- *Corresponding Author:
- Irene J. Furtado
Department of Microbiology, Goa University, Taleigao Plateau, Goa-403 203, India
E-mail: ijffurtado@unigoa.ac.in
| Date of Submission | 29 April 2014 |
| Date of Revision | 16 November 2014 |
| Date of Acceptance | 26 March 2015 |
| Indian J Pharm Sci 2015; 77(2):183-189 |
Abstract
Marine invertebrates exposed to high levels of reactive oxygen species in the oceans have been reported to produce antioxidants as a major defense against free radical mediated toxicity; protecting their tissues from the damage associated with the oxidative stress. In view of this, the present study was carried out to determine the antioxidant activity of 100 bacterial bionts isolated from marine sponges, corals and a single bivalve. Methanol extract of biont GUVFCFM-3 produced 67.83% scavenging of 2,2-diphenyl-2-picrylhydrazyl free radicals and 65.87% scavenging of superoxide free radicals. Preliminary tests leading to the identification of the extracellular antioxidant factor produced by GUVFCFM-3 revealed that it is a peptide. We report that the genera Chromohalobacter sp. primarily known for its unique salt tolerating abilities by virtue of the production of osmolytes is an excellent scavenger of free radicals.
Keywords
Antioxidant, bacterial bionts, Chromohalobacter sp., invertebrates
Globally efforts are underway to isolate microbes and harness their pharmacological principles. Flavonoids, phenols, lycopene, carotenes, lutein, resveratrol exhibiting antioxidant properties have been reported from grains, cereals, medicinal herbs, plants, eggs, meat, legumes, nuts [1,2]. However it is only in the recent years that reports are emerging on the antioxidant potential of marine invertebrates [3‑5], although antioxidant factors have been reported from microorganisms such as B. cereus, Lactobacillus sp., Micrococcus freudenreichii, S. lutea, Streptomyces sp. isolated from milk, feather protein hydrolysate and marine sediments [6,7]. Marine organisms such as the invertebrates; particularly those inhabiting the intertidal reaches are exposed to stressful vagaries of nature. To fight the stress posed by the high level of ROS, which is triggered through a combination of photosynthesis, symbiont oxygen production, intense light intensities and UV radiation [8‑10], they are known to produce antioxidant compounds. In reality, the microorganisms living as exobionts and endobionts in the tissue of such organisms are implicated in combating the oxidative stress that causes damage to host cells and tissue.
Despite the increasing importance, the ability of marine bacterial bionts producing antioxidant compounds has been sparingly studied [11,12]. In the light of the above, we report a major screening effort to quantitatively assess the antioxidant potential of bacterial bionts (marine halophiles/archaea) from several dominant marine invertebrates (sponges, corals and bivalve) collected from the Bay of Mandapam, an important coral reef ecosystem of Southern India.
Materials and Methods
Marine Invertebrates
Nine marine sponges namely Petrosia testudinaria, Cinachyra cavernosa, Haliclona sp., Unidentified sponge (MAM 5), Callyspongia fibrosa, Heteronema erecta, Callyspongia reticutis var solomonensis, Fasciospongia cavernosa, Unidentified sponge (NIO 3), five marine corals namely Telesto sp., Echinogorgia reticulata, Echinomuricea indica, Echinogorgia complexa, Acropora formosa and one marine bivalve Perna viridis were sampled from the subtidal regions of India. The invertebrates were collected from Mandapam, Tamil Nadu on the South Eastern coast of the Indian peninsula situated at (Lat. 09°19' 37.3'' N and Long. 79° 10' 20.5'' E), by scuba diving at depths of 8‑10 m, 2 nautical miles off the South Eastern Coast in the Gulf of Mannar (GoM).
Isolation of halophilic bacterial bionts
Hundred halophilic bacterial bionts associated with marine invertebrates isolated previously by the authors [13], and earlier reported for its antibacterial activity [14], were used in this study. The bacterial strains were isolated and maintained on halophilic medium namely, tryptone yeast extract agar slopes (TYE) [15] consisting of (g/l) MgSO4‑20; CaCl2‑0.2; tryptone‑5; yeast extract‑3, pH adjusted to 7, using 1N NaOH, supplemented with 3% NaCl (3% TYE) and TYE supplemented with 25% NaCl (NTYE) [16], respectively.
Screening of isolates for antioxidant activity‑qualitative assessment
Hundred halophilic bionts were individually inoculated on 3% TYE and NTYE agar plates using a template of 15 squares. Plates were incubated at ambient temperature (27‑30°) for a period of 7 days for cultures growing on NTYE, and for 24 h, for cultures growing on 3% TYE. Thereafter, a sterile filter paper (Whatman no. 1) was placed on the growth on agar plates and further incubated for a day at room temperature. The filter paper having replicate of growth was then taken out and sprayed with 2,2‑diphenyl‑2‑picrylhydrazyl (DPPH) solution (80 μg/ml in ethanol), allowed to dry at ambient temperature (27‑30°) until white spots were formed around colonies against a purple background. The isolates selected for quantitative assessment based on the initial screening results were subjected to identification following sequencing of the 16S rDNA gene.
Preparation of crude extracts for demonstration of extracellular antioxidant factor
Isolates screened positive in the preliminary screening efforts were selected for further quantitative assays for which individual bacterial bionts were grown in 3% TYE and NTYE at (27‑30°) for a minimum of 2 days for cultures growing in 3% TYE and a maximum of 7 days for cultures growing on NTYE at 150 rpm (RS 24 BL; Remi, India). The cell free supernatant obtained on separation of cells by centrifuging at 12 000×g for 20 min at 4° (C‑24BL, Remi, India) was extracted three times in ethyl acetate (EA), concentrated to dryness under vacuum using rotary evaporator (Buchi, Germany). Individual crude extracts were weighed and dissolved in methanol to get a 1000 μg/ml test solution of crude extracts. All the experiments were carried out in triplicate and appropriate solvent control was maintained.
Evaluation of 1,1‑diphenyl‑2‑picryl hydrazyl (DPPH) free radical scavenging ability of crude biont extracts; quantitative assessment
The free‑radical scavenging capacity of various extracts of bionts was tested by its ability to bleach the stable DPPH radical according to the method of Zho and Elledge 2000 [17]. To 1.5 ml of 0.1 mM DPPH solution in ethanol, 1.5 ml of 1 mg/ml concentration of individual extracts or L‑ascorbic acid (the standard compound), were added to make the working volume of 3 ml. The mixture was shaken vigorously and allowed to reach a steady state for 30 min at (27‑30°) Decolourization of DPPH was determined by measuring the decrease in absorbance at 517 nm, and the free radical scavenging activity was calculated using the formula, % DPPH scavenging activity = (AControl−ATest)/(AControl)×100, where, AControl is the absorbance of control, ATest is the absorbance of crude extracts.
Absorbance of DPPH solution (AControl) was measured against 95% ethanol blank. Absorbance of test reagent (ATest) was read immediately after 30 min. L‑ascorbic acid (0.1%) was used as the standard for comparison. Experiments were performed in triplicate, and the results averaged. Extracts of bionts showing highest scavenging activity were prepared from 0.1% stock solution to find the effective concentration or EC50/IC50 value, which is the concentration of substrate that causes 50% loss of the DPPH activity.
Evaluation of superoxide anion free radical scavenging ability of crude extracts of biontsquantitative assessment
Superoxide anion scavenging ability of crude extracts of bionts was carried out according to Liu et al [18]. Superoxide radicals were generated in phenazine methosulphate‑nicotinamide adenine dinucleotide (PMS‑NADH) systems by oxidation of nicotinamide adenine dinucleotide (NADH) and assayed by the reduction of nitrobluetetrazolium (NBT). The superoxide radicals were generated by taking 1.0 ml of (50 μM) NBT solution and 1 ml (78 μM) NADH solution each prepared in 3 ml of 16 mM of Tris HCl buffer pH 6. To this system crude methanol extracts were added (1 mg/ml). The reaction was initiated by adding 1.0 ml of 10 μM PMS solution to the mixture, incubated at 25° for 5 min, and monitored for absorbance at 560 nm against blank samples. L‑ascorbic acid (0.1%) was used as a reference compound. Decreased absorbance of the reaction mixture was indicated as an increased superoxide anion scavenging activity. The percent inhibition of superoxide anion generation was calculated by considering the absorbance without the extract as 100%. Scavenging capacity of crude extracts was calculated using the formula, SOD scavenging activity (%) = (AControl‑ATest)/AControl) ×100, where AControl is the absorbance of the control, ATest is the absorbance of crude extracts.
Extracts of bionts showing highest antioxidant activity were prepared from 0.1% stock solution to find the effective concentration or EC50/IC50 value, which is the concentration required for a 50% inhibition (IC50) of formation of superoxide radicals.
Evaluation of total reducing capacity of crude extracts‑quantitative assessment
Crude extracts of culture supernatant of bacterial bionts (100‑1000 μg/ml) were mixed with 2.0 ml of 0.2 M phosphate buffer pH 6.6 and 2.0 ml of 1% potassium ferricyanide [K3Fe(CN)6], incubated at 50°. After 20 min, a 2.0 ml aliquot of 1% trichloroacetic acid was added to the mixture and centrifuged at 1036×g for 10 min. 2.5 ml of the upper layer of the solution was mixed with 0.5 ml of freshly prepared 1% FeCl3, and the absorbance was measured at 700 nm against reagent blank [19]. Measurements were performed in triplicate.
Chemical characterization of antioxidant factor produced by GUVFCFM‑3
Crude extract of biont GUVFCFM‑3 showing maximum scavenging activity, was spotted onto four thin layer aluminum sheets pre‑coated with silica (SD fine‑chem limited Aluchrosep silica gel60/UV254). The chromatograms were resolved using solvent system consisting of toluene:cyclohexane (8:2) (v/v). The plates were dried at room tempertaure and one plate was sprayed with 0.02% DPPH in ethanol, allowed to stand at room temperature. After 10 min white bands appearing against a purple background were noted and Rf determined [20]. The other three plates were individually sprayed with 0.25% ninhydrin prepared in acetone for the detection of amino acids; phenol‑sulphuric acid reagent for detection of sugars and lipids were visualized using an iodine‑saturated chamber, respectively. The IR spectrum of the antioxidant factor obtained by preparative TLC was recorded on a Fourier Transform IR spectrometer (Shimadzu IR Prestige‑21) in the 4000 to 400 cm‑1 spectral region at a resolution of 1902 cm‑1 after grinding with a 0.23 mm KBr pellet.
Statistical analysis
All experiments were carried out in triplicate, and the results expressed as a mean of three independent analysis±standard deviation (n=3).
Results and Discussion
Analysis of antioxidant activity using different in vitro methods led to the evaluation of antioxidant potential of bacterial bionts obtained from marine invertebrates. Marine bacteria constitute a diverse group, which has received attention only in recent years, as potential natural antioxidant producers in terms of their ability to act as efficient radical scavengers and it is believed to be mainly due to their redox properties [21]. Despite the increasing research interest in invertebrate associated bionts in the last decade, there are only few reports of sponge associated bacteria possessing antioxidant activity [11,12]. This study therefore, aimed at screening for potential antioxidant producing bionts associated with sponges, bivalve and corals from Mandapam in the Indian subcontinent and evaluation of its antioxidant activity.
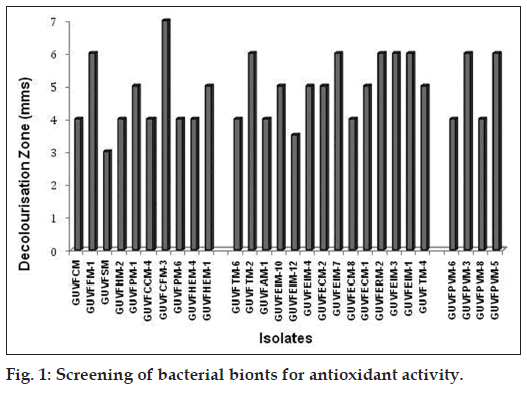
Out of the 100 bionts screened as filter paper replicates for antioxidant activity, 55 were positive. Based on the initial screening, 28 bionts exhibiting a white zone greater then 3 mm on a purple background (fig. 1), were selected and identified upto species level using 16S rRNA gene sequencing as previously described by Velho‑Pereira and Furtado 2014 (unpublished observations). The sequences are deposited in the Genbank under the accession number JX839255‑JX839279. Of these 10 were sponge bionts, 14 were coral bionts and the remaining 4 were bivalve bionts.
Chromohalobacter israelensis dominated the cultural assemblage of bacteria with antioxidant activity at 46.42%, which was followed by Psychrobacter celer (14.28%) and Chromohalobacter canadensis (7.14%). The isolates Burkholderia sp., Salinicoccus luteus, Staphylococcus equorum, Lysinibacillus boronitolerans, Virgibacillus salexigens, Chromohalobacter saresensis, Psychrobacter okhotskensis, Bacillus lehensis and Chromohalobacter nigrandesensis were least represented with a mere 3.57%. Thus, it can be clearly inferred that Gram ‑ve bacteria (82.14%) showed greater antioxidant activity as compared to the Gram +ve counterparts (17.85%).
The bacteria belonging to genera Pseudoalteromonas haloplanktis [22], Pseudomonas agglomerans [23], bacteria belonging to the family Flavobacteriaceae [21] and more recently Virgibacillus sp. and Rubritalea squalenifasciens are some marine bacteria reported for their antioxidant activity. Hence the finding that bacteria belonging to genera Chromohalobacter sp., Psychrobacter sp., Bacillus sp., Staphylococcus sp., Lysinibacillus sp. are also antioxidant producers is a first report and thus of much importance and relevance.
This is also the first report that delves with the antioxidant capacity of halotolerant bionts from three different marine invertebrates namely sponges, corals and bivalves from the same econiche. Interestingly, Chromohalobacter sp. clearly dominated the cultural bacterial assemblage associated with marine invertebrates having antioxidant activity. This euryhaline bacterium best known for its unique salt tolerating abilities by virtue of the production of osmolytes is not much known for its antioxidant capabilities. Though there are no published reports of antioxidant production by the bacteria of this genera, proteonomic studies have inferred from electronic annotation that there exists a putative sequence of 182 AA having antioxidant activity.
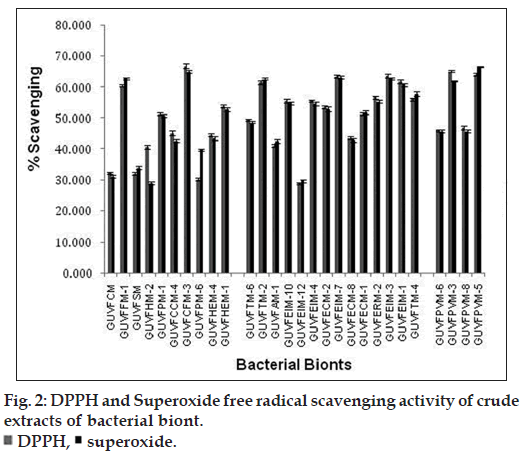
Among the sponge bionts, GUVFCFM‑3 (bearing accession number JX839255) showed the highest percentage scavenging at 67.05% (fig. 2) and was identified as C. israelensis. Among the coral bionts, GUVFEIM‑3 identified as C. israelensis (bearing accession number JX839277) showed the highest percentage scavenging at 63.77%. It was followed closely by biont GUVFEIM‑7 (bearing accession number JX839263) identified again as C. israelensis at 63.52%. Amongst the bivalve bionts isolate GUVFPVM‑3 identified as S. luteus (bearing accession number JX839272) showed the highest percentage scavenging at 65.21% followed by GUVFPVM‑5 identified as P. celer (bearing accession number JX839269) at 64.17%. In our study, we found that the extract of sponge biont C. israelensis (GUVFCFM‑3) showed the maximum DPPH radical scavenging activity 67.05% among all the other extracts tested at a similar concentration of 1000 μg/ml while the lowest was seen in the coral biont V. salexigens (GUVFEIM‑4) (bearing accession number JX839261) at 28.56%.
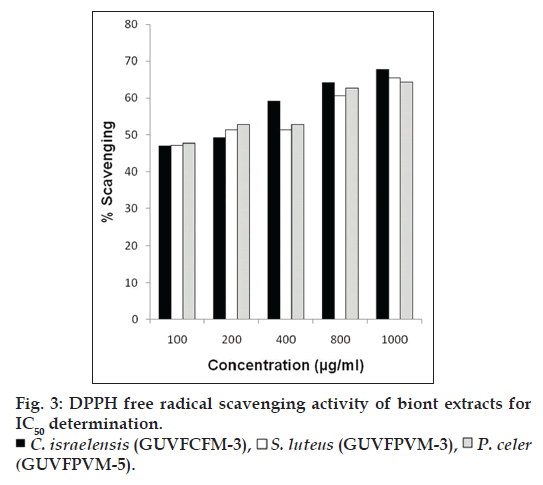
Extracts of the three cultures C. israelensis (GUVFCFM‑3), S. luteus (GUVFPVM‑3), P. celer (GUVFPVM‑5) showing maximum scavenging activity among all the invertebrate bionts tested, were further assayed at different concentrations of 100, 200, 400, 800 and 1000 μg/ml (fig. 3) for determination of IC50 values. The scavenging of the free radical DPPH occurred in a concentration dependent manner. The IC50 values of the extracts for inhibition of free radicals were found to be 169.96, 187.59 and 161.00 μg/ml, respectively. It was interesting to note that in our study the sponge biont GUVFCFM‑3 identified as Chromohalobacter israelensis showed an approximately two times higher scavenging potential of DPPH free radicals at half the concentration reported by the sponge biont identified as Virgibacillus sp. in the research efforts of Arunachalam and Appadorai [11]. The IC50 values of Virgibacillus sp. isolate was also five times greater than that of our isolate GUVFCFM‑3, thereby clearly implicating its greater efficiency as a DPPH free radical scavenger.
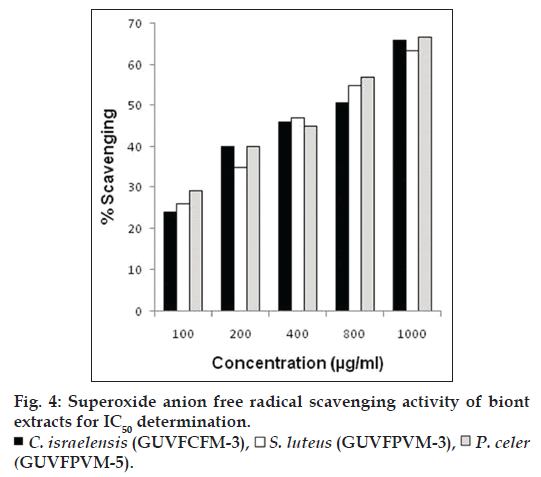
The highest scavenging of superoxide free radicals among all the invertebrate associated bionts was shown by culture GUVFCFM‑3 (bearing accession number JX839255) with a percentage scavenging of 65.21% while the lowest was seen in culture GUVFHM‑2 (bearing accession number JX839262) at 29.09% (fig. 2). Among the sponge bionts culture GUVFCFM‑3 identified as C. israelensis showed the highest superoxide scavenging at 67.21%. Amongst the coral bionts culture GUVFECM‑2 (bearing accession number JX839256) identified as C. israelensis showed the highest scavenging activity at 63.59%. Isolate GUVFPVM‑5 (bearing accession number JX839269) showed the highest superoxide scavenging activity among the bivalve bionts at 66.57%.
At different concentrations (10‑1000 μg/ml) scavenging of superoxide free radicals by the three cultures namely, C. israelensis (GUVFCFM‑3), S. luteus (GUVFPVM‑3), P. celer (GUVFPVM‑5) showing maximum scavenging activity, occurred in a concentration dependent manner (fig. 4). IC50 values of C. israelensis (GUVFCFM‑4), S. luteus (GUVFPVM‑3), P. celer (GUVFPVM‑5) were found to be 629.84, 630.98 and 569.43 μg/ml respectively. There was a remarkable difference in the scavenging of superoxide free radicals by culture GUVFCFM‑3 which was later identified as Chromohalobacter israelensis in our study and the isolate Virgibacillus reported in the work of Arunachalam and Appadorai [11] in that, GUVFCFM‑3 has a scavenging effect that was five times more than that of Virgibacillus sp. at half the concentration. The IC50 value of Virgibacillus sp. was also two times greater than that of our isolate GUVFCFM‑3. This finding is suggestive, that the isolate in our study, could scavenge greater number of superoxide free radicals at the same concentration.
Our results clearly showed the presence of reductants in the crude biont extracts which caused the reduction of the ferric/ferricyanide complex to the ferrous form. The higher absorbance of the reaction mixture was a clear indicant of greater reducing power. The reducing power of the extracts increased as concentration increased, indicating the presence of electron donors that can react with free radicals to convert them to stable products and terminate the radical chain reactions.
The reducing capacities of the extracts were nearly comparable to that of ascorbic acid. The methanol bacterial extracts showed the highest activity at the tested concentration of 1000 μg/ml. The reducing capacity of a compound serves as a significant indicator of its potential antioxidant activity and hence of immense value. The antioxidant activity has been attributed to various chain mechanisms, among which are the prevention of chain initiation, the binding of transition metal ion catalysts, decomposition of peroxides, the prevention of continued hydrogen abstraction, the reductive capacity and radical scavenging [24].
Thin layer chromatography showed a single purple spot with Rf=0.6 on spraying with ninhydrin which coincided with the Rf value of the bleached spot observed on spraying with DPPH when developed in the same solvent system. No spots were detected with phenol‑sulphuric acid reagent and on exposure to iodine vapours. The infrared spectrum of the partially purified extracellular antioxidant factor, showing the bleached band with DPPH had a broad absorption at 3535–3000 cm‑1 due to N–H stretching, bands at 1658.78 cm‑1 and 1529.551 cm‑1 were due to C–O stretching and N–H bending, implicating the presence of peptide bonds. Band at 2954.95 cm‑1 indicated C–H stretching of aliphatic chains. Vibrations observed at 1091.71 cm‑1 were characteristic of carbonyl groups while vibration at 1408.4 cm‑1 is characteristic of the CN stretch of the amide. From the above observations it could be clearly inferred that the extracellular antioxidant factor isolated from culture C. israelensis (GUVFCFM‑3) was a peptide.
Proteinaceous extracellular peptides such as diketopiperazines (DKPs) have been reported for their antioxidant activity by virtue of scavenging of the DPPH free radicals from an Antarctic psychrophile Pseudoalteromonas haloplanktis. Their free radical scavenging properties could be ascribed to the presence of a phenyl group in their structure [22]. However, previous reports [25] indicated that antioxidant activity of DKPs, and their free radical scavenging properties were not correlated.
In conclusion, we report that the members of the genus Chromohalobacter sp. isolated from all the three invertebrates showed excellent scavenging of free radicals that is comparable to that of standard antioxidants. C. israelensis (GUVFCFM‑3) a sponge biont of Callyspongia fibrosa produces extracellular antioxidant factor which scavenges 67.05% of DPPH free radicals and 67.21% of superoxide radicals. Preliminary characterization of the extracellular antioxidant factor suggests it to be of a peptide nature. Overall the invertebrate bionts and in particular C. israelensis (GUVFCFM‑3) holds promise for large scale production of antioxidant compounds that is gaining importance in effectively treating neurodegenerative and other disorders caused due to the activity of free radicals.
References
- Diaz P, Jeong SC, Lee S, Khoo C, Koyyalamudi SR. Antioxidant and antiinflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin Med 2012;7:26.
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 2005;53:4290-302.
- Dunlap W, Llewellyn L, Doyle J, Yamamoto Y. A microtiter plate assay for screening antioxidant activity in extracts of marine organisms. Mar Biotechnol (NY) 2003;5:294-301.
- Chairman K, Singh AJ, Alagumuthu G. Cytotoxic and antioxidant activity of selected marine sponges. Asian Pac J Trop Dis 2012;2: 234-8.
- Bhimba BV, Vinod V, Beulah MC. Biopotential of secondary metabolites isolated from marine sponge Dendrillanigra. Asian Pac J Trop Dis 2011;1:299-303.
- Abubakr MA, Hassan Z, Imdakim MM, Sharifah NR. Antioxidant activity of lactic acid bacteria (LAB) fermented skim milk as determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) and ferrous chelating activity (FCA). Middle East J Sci Res 2012;11:900-7.
- Spandana M, Madhuri TR, Kumari DA, Sushmitha K. Investigation on antioxidant and antimicrobial activities of marine Actinomycetes. Int J Pharm PharmSci 2012;3:563-6.
- Almeida EA, Dias Bainy AC, Dafre AL, Gomes OF, Madeiros M, di Mascio P. Oxidative stress in digestive gland and gill of the brown mussel (Pernaperna) exposed to air and re-submersed. J Exp Mar BiolEcol 2005;318:21-30.
- Brouwer M, Larkin P, Brown-Peterson N, King C, Manning S, Denslow N. Effects of hypoxia on gene and protein expression in the blue crab Callinectessapidus.Mar Environ Res 2004;58:787-92.
- Jiang H, Li F, Xie Y, Huang B, Zhang J, Zhang J, et al. Comparative proteomic profiles of the hepatopancreas in Fenneropenaeuschinensisresponse to hypoxic stress. Proteomics 2009;9:3353-67.
- Arunachalam K, Appadorai RA. Antioxidant potential and biochemical evaluation of metabolites from the marine bacteria Virgibacillussp.associated with the sponge Callyspongiadiffusa. Free Rad Antiox 2013;3:48-51.
- Shindo K, Asagi E, Sano A, Hotta E, Minemura N, Mikami K, et al.Diapolycopenedioic acid xylosyl esters A, B, and C, novel antioxidativeglyco-C30-carotenoic acids produced by a new marine bacterium Rubritaleasqualenifaciens. J Antibiot (Tokyo) 2008;61:185-91.
- Velho-Pereira S, Furtado I. Retrieval of Euryhaline, Eubacterial and Haloarchaealbionts from nine different benthic Sponges: Reflection of the bacteriological health of waters of Mandapam in India. Indian J Mar Sci 2014;43:773-83.
- Velho-Pereira S, Furtado I. Antibacterial activity of halophilic bacterial bionts from marine invertebrates of Mandapam-India. Indian J Pharm Sci 2012;74:331-8.
- Raghavan TM, Furtado I. Tolerance of an estuarine halophilicarchaebacterium to crude oil and constituent hydrocarbons. Bull Environ ContamToxicol 2002;65:725-31.
- Steensland H, Larsen H. A study of cell envelopes of Halobacteria. J Gen Microbiol 1968;55:325-36.
- Zho BB, Elledge SJ. The DNA damage response: Putting checkpoints in perspective. Nature 2000;408:433-9.
- Liu F, Ooi VE, Chang ST. Free radical scavenging activity of mushroom polysaccharide extracts. Life Sci 1997;60:763-71.
- Ulc IG, Elia IR, Gepdiremen AA, Boyer L. Antioxidant activity of lignans from fringe tree (ChionanthusvirginicusL.) Eur Food ResTechnol 2006;223:759-67.
- Sadhu SK, Okuyama E, Fujimoto H, Ishibashi M. Separation of Leucasaspera, a medicinal plant of Bangladesh, guided by prostaglandin inhibitoryand antioxidant activities. ChemPharma Bull (Tokyo) 2003;51:595-8.
- Shindo K, Kikuta K, Suzuki A, Katsuta A, Kasai H, Yasumoto-Hirose M, et al. Rare carotenoids, (3R)-saproxanthinand (3R, 2'S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. ApplMicrobiol Biotechnol 2007;74:1350-7.
- Mitova M, Tutino ML, Infusini G, Marino G, De Rosa S. Exocellularpeptides from Antarctic psychrophilePseudoalteromonashaloplanktis. Mar Biotech (NY) 2005;7:523-31.
- Wang H, Jiang X, Mu H, Liang X, Guan H. Structure and protective effect of exopolysaccharide from P. agglomerans strain KFS-9 against UV radiation. Microbiol Res 2007;162:124-9.
- Diplock AT. Will the ?good fairies? please proves to us that vitamin E lessens human degenerative of disease? Free Rad Res 1997;27:511-32.
- Boldyrev AA, Dupin AM, Batrukova MA, Bavykina NI, Korshunova GA, Shvachkin YP. A comparative study of synthetic carnosine analogs as antioxidants. Comp BiochemPhysiol 1989;94:237-40.
 DPPH,
DPPH,  superoxide.
superoxide.