- *Corresponding Author:
- D. Liu
Shenzhen Key Laboratory of Fermentation, Purification and Analysis, Shenzhen Polytechnic, Shenzhen-518 055, China
E-mail: liudongsz@szpt.edu.cn
| Date of Submission | 19 March 2014 |
| Date of Revision | 02 January 2015 |
| Date of Acceptance | 21 May 2015 |
| Indian J Pharm Sci 2015;77(3):274-282 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The cell-based antioxidant activity assay as more biological relevant assay was considered to be more accurate to predict antioxidant activity in vivo than chemical activity assays. In the present study, the five main Phyllanthus emblica L. cultivars in China were subjected for cellular antioxidant activity based on HepG 2 cells as well as antiproliferative activity. Total phenolics, total flavonoids and oxygen radical absorbance capacity were also measured. The results showed that Qingyougan, Binggan and Boligan (832±100, 774±52 and 704±28 μmol of quercetin equivalents/100 g) had higher cellular antioxidant activity than Tianyougan and Yougan (553±50 and 457±24 μmol of quercetin equivalents/100 g) in phosphate buffered saline wash protocol whereas, Boligan (3735±217 μmol of quercetin equivalents/100 g) had the highest cellular antioxidant activity and Tianyougan (2025±171 μmol of quercetin equivalents/100 g) had the lowest cellular antioxidant activity in no phosphate buffered saline wash protocol. The highest and lowest antiproliferative activities were observed in Binggan and Tianyougan (median effective dose: 6.95±0.11 and 14.03±0.10 mg/ml), respectively. The significant correlation was only observed between total flavonoids and cellular antioxidant activity from no phosphate buffered saline wash protocol (R 2 =0.908, P<0.05), and total flavonoids and antiproliferative activity (R 2 =0.887, P<0.05), suggesting the major contribution of flavonoids to the bioactivities of emblica. Overall, the data obtained revealed that different Phyllanthus emblica L. cultivars had strong cellular antioxidant and antiproliferative activities, thus should be recommended to increase consumption for health.
Keywords
Phyllanthus emblica L., cellular antioxidant activity , antiproliferative activity, oxygen radical absorbance capacity, phenolics, flavonoids
Phyllanthus emblica L., also known as Indian gooseberry, gooseberry, Emblica officinalis, emblica, amla, amalki, or aonla, is widely distributed in the tropical and subtropical areas of China, India, Indonesia and Thailand [1,2]. This plant has been widely used in many traditional medicinal systems, such as Chinese herbal medicine, Tibetan medicine and Ayurveda medicine [3-5]. Various parts of the plant have been used to treat a range of ailments, but the most important part is the fruit. The fruit extract of emblica has been reported to possess anticancer [6], antiinflammatory [7], antiulcer [8], antidiabetic [9], cardioprotective [10], and heptoprotective activities [11] which, directly or indirectly attribute to its antioxidant activity since numerous diseases or disorders are associated with free radicals and oxidative stress [12-14].
Numerous studies have shown that the emblica fruit exhibits a high level of antioxidant capacity to inhibit lipid peroxidation [15,16] and scavenge free radicals [5,17-19] such as superoxide anion radical, hydrogen radical, hydrogen peroxide, nitric oxide radical, which may be due to high contents of phenolics, such as flavonoids (e.g. quercetin) and hydrolysable tannins (e.g. gallic acid and its derivatives, ellagic acid and its derivatives, isocorilagin, chebulanin, chebulagic acid, furosin, geraniin, mallotusinin) [20-22]. Total phenolic content, as well as total flavonoid content, can vary among cultivars, which, may ultimately affect the overall antioxidant activity as well as other good therapeutic properties. Thus, it is of interest to explore the differences in phenolic content and antioxidant activity between different emblica cultivars so as to provide a more complete characterization on the emblica.
Previous studies on the antioxidant activity of emblica fruit have focused mainly on the chemical antioxidant activity assays, such as DPPH radical/ABTS radical/hydroxyl radical/superoxide anion radical scavenging assays [17,23], the ferric reducing/antioxidant power (FRAP) assay [23], and ferrous ion chelating ability assay [20]. However, the ability of the chemical antioxidant activity assay to predict in vivo activity is questioned [24] since biological systems are much more complex than the simple chemical mixtures employed. The cellular antioxidant activity (CAA) assay, a more biological representative method, has been developed to measure the antioxidant activity of antioxidants, dietary supplements, and foods in cell culture [25]. The CAA assay utilizes 2’,7’-dichlorofluorescin diacetate (DCFH-DA) as a probe in cultured human HepG2 cells, which is deacetylated by cellular esterases to form polar 2’,7’-dichlorofluorescin (DCFH) and then fluoresces when oxidized by peroxyl radicals to dichlorofluorescein (DCF). The HepG2 cell-based CAAs of a variety of fruits and vegetables have been determined [26,27], but there is no report on the HepG2 cell-based CAA of different kinds of emblica, despite that the CAA of emblica from Thailand was determined by using human myelokeukemic U937 cells, which showed that the water extract of emblica possess stronger cellular antioxidant effect than gallic acid [23].
The objective of this study was to measure the HepG2 cell-based CAAs of the five main Phyllanthus emblica L. cultivars in China (Qingyougan, Yougan, Tianyougan, Boligan and Binggan), as well as the oxygen radical absorbance capacity (ORAC) and the antiproliferative activity on HepG2 human liver cancer cell growth. Besides, total phenolics and total flavonoids were also measured to further examine the correlations between antioxidant activity or antiproliferative activity and total phenolics or total flavonoids.
Materials and Methods
Folin-Ciocalteu reagent, gallic acid, catechin hydrate, fluorescein disodium salt, 6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid (Trolox), 2,2’-azobis(2-amidinopropane)dihydrochloride (AAPH), 2’,7’-dichlorofluorescin diacetate (DCFH-DA), 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid (Hepes), Williams’ Medium E (WME), trypan blue, dimethyl sulfoxide, and methylene blue were purchased from Sigma-Aldrich, St. Louis, MO, USA. Sodium hydroxide, sodium carbonate, aluminium chloride, sodium nitrite, acetone, ethanol, ethyl acetate, glutaraldehyde and acetic acid were obtained from Guangzhou Chemical, Guangzhou, China. Fetal bovine serum (FBS), 0.05% Trypsin- EDTA and Hanks’ balanced salt solution (HBSS) were purchased from Gibco Life Technologies, Grand Island, NY, USA. The HepG2 human liver cancer cells were obtained from the American Type Culture Collection (ATCC), Rockville, MD, USA.
Preparation of Phyllanthus emblica L. extracts
The five main Phyllanthus emblica L. cultivars in China (Qingyougan, Yougan, Tianyougan, Boligan and Binggan), harvested at the edible maturity stage in Zhangpu County, Fujian Province between September and October, and authenticated by Fruit Tree Research Institute (Guangzhou, China), were purchased from a local market (Buji, Shenzhen, China). Extracts were prepared from the edible portions of fresh fruits using a modified method, as reported previously [26]. Briefly, in triplicate, a weight of 100 g of fresh emblica fruit was homogenized in 200 ml of 70% chilled acetone with a high speed homogenizer for 5 min. The mixture was then centrifuged at 12 000 g for 10 min. The supernatant was removed and the residue was again extracted with 200 ml of 70% chilled acetone. The centrifugation and the supernatant removal were also repeated again. The two aliquots of supernatant were pooled and evaporated at 45º to dryness. The final extracts were diluted with deionized water to a final volume of 10 ml and stored at -80º until use. Control extracts were prepared using the same extraction solvents and procedures without emblica fruits.
Determination of total phenolic content
The total phenolic content was measured using the colorimetric Folin-Ciocalteu method reported previously [28] with slight modifications. Briefly, volumes of 0.4 ml of deionized water and 0.1 ml of diluted extracts were added to a test tube. A volume of 0.1 ml of Folin-Ciocalteu reagent was then added to the solution and allowed to react for 6 min. After reaction, volumes of 1 ml of 7% sodium carbonate and 0.8 ml of deionized water were added to the test tube. The color was developed for 90 min, and the absorbance was read at 760 nm using Spectra Max M2 spectrophotometer (MD, USA). The measurement was compared to a standard curve of gallic acid, obtained from a known concentration range of gallic acid standards prepared similarly as the sample. All results were expressed as grams of gallic acid equivalents (GAE) per 100 g fresh emblica fruit. Data were shown as mean±SD for three replications.
Determination of total flavonoid content
The total flavonoid content was measured using aluminium chloride-sodium nitrite method [29] with some modifications in this study. That is, volumes of 2 ml of diluted extracts and 75 μl of 5% sodium nitrite was added to the test tube and allowed to react for 6 min. Then, a 150 μl aliquot of 10% aluminium chloride was added to the test tube. After 5 min, a 0.5 ml aliquot of 1 M sodium hydroxide was added, and the mixture was diluted to a final volume of 3 ml with deionized water. The absorbance of the final mixture was read immediately at 510 nm using Spectra Max M2 spectrophotometer (MD, USA). The measurement was compared to the standard curve of catechin and expressed as milligrams of catechin equivalents per 100 g fresh emblica fruit. Data were shown as mean±SD for three replications.
Determination of total antioxidant activity
The total antioxidant activity of the five emblica cultivars extracts was measured using the oxygen radical absorbance capacity (ORAC) assay [26]. That is, a 20 μl aliquot of blank, Trolox standard, or emblica extracts in 75 mM potassium phosphate buffer (pH 7.4, working buffer), was added to triplicate wells in a black, clear-bottom, 96-well microplate. The triplicate samples were distributed throughout the microplate and were not placed side-by-side, to avoid any effects on readings due to location. A volume of 200 μl of 0.96 μM fluorescein in working buffer was added to each well and incubated at 37° for 20 min, with intermittent shaking, before the addition of 20 μl of freshly prepared 119 mM AAPH in working buffer. The microplate was immediately inserted into a Fluoroskan Ascent FL plate reader (MD) at 37°. The decay of fluorescence at 538 nm was measured with excitation at 485 nm every 4.5 min for 2.5 h. The areas under the fluorescence versus time curve for the samples minus the area under the curve for the blank were calculated and compared to a standard curve of the areas under the curve for 6.25, 12.5, 25, and 50 μM Trolox standards minus the area under the curve for blank. ORAC values were expressed as micromoles of Trolox equivalents (TE) per 100 g fresh emblica fruit. Data were shown as mean±SD for three replications from one experiment.
Cell culture
HepG2 cells were grown in growth medium (WME supplemented with 5% FBS, 10 mM Hepes, 5 μg/ml insulin, 0.05 μg/ml hydrocortisone, 50 units/ml penicillin, 50 μg/ml streptomycin and 100 μg/ml gentamicin) and were maintained at 37º in 5% CO2 as described previously [26]. Cells used in this study were between passages 12 and 35.
Cytotoxicity
The cytotoxicity of the five emblica cultivars extracts toward HepG2 cells was measured using the colorimetric methylene blue assay reported previously [25]. HepG2 cells were seeded at 4×104/well on a 96-well microplate in 100 μl of growth medium at 37°. Twenty-four hours after seeding, the growth medium was removed, and the cells were washed with 100 μl of phosphate buffered saline (PBS). Then, a volume of 100 μl of treatment medium (WME supplemented with 2 mM L-glutamine and 10 mM Hepes) containing various concentrations (10, 25, 50, 100, 150, 200 and 250 mg/ml) of extracts were applied to the cells, and the microplates were incubated at 37° for 24 h. The treatment medium was removed, and the cells were washed with PBS. A volume of 50 μl/well methylene blue staining solution (98% HBSS, 0.67% glutaraldehyde, 0.6% methylene blue) was applied to each well, and the microplate was incubated at 37° for 1 h. The dye was removed, and the plate was immersed in fresh deionized water until the water was clear. The water was tapped out of the wells, and the microplate was allowed to air-dry briefly before 100 μl of elution solution (49% PBS, 50% ethanol, 1% acetic acid) was added to each well. Then the microplate was placed on a bench-top shaker for 20 min to allow uniform elution. The absorbance was read at 570 nm with blank subtraction using the Spectra Max M2 spectrophotometer (MD, USA). Concentrations of extracts that decreased the absorbance to a significant difference (P<0.05) when compared with the control were considered to be cytotoxic.
Cellular antioxidant activity of emblica extracts
The CAA assay was reported previously [25-27]. Briefly, HepG2 cells were seeded at a density of 6×104/well on a 96-well microplate in 100 μl of growth medium/well. Twenty four hours after seeding, the growth medium was removed, and the wells were washed with 100 μl of PBS. Wells were treated in triplicate for 1 h with 100 μl of treatment medium containing solvent control, control extracts or extracts plus 25 μM DCFH-DA. When a PBS wash was utilized, wells were washed with 100 μl of PBS. Then 600 μM AAPH was added to the cells in 100 μl of HBSS, and the 96-well microplate was placed into a Fluoroskan Ascent FL plate reader at 37º. Emission at 538 nm was measured after excitation at 485 nm every 5 min for 1 h.
Quantification of CAA
As described previously [25], the area under fluorescence versus time curve was integrated after blank subtraction and subtraction of the initial fluorescence values. The CAA values of extracts at each concentration were calculated using the following formula, CAA unit=1- ∫SA/∫CA, where, ∫ SA is the integrated area under the sample fluorescence versus time curve and ∫CA is the integrated area from the control curve. The median effective dose (EC50) was determined from the median effect plot of log(fa/ fu) versus log(dose), where fa is the fraction affected (CAA unit) and fu is the fraction unaffected (1-CAA unit) by the treatment. The EC50 values were reported as mean±SD for triplicate sets of data obtained from the same experiment. EC50 values were converted to CAA values, expressed as micromoles of quercetin equivalents (QE) per 100 g fresh emblica fruit, using the mean EC50 value for quercetin from five separate experiments. Measurement
Measurement of antiproliferative activity
The antiproliferative activity of the five emblica cultivars extracts was determined by measurement of the inhibition of HepG2 human liver cancer cell proliferation using the colorimetric methylene blue assay reported previously [30]. Briefly, HepG2 cells were seeded at a density of 2.5×104/well on a 96-well microplate in 100 μl of growth medium/well. Four hours after seeding, the growth medium was removed, and media containing various concentrations (0, 5, 10, 25, 50, 100, 150 and 200 mg/ml) of extracts were added to the wells. Control cultures received the extraction solution minus the extracts, and blank wells contained 100 μl of growth medium without cells. After 96 h of incubation, the treatment medium was removed, the cells were washed with PBS, dyed, washed and eluted. The absorbance was read at 570 nm with blank subtraction. Cell proliferation (percent) was calculated from the comparison between the sample absorbance reading at each concentration and the control absorbance, using at least three replications for each sample. The median effective dose (ED50) was determined and expressed as mg of extract per ml. Data were shown as mean±SD.
Statistical analysis
All data were reported as mean±SD for three replications. Statistical analysis was performed using SPSS V20.0 software (SPSS Inc, Chicago). Differences between means were analyzed by One-Way ANOVA test. Correlations between various parameters were also investigated using regression analysis. Significance was determined at P<0.05.
Results and Discussion
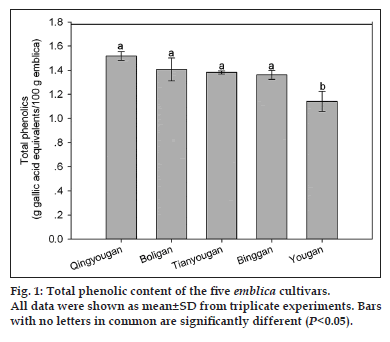
The total phenolic content of the five main Phyllanthus emblica L. cultivars in China was determined from their 70% acetone extracts by using the Folin-Ciocalteu method (fig. 1). Seventy percent acetone was used as extraction solvent in this study based on our preliminary experiments, which, showed that the emblica extract from 70% acetone had higher content of phenolics and higher level of antioxidant activity than the extract from other solvents. Singh et al. [31], also reported that the acetone extract exhibited better hydroxyl radical scavenging activity than methanol or chloroform extract for the emblica containing triphala. It could be found from fig. 1 that Yougan had the lower phenolic content (1.142±0.084 g of GAE/100 g) as being compared with other four emblica cultivars extracts (P<0.05), and there was no significant difference in the total phenolic contents between Qingyougan (1.518±0.037 g of GAE/100 g),Boligan (1.407±0.095 g of GAE/100 g), Tianyougan (1.382±0.013 g of GAE/100 g) and Binggan (1.362±0.037 g of GAE/100 g), averaging 1.417 g of GAE/100 g. There was a 75% difference in phenolic content between the highest and the lowest cultivars (P<0.05). It should be noted that the phenolic content determined in this study was lower than the previous reports [18,32]. The main reason lied in that data expressed here were based on the 100 g fresh fruit rather than 100 g dried fruit.
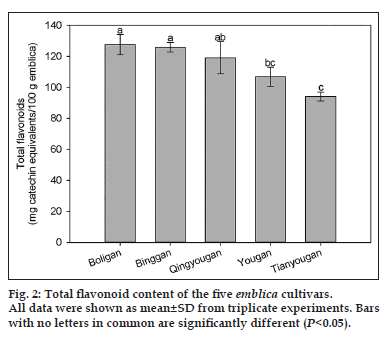
Flavonoid contents of the five Phyllanthus emblica L. cultivars are shown in fig. 2. Boligan, Binggan and Qingyougan had the highest total flavonoid content (127.6±6.5, 125.8±3.1, and 117.8±10.3 mg of catechin equivalents/100 g, respectively), followed by Yougan (106.7±6.1 mg of catechin equivalents/100 g), which was statistically similar to Qingyougan. The lowest flavonoid content was observed in Tianyougan (94.1±2.9 mg of catechin equivalents/100 g), but statistically, this cultivar was similar to Yougan. There was a 74% difference in flavonoid content between the highest and the lowest cultivars (P<0.05), similar to phenolic content, despite of the different relative ranking of cultivars for phenolics and flavonoids.
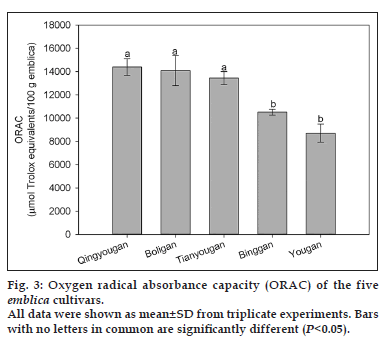
The total antioxidant activities of the five Phyllanthus emblica L. cultivars were measured by the ORAC assay and expressed as micromoles of Trolox equivalents (TE) per 100 g of emblica (fig. 3). Qingyougan (14387.0±707.2 μmol of TE/100 g), Boligan (14087.5±1289.5 μmol of TE/100 g) and Tianyougan (13433.6±558.6 μmol of TE/100 g) (P>0.05) which, were not significantly different from each other (P>0.05), exhibited higher peroxyl radical scavenging ability than Binggan and Yougan (10519.2±237.2, 8708.1±784.7 μmol of TE/100 g, respectively), which were also statistically similar to each other (P>0.05). There was a 60% difference in antioxidant activity between the highest and the lowest cultivars (P<0.05). Besides, the relative ranking of cultivars for ORAC values was the same as that for total phenolic content. Additionally, a significantly positive relationship was observed between total phenolics and ORAC values (R2=0.891, P<0.05), indicating the major contribution of the phenolics to the peroxyl radical scavenging ability. The positive correlation between phenolics and antioxidant activity was also reported in many other fruits and vegetables [26,27].
Since the consideration of some aspects of cell uptake, metabolism, and distribution of bioactive compounds, the CAA assay is an improvement over the traditional chemical antioxidant activity assays [25]. Thus, the cellular antioxidant activities of the five Phyllanthus emblica L. cultivars were evaluated using the CAA assay. The EC50 for the five cultivars, along with their median cytotoxicity doses (CC50), is listed in Table 1. The CAA was measured using two protocols, as described previously [25-27] in the PBS wash, the HepG2 cells were washed with PBS between the emblica extract and AAPH treatments; in the no PBS wash, the HepG2 cells were not washed between the emblica extract and AAPH treatments.
| Cultivars | EC50(mg/ml) | Cytotoxicity(mg/ml) | ||
|---|---|---|---|---|
| PBS wash | no PBS wash | |||
| Qingyougan | 1.11±0.13 | 0.278±0.040 | >20 | |
| Binggan | 1.12±0.07 | 0.271±0.023 | >20 | |
| Boligan | 1.24±0.05 | 0.212±0.013 | >20 | |
| Tianyougan | 1.67±0.16 | 0.393±0.032 | >20 | |
| Yougan | 2.01±0.10 | 0.367±0.013 | >20 | |
Table 1: Cellular antioxidant activity and cytotoxicity of the five Phyllanthus emblica L. Cultivars
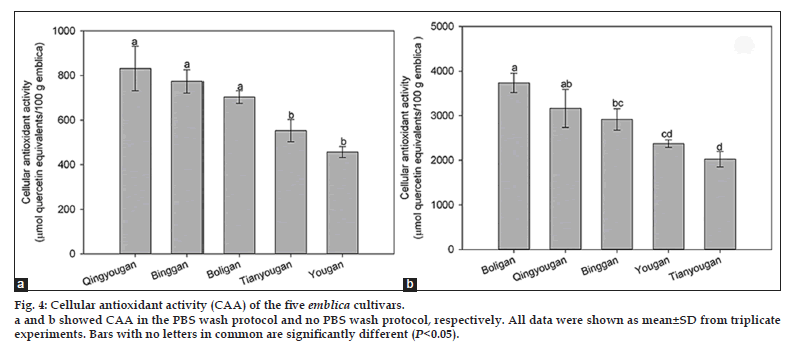
The CAA values of the five Phyllanthus emblica L. cultivars in the PBS wash protocol are shown in fig. 4a. Qingyougan (832±100 μmol of QE/100 g), Binggan (774±52 μmol of QE/100 g) and Boligan (704±28 μmol of QE/100 g), which were not significantly different from each other (P>0.05), had the greater cellular antioxidant activity than Tianyougan (553±50 μmol of QE/100 g) and Yougan (457±24 μmol of QE/100 g), which were also not significantly different from each other (P>0.05). There was significant difference between Boligan and Tianyougan (P<0.05). There was a 59% difference in CAA values from the PBS wash protocol between the highest and the lowest cultivars (P<0.05), similar to the ORAC values. Additionally, for ORAC values and CAA values from the PBS wash, the highest and the lowest cultivars were identical, that is, Qingyougan of the highest while Yougan of the lowest.
The CAA values of the five Phyllanthus emblica L. cultivars in the no PBS wash protocol are shown in fig. 4b. Boligan showed the highest cellular antioxidant activity with a CAA value of 3735±217 μmol of QE/100 g, followed by Qingyougan (3164±426 μmol of QE/100 g), Binggan (2918±240 μmol of QE/100 g), Yougan (2373±85 μmol of QE/100 g) and Tianyougan (2025±171 μmol of QE/100 g). There were no significant differences between Boligan and Qingyougan, Qingyougan and Binggan, Binggan and Yougan, Yougan and Tianyougan (P>0.05). There was a 54% difference in CAA values from the no PBS wash protocol between the highest and the lowest cultivars (P<0.05). Similar to the CAA values from the no PBS wash protocol, the highest and the lowest flavonoid contents were also observed in Boligan and Tianyougan, respectively. Besides, correlation analysis also showed that total flavonoids were significantly related to CAA values from the no PBS wash protocol (R2=0.908, P<0.05), suggesting that flavonoids might be responsible for the cellular antioxidant activity of the emblica. Quercetin, the major flavonoid in emblica [32], was reported to possess strong cellular antioxidant activity [25].
In addition, all the five emblica cultivars showed much higher level of CAAs than previously reported vegetables and fruits [26,27], consistent with the ORAC values, which might be attributed to much higher phenolic content in emblica. However, no significant correlation was observed in the five emblica cultivars between total phenolics and CAA values from the PBS wash protocol (R2=0.835, P>0.05) and no PBS wash protocol (R2=0.483, P>0.05), which, might be due to the synergistic or antagonistic interactions between different phenolics [33]. Besides, all the five emblica cultivars extracts displayed dramatically lower CAA values when a PBS wash was done between the emblica extract and AAPH treatments, implying that the some bioactive compounds in the tested extract adsorbed loosely to the membrane and were taken up less readily. The similar results were shown in gallic acid and ascorbic acid [25], which, were of large amount in emblica [22,34].
The antiproliferative activities of the five Phyllanthus emblica L. cultivars were determined from the inhibition of their extracts toward the growth of HepG2 cells in vitro using the methylene blue assay. In fig. 5, the proliferation of HepG2 cell was inhibited in a dose-dependent manner after exposure to all five extracts. The antiproliferative activity was also expressed as the median effective dose, ED50 (fig. 6) where, a lower ED50 value signified a higher antiproliferative activity. Binggan had the greatest antiproliferative activity with the lowest ED50 value (6.95±0.11 mg/ml), followed by Qingyougan and Boligan (9.95±0.30 and 10.06±0.49 mg/ml, respectively), and then Yougan (11.83±0.12 mg/ml). The ED50 of Tianyougan (14.03±0.10 mg/ml) was significantly higher than all other cultivars (P<0.05), in other words, Tianyougan was of the lowest antiproliferative activity. Correlation analysis showed that the antiproliferative activity was only significantly correlated to the total flavonoid content (R2=0.887, P<0.05), suggesting the contribution of flavonoid to the antiproliferative activity. Liu et al. [2] reported that quercetin, kaempferol and their glycosides exhibited inhibitory effect against MCF-7 cells. Acylated apigenin glucoside, a flavonoid compound from emblica, was also reported to possess potent inhibitory on the growth of tumor cell lines [35].
In this study, the antioxidant and antiproliferative activities of the five main Phyllanthus emblica L. cultivars in China (Qingyougan, Yougan, Tianyougan, Boligan and Binggan), as well as total phenolics and flavonoids, were evaluated from their 70% acetone extracts. The results indicated that total phenolics were significantly correlated to ORAC values (R2=0.891, P<0.05), while total flavonoids were significantly correlated to CAA values from no PBS wash protocol (R2=0.908, P<0.05) and antiproliferative activiy (R2=0.887,P<0.05). Although the CAA values, ORAC values and antiproliferative activities were not significantly correlated with each other for the five emblica cultivars in this study, the significant differences were observed among cultivars in respect to the above activities as well as the total phenolics and flavonoids. This knowledge could be useful for consumers to estimate the value of each cultivar. However, a more detailed investigation on the individual phenolic compound present in different emblica cultivars needs to be carried out so as to provide a more complete characterization on the health benefits of antioxidant and antiproliferative activities of different emblica cultivars. Overall, this study shows that different Phyllanthus emblica L. cultivars are of strong antioxidant and antiproliferative activities, thus should be recommended to eat daily for health.
Financial support and sponsorship
The authors are grateful for the financial support from Guangdong Natural Science Foundation (No. S2011010004455), Shenzhen Science and Technology Plan Project (No. JC201005280530A) and GDHVPS (2011).
Conflict of interest
There are no conflicts of interest.
References
- Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol 2000;71:23-43.
- Liu X, Zhao M, Wu K, Chai X, Yu H, Tao Z, et al.Immunomodulatoryand anticancer activities of phenolics from emblicafruit (PhyllantliusemblicaL.). Food Chem 2012;131:685-90.
- Xiang Y, Pei Y, Qu C, Lai Z, Ren Z, Yang K, et al. In vitroAntiherpessimplex virus activity of 1,2,4,6-tetra-O-galloyl-beta-D-glucose from PhyllanthusemblicaL. (Euphorbiaceae).Phytother Res 2011;25:975-82.
- Zhang LZ, Zhao WH, Guo YJ, Tu GZ, Lin S, Xin LG. Studies on chemical constituents in fruits of Tibetan medicine Phyllanthusemblica. China J Chin Mater Med 2003;28:940-3.
- Poltanov EA, Shikov AN, Dorman HJ, Pozharitskaya ON, Makarov VG, Tikhonov VP, et al. Chemical and antioxidant evaluation of Indian gooseberry (EmblicaofficinalisGaertn., syn.PhyllanthusemblicaL.) supplements. Phytother Res 2009;23:1309-15.
- Mahata S, Pandey A, Shukla S, Tyagi A, Husain SA, Das BC, et al.Anticancer activity of PhyllanthusemblicaLinn. (Indian Gooseberry):Inhibition of transcription factor AP-1 and HPV gene expression in cervical cancer cells. Nutr Cancer 2013;65:88-97.
- Dang GK, Parekar RR, Kamat SK, Scindia AM, Rege NN. Antiinflammatory activity of Phyllanthusemblica,Plumbagozeylanicaand Cyperusrotundusin acute models of inflammation. Phytother Res2011;25:904-8.
- Pakrashi A, Pandit S, Bandyopadhyay SK, Pakrashi SC. Antioxidant effect of Phyllanthusemblica fruits on healing of indomethacin-induced gastric ulcer in rats. Indian J ClinBiochem 2003;18:15-21.
- Akhtar MS, Ramzan A, Ali A, Ahmad M. Effect of amla fruit (EmblicaofficinalisGaertn.) on blood glucose and lipid profile of normal subjectsand type 2 diabetic patients. Int J Food SciNutr 2011;62:609-16.
- Ojha S, Golechha M, Kumari S, Arya DS. Protective effect of Emblicaofficinalis(amla) on isoproterenol-induced cardiotoxicity in rats. ToxicolInd Health 2012;28:399-411.
- Damodara Reddy V, Padmavathi P, Gopi S, Paramahamsa M, Varadacharyulu NC. Protective effect of Emblicaofficinalisagainst alcohol-induced hepatic injury by ameliorating oxidative stress in rats. Indian J ClinBiochem 2010;25:419-24.
- Nain P, Saini V, Sharma S, Nain J. Antidiabetic and antioxidant potential of EmblicaofficinalisGaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J Ethnopharmacol 2012;142:65-71.
- Golechha M, Bhatia J, Arya DS. Studies on effects of Emblicaofficinalis(amla) on oxidative stress and cholinergic function inscopolamine-induced amnesia in mice. J Environ Biol 2012;33:95-100.
- Chen TS, Liou SY, Chang YL. Supplementation of Emblicaofficinalis (amla) extract reduces oxidative stress in uremic patients. Am J Chin Med 2009;37:19-25.
- Nampoothiri SV, Prathapan A, Cherian OL, Raghu KG, Venugopalan VV, Sundaresan A. In vitro antioxidant and inhibitory potential of TerminaliabellericaandEmblicaofficinalisfruits against LDL oxidation and keyenzymes linked to type 2 diabetes. Food ChemToxicol 2011;49:125-31.
- Jayasinghe C, Gotoh N, Wada S. Pro-oxidant/antioxidant behaviours of ascorbic acid, tocopherol, and plant extracts in n-3 highly unsaturated fatty acid rich oil-in-water emulsions. Food Chem 2013;141:3077-84.
- Hazra B, Sarkar R, Biswas S, Mandal N. Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminaliachebula, Terminaliabelerica and Emblicaofficinalis. BMC Complement Altern Med 2010;10:20.
- Liu X, Zhao M, Wang J, Yang B, Jiang Y. Antioxidant activity of methanolic extract of emblica fruit (Phyllanthusemblica L.) from six regions in China. J Food Compos Anal 2008;21:219-28.
- Kumaran A, Karunakaran RJ. Nitric oxide radical scavenging active components from PhyllanthusemblicaL. Plant Food Hum Nutr2006;61:1-5.
- Luo W, Zhao M, Yang B, Ren J, Shen G, Rao G. Antioxidant and antiproliferative capacities of phenolics purified from PhyllanthusemblicaL. fruit. Food Chem 2011;126:277-82.
- Liu X, Zhao M, Wang J, Luo W. Antimicrobial and antioxidant activity of emblica extracts obtained by supercritical carbon dioxide extraction and methanol extraction. J Food Biochem 2009;33:307-30.
- Salimath PV, Kumar GS, Nayaka H, Dharmesh SM. Free and bound phenolic antioxidants in amla ( Emblicaofficinalis) and turmeric (Curcuma longa). J Food Compos Anal 2006;19:446-52.
- Charoenteeraboon J, Ngamkitidechakul C, Soonthornchareonnon N, Jaijoy K, Sireeratawong S. Antioxidant activities of the standardized water extract from fruit of PhyllanthusemblicaLinn. Songklanakarin J SciTechnol2010;32:599-604.
- Liu RH, Finley J. Potential cell culture models for antioxidant research. J Agric Food Chem 2005;53:4311-4.
- Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 2007;55:8896-907.
- Song W, Derito CM, Liu MK, He X, Dong M, Liu RH. Cellular antioxidant activity of common vegetables. J Agric Food Chem 2010;58:6621-9.
- Wolfe KL, Kang X, He X, Dong M, Zhang Q, Liu RH. Cellular antioxidant activity of common fruits. J Agric Food Chem 2008;56:8418-26.
- Chu YF, Sun J, Wu XZ, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem 2002;50:6910-6.
- Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH. Antioxidant and antiproliferative activities of raspberries. J Agric Food Chem 2002;50:2926-30.
- Felice DL, Sun J, Liu RH. A modified methylene blue assay for accurate cell counting. J Funct Foods 2009;1:109-18.
- Singh R, Singh B, Kumarb N, Arora S. Antioxidant activity of triphalaa combination of Terminaliachebula, Terminaliabellerica and Emblicaofficinalis. J Food Biochem 2010;34:222-32.
- Judprasong K, Charoenkiatkul S, Thiyajai P, Sukprasansap M. Nutrients and bioactive compounds of Thai indigenous fruits. Food Chem 2013;140:507-12.
- Heinonen IM, Meyer AS, Frankel EN. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation.
- Raghu V, Platel K, Srinivasan K. Comparison of ascorbic acid content of Emblicaofficinalis fruits determined by different analytical methods.
- El-Desouky SK, Ryu SY, Kim YK. A new cytotoxic acylatedapigeninglucoside from PhyllanthusemblicaL. Nat Prod Res 2008;22:91-5.
 Control,
Control, Tianyougan,
Tianyougan, Yougan,
Yougan, Boligan,
Boligan, Qingyougan,
Qingyougan, Binngan.
Binngan.



