β-stimulated collagen production in fibroblasts. Am J Physiol Lung Cell Mol Physiol 2004;286(1):L121-8.
[Crossref] [Google Scholar] [PubMed]
- Trowbridge JM, Gallo RL. Dermatan sulfate: New functions from an old glycosaminoglycan. Glycobiology 2002;12(9):117-25R.
[Crossref] [Google Scholar] [PubMed]
- Steiling H, Munz B, Werner S, Brauchle M. Different types of ROS-scavenging enzymes are expressed during cutaneous wound repair. Exp Cell Res 1999;247(2):484-94.
[Crossref] [Google Scholar] [PubMed]
- Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol 1998;59(3):195-201.
[Crossref] [Google Scholar] [PubMed]
- Singh R, Ali A, Jeyabalan G, Semwal A. An overview of the current methodologies used for evaluation of aphrodisiac agents. J Acute Dis 2013;2(2):85-91.