- *Corresponding Author:
- C. T. Hoang
Department for Management of Science and Technology Development, Vietnam
E-mail: hoangthanhchi@tdtu.edu.vn
| Date of Submission | 24 January 2019 |
| Date of Revision | 23 March 2019 |
| Date of Acceptance | 15 July 2019 |
| Indian J Pharm Sci 2019;81(5):975-980 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study was undertaken to evaluate the phytoconstituents present in the seed extract of Dialium cochinchinensis for antioxidant, antimicrobial and cytotoxic properties. Results showed the presence of polyphenols, flavonoids, alkaloids, saponins, coumarins and a polyuronid compound in the D. cochinchinensis seed extract. The total polyphenol and flavonoid contents of the dry extract were 133.5±2.87 mg GAE/g and 227.0±23.20 mg Rutin equivalent/g, respectively. D. cochinchinensis seed extract exhibited antioxidant property in 2,2-diphenyl-1-picrylhydrazyl, 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid and potassium ferricyanide reducing antioxidant power methods. Moreover, D. cochinchinensis seed extract exerted inhibitory effect on Gram-positive bacteria. Finally, D. cochinchinensis seed extract was found to be toxic to Vero cell lines and the pathogens with selectivity index ranging from 0.01 to 0.04. In conclusion, D. cochinchinensis seed extract could be considered as a new source of natural antioxidant and antimicrobial agents for research and application.
Keywords
Dialium cochinchinensis, antioxidant activity, phytochemical screening, antimicrobial, cytotoxicity
Dialium cochinchinensis Pierre belongs to the genus Dialium and consists of about 30 species, in which 10 species are found in the tropical rain forests of Southeast Asian including Myanmar, Thailand, Indochina, Malaysia, Laos and Vietnam (Nghe An, Ha Tinh, Kon Tum, Dong Nai, Binh Duong, Binh Phuoc, Ho Chi Minh, and Ninh Thuan provinces)[1]. In Vietnam, D. cochinchinensis is listed in IUCN Red list and Vietnam Red Book[2].
D. cochinchinensis can grow up to 35 meters high with the bole is about 16-18 m high, 80-100 cm diameter, and rarely 150 cm. The bark generally is grey or whitish, the inner bark is 6-8 mm thick, hard, brownish, exuding a little transparent resin, soon turning red. This is one of the valuable woody trees, being used for furniture, railway sleepers, heavy construction (staircases, doors and window frames), bridges, ship- and boat-building, vehicles, chopping board, sports equipment, cart axles, agriculture implements, and oil and sugar mills. Fruit is a pod, ovoid, slightly laterally compressed, 15-20×8- 15 mm, finely pubescent to velvety[1]. The edible pulp is sweet and sour that has laxative effect[2]. Seeds are either 1 or 2, elliptic, 9×6×3 mm, longitudinally striate and covered by whitish pulp[1]. The seeds have usually been thrown out after using fruits that can become a potential resource for industry. To our knowledge, there is still no publication about the bioactivity of D. cochinchinensis. However, there are some studies about the bioactivity of D. guineense, one of the other Dialium genus species. The bark extract of D. guineense exhibited antibacterial activity[3], while the leaf extracts have antioxidant and antimicrobial activities[4]. The dichloromethane fraction of D. guineense fruit coat exerted wound-healing and antimicrobial properties[5]. Moreover, the phenolic extract of D. guineense pulp enhanced reactive oxygen species detoxification in aflatoxin B(1) hepatocarcinogenesis[6]. In this study, the antioxidant, antimicrobial and cytotoxicity of D. cochinchinensis seeds (DCS) were evaluated for the first time.
DCS were collected from trees at Kon Chư Răng conservation garden, Son Lang, Kbang District, Gia Lai-Kon Tum Province, Vietnam. DCS were washed and oven-dried until fully dry after a week at 40°. The dry DCS were ground to a fine powder in a blender. The powder was macerated with 80 % ethanol in the ratio of 1:10 (w/v). The mixture of powder and ethanol was continuously swirled for 4 d at room temperature and filtered through a Whatman filter paper. The filtrate concentrated in a rotary evaporator at 40° to obtain the crude extract. The crude extract was kept at –20° till further analysis. The phytoconstituents were evaluated according to the method described by Ciulei[7].
The total polyphenol content (TPC) of the DCS extract was determined using Folin-Ciocalteu reagent[8] following a slightly modified method of Nunzia et al.[9]. A volume of 200 μl extract in concentration of 1000 μg/ml was mixed with 200 μl Folin-Ciocalteu reagent 100 %. The mixture was incubated at room temperature for 5 min and 1600 μl of sodium carbonate solution (5 %, w/v) was added. The reaction mixture was incubated at 40° for 20 min and the absorbance was measured at 765 nm. Gallic acid 0 to 500 μg/ml was used to develop a standard curve. The content of TPC was expressed as mg/g gallic acid equivalent (GAE) of dry extract.
The total flavonoid content (TFC) of the DCS extract was determined using aluminium chloride colorimetric method[10]. Rutin 0 to 500 μg/ml was used to develop a standard curve. Rutin solution (500 μl) was added to 2000 μl distilled water and 150 μl of 5 % NaNO2. After incubated at room temperature for 6 min, 150 μl of 10 % AlCl3 solution was added and then incubated for 6 min at room temperature. A volume of 2000 μl of 4 % NaOH and 200 μl distilled water was added to the mixture and incubated for 15 min at room temperature before distributed into 96-well plates and absorbance was measured at 510 nm. The extract with concentration of 1000 μg/ml was determined to determine TFC as mg/g rutin equivalent (RE) of dry extract.
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was carried out according to the method of Ghatak et al. with slightly modification[11]. DPPH solution (0.3 mM) was prepared and reacted with the DCS extract at ratio of 1:1. The absorbance of the mixture was measured at 517 nm after incubation at 37° for 30 min. Vitamin C was used as a positive control for this assay. The ability to scavenge DPPH radical was calculated using following formula, % DPPH radical scavenging = (sample–negative control)/negative control)×100.
2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS, 2.6 mM) was allowed to reacted with K2S2O8 (7.4 mM) at ratio of 3:1 in a dark room for 16 h at room temperature to become the solution of ABTS*(+). ABTS*(+) solution was diluted with distilled water to obtain an absorbance of 1.00±0.02 at 734 nm. After mixing 750 μl of diluted ABTS*(+) solution with 150 μl of extract, the absorbance was measure at 734 nm. Vitamin C was used as positive control for this assay. The ability to scavenge ABTS*(+) radical was calculated using following formula, % ABTS*(+) radical scavenging = ((sample–negative control)/ negative control)×100.
Reducing power of DCS extract was detected based on potassium ferricyanide reducing antioxidant power (PFRAP) assay[12]. A volume 1 ml of extract was added 2.5 ml of phosphate-buffered saline (PBS, pH 6.6) and 2.5 ml potassium ferricyanide 1 %, mixed well and incubated for 20 min at 50°. The mixture was added to 2.5 ml of 10 % trichloroacetic acid and incubated for 10 min at room temperature. The upper layer (2.5 ml) was taken, 2.5 ml distilled water was added followed by 0.5 ml of 0.1 % FeCl3. The absorbance of the resulting blue-green colour was measured at 700 nm. Vitamin C was used for positive control.
Four Gram-positive bacteria (Staphylococcus aureus ATCC 6538 and ATCC 25923, Rhodococcus equi ATCC 6939 and Listeria monocytogenes ATCC 13932) and 5 Gram-negative bacteria (Escherichia coli ATCC 25922 and ATCC 8739, Listeria monocytogenes ATCC 13932, Proteus mirabilis ATCC 25933, Citrobacter freundii ATCC 8090 and Salmonella enterica ATCC 14028) were used. Agar well diffusion method was adopted for antibacterial activity assessment[13]. The bacteria were reactivated in tryptic soy broth (Acumedia, Neogen, US) at 37° for 20 h before adjusted turbidity to 0.5 McFarland equivalent to (1-2)×108 CFU/ml[14]. The adjusted bacterial biomass was spread out as a microbial inoculum over the entire agar surface. Then, a hole with a diameter of 6 mm is punched aseptically with a sterile cork borer and a volume of 50 μl of the extract at different concentration is introduced into the well. The agar plates were incubated at 37° overnight. The appearance of the inhibition zones indicated antibacterial property of extracts. Ampicillin was used as positive control and ethanol was used as a solvent control.
The microbial biomass prepared in Mueller-Hinton broth (MHB; HiMedia, India) after dilution of standardized microbial suspension adjusted to 0.5 McFarland scale[14]. Then, the adjusted biomass was diluted with MHB to obtain a concentration of (1-2)×105 CFU/ml. A volume of 50 μl diluted bacterial biomass was transferred into each well in 96 plates. Fifty microlitres of extracts in different concentration was added into each well. The plate was incubated at 37° for 20 h. The MIC was read as the least concentration that inhibited the growth of the test organisms. In this study a volume of 30 μl resazurin 0.02 % (Sigma- Aldrich, Merck, Germany) was added to each well to identify the MIC which was the least concentration that still in the blue colour[15]. The MBC was determined by sub-culturing the test dilutions onto TSA dish and incubated further for 18-24 h. The lowest dilution that yielded no single bacterial colony on the TSA dish was taken as MBC[16].
The toxic effects of the DCS extract on African green monkey kidney (Vero) cells Vero cell lineage (ATCC CCL-81™) was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Vero cells were grown in Dulbecco’s modified Eagle’s medium (Sigma- Aldrich, Ho Chi Minh City, Vietnam) supplemented with 10 % foetal bovine serum, 100 IU/ml penicillin, 0.1 mg/ml streptomycin, 1 % sodium pyruvate and 1 % L-glutamine (Biobasic, TBR Technology Corporation, Ho Chi Minh City, Vietnam) in a humidified incubator of 5 % CO2 at 37°. The Vero cells were distributed into 96-wells microplates (3×104 cells per 100 μl per well) and incubated at 37° for 24 h. Then, 100 μl of DCS extract at different concentrations was added starting with 400 μg/ml in successive serial dilutions to 25 μg/ml. Dimethyl sulfoxide (DMSO) 0.1 % was used as a control. After 48 h incubation, 20 μl of MTT solution (5 mg/ml in PBS) was added and the plates were incubated at 37° for 2 h. Then, 100 μl DMSO was added to each well to dissolve the MTTformazan crystals. Cytotoxicity was expressed as the concentration of test sample resulting in a 50 % reduction of absorbance compared to untreated cells (LC50 values). The selectivity index (SI) was expressed as LC50/MIC value. The SI values indicate the plant extract’s relative safety. The index value is a measure of the extract’s beneficial affects at a low dose versus its harmful effects a high dose. A high SI is an indication of a large safety margin between beneficial and toxic dose.
All experiments were conducted in triplicate. The data were analysed using GraphPad Prism version 7.04. Significant differences were determined by p-value with p-value was less than 0.001. Data were presented as mean±standard error of mean (SEM).
The traditional medicinal herbs are used as cures because of pharmacological actions, which could be attributed to phytoconstituents such as alkaloids, flavonoids and tannins. Some of them have potential to reduce the risk and progression of acute and chronic diseases such as cancer, cardiac and stroke mostly due to antioxidant effect[17].
All results of phytochemical analysis are showed in the Table 1. The results showed the presentation of polyphenols, flavonoids, alkaloids, saponins, coumarins and a polyuronid compound in the DCS extract. Plant polyphenols are well known antioxidants due to suitable structure for scavenging free radicals[18]. The tests also indicated that cardiac glycoside, anthocyanin and reducing sugar compound have a high probability of appearing in the sample.
| Natural products | Fractions | ||
|---|---|---|---|
| Diethyl ether | Ethanol | Water | |
| Alkaloid | + | + | + |
| Coumarin | + | || | || |
| Flavonoid | + | + | + |
| Cardiac glycoside | || | + | - |
| Saponin | || | + | + |
| Polyphenol | || | + | + |
| Anthocyanin | || | ± | - |
| Polyuronid | || | || | + |
| Reducing sugar | || | + | - |
| Organic acid | || | - | - |
‘+’ Present, ‘-’ absent ‘||’ no need to detect
Table 1: Secondary metabolites groups existing in DCS
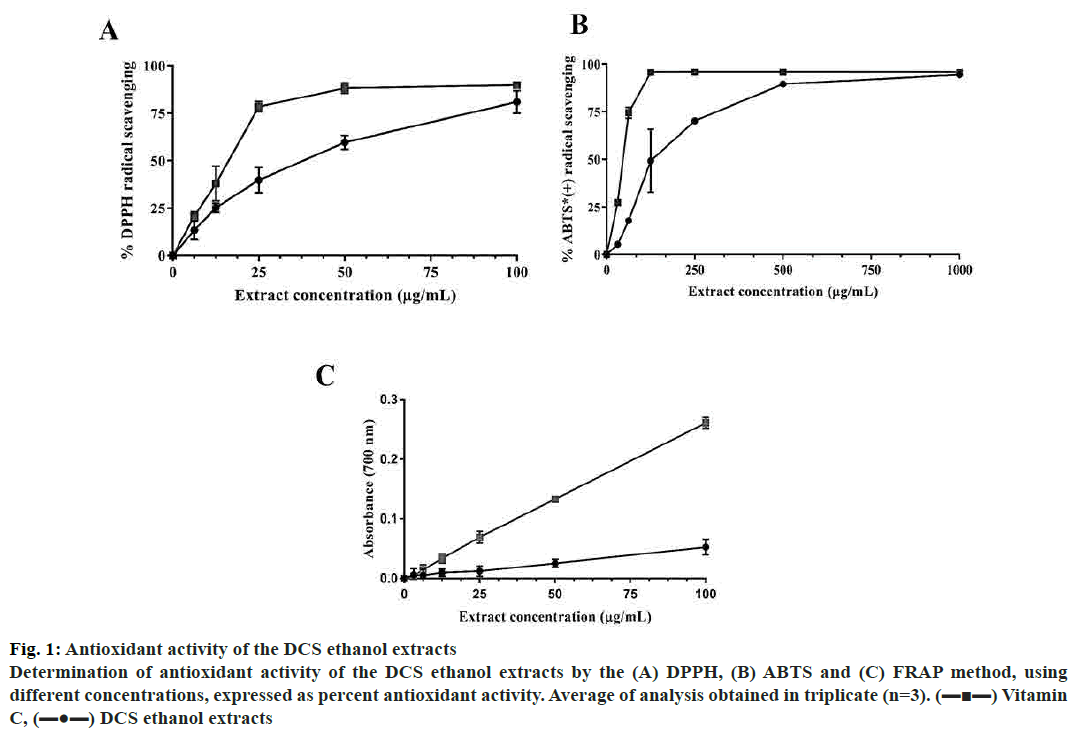
Moreover, the total TPC and TFC of dry extract were found to be 133.5±2.87 mg GAE/g and 227.0±23.20 mg RE/g dry, respectively. These results indicated that DCS has a high level of total polyphenol and flavonoid. The antioxidant property of DCS was determined using DPPH and ABTS radical scavenging methods. DPPH free radical scavenging activity is one of the most common methods based on the change of colour from purple into yellow when the radicals contact the antioxidants[18]. DCS extract potential to scavenge DPPH free radical was evaluated and compared with vitamin C. The results showed in fig. 1A and B indicated that DCS extract exhibited antioxidant effect as compared to vitamin C. The half maximal inhibitory concentration (IC50) of DPPH radical scavenging assays were inferred by nonlinear regression analysis. Although vitamin C exhibited higher scavenging activity, the higher the DCS extract concentration the stronger effect was expressed and approached asymptote vitamin C curve with quite low IC50 (33.6±1.66 μg/ml, fig. 1).
Figure 1: Antioxidant activity of the DCS ethanol extracts
Determination of antioxidant activity of the DCS ethanol extracts by the (A) DPPH, (B) ABTS and (C) FRAP method, using
different concentrations, expressed as percent antioxidant activity. Average of analysis obtained in triplicate (n=3). (▬■▬) Vitamin
C, (▬●▬) DCS ethanol extracts
ABTS radical scavenging assay was based on the change from dark green into colourless was used. At a concentration of 1000 μg/ml the ABTS scavenging activity of vitamin C were equal to the DCS extract. The IC50 for ABTS scavenging curve was determined as 140.0±7.47 μg/ml (fig. 1).
The reducing power of DCS extract was further investigated by performing PFRAP assay. The result (fig. 1C) showed that the reducing power increased with increasing concentration. However, the reducing power of DCS was much lower than that of vitamin C at the same concentration. The results of this study suggested that DCS contained polyphenols and flavonoids that cause the strong antioxidant property. It can be used as a natural source of antioxidants to prevent the progression of diseases.
Many seeds from the plants have proven to be an extremely good source of natural antioxidants, such as grape seeds (Vitis vinifera), soybean (Glycin max), tamarind (Tamarindus indica) seeds[19], Emblica officinalis (amla) fruit pulp[20] and crab eye seeds (Abrus precatorius)[21]. Compared to these seeds (via vitamin C), DCS has a stronger antioxidant activity.
The free radical scavenging activity, reducing power of extract appeared to depend on the present of phenolics, flavonoids or tannin and their concentration[22]. In this investigation, it was found that DCS is rich of TPC and TFC (133.5±2.87 mg GAE/g and 227.0±23.20 mg RE/g, respectively)
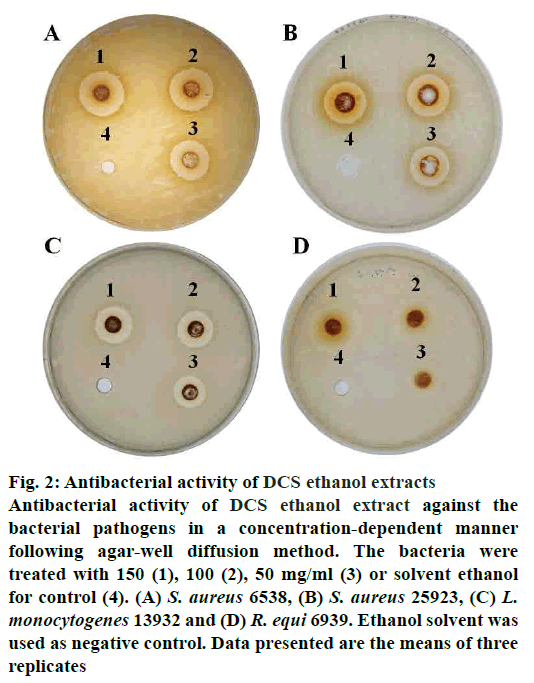
The antibacterial activity of DCS ethanol extract is demonstrated by the inhibition zones (Table 2). DCS ethanol extract was more effective on Gram-positive bacteria than the Gram-negative ones (fig. 2). The Grampositive bacteria such as S. aureus, L. monocytogenes and R. equi showed greater sensitivity to the extracts than the Gram-negative bacteria, E. coli, P. mirabilis, S. enterica and C. freundii, which could be due to the presence of outer membrane[23]. Outer membrane of Gram-negative bacteria provides a formidable barrier that blocks the penetration of extracts[23].
| Organism | Extract concentration (mg/ml) | |||
|---|---|---|---|---|
| 0 | 50 | 100 | 150 | |
| S. aureus ATCC 6538 | - | 9.00±0.58 | 10.60±0.58 | 12.18±0.43 |
| S. aureus ATCC 25923 | - | 7.13±0.42 | 8.46±0.49 | 10.47±0.44 |
| R. equi ATCC 6939 | - | 6.02±0.19 | 8.34±0.23 | 10.50±0.32 |
| L. monocytogenes ATCC 13932 | - | 6.83±0.42 | 8.14±0.19 | 9.03±0.34 |
| P. mirabilis ATCC 25933 | - | - | 3.49±1.29 | 5.17±1.00 |
| C. freundii ATCC 8090 | - | - | - | - |
| S. enterica ATCC 14028 | - | - | - | - |
| Escherichia coli ATCC 8739 | - | - | - | - |
| Escherichia coli ATCC 25922 | - | - | - | - |
Table 2: Antimicrobial ability of DCS expressed as inhibition zones (mm)
Figure 2: Antibacterial activity of DCS ethanol extracts
Antibacterial activity of DCS ethanol extract against the
bacterial pathogens in a concentration-dependent manner
following agar-well diffusion method. The bacteria were
treated with 150 (1), 100 (2), 50 mg/ml (3) or solvent ethanol
for control (4). (A) S. aureus 6538, (B) S. aureus 25923, (C) L.
monocytogenes 13932 and (D) R. equi 6939. Ethanol solvent was
used as negative control. Data presented are the means of three
replicates
The effectiveness of an antimicrobial agent is determined by its ability to inhibit or kill bacteria[3]. DCS extract showed strong inhibition effect on some bacteria tested. The MICs of DCS extract ranged from 1.56 to 6.25 mg/ml (Table 3). MBCs ranged from 25 to upper 50 mg/ml (Table 3). The observed antibacterial ability of DCS seed extract on the selected bacterial pathogen in vitro could imply that the extract may indeed be effective in vivo as claimed by traditional healers.
| DCS extract | Ampicillin | ||||
|---|---|---|---|---|---|
| Organism | MIC (mg/ml) | MBC (mg/ml) | Selectivity index (SI) | MIC (µg/ml) | MBC (µg/ml) |
| S. aureus ATCC 6538 | 3.13 | 25 | 0.02 | 1.56 | 12.5 |
| S. aureus ATCC 25923 | 6.25 | 25 | 0.01 | 1.56 | 3.13 |
| R. equi ATCC 6939 | 1.56 | 50 | 0.04 | 25 | >25 |
| L. monocytogenes ATCC 13932 | 3.13 | >50 | 0.02 | 0.78 | 6.25 |
Table 3: MICS, MBCS and SI of DCS extract
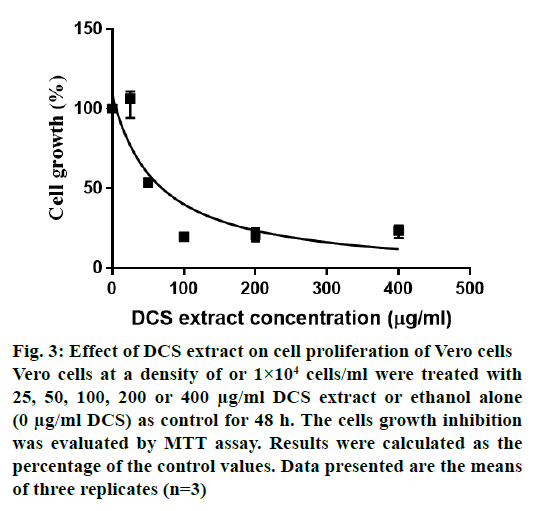
Fig. 3 shows the cytotoxic effects of DCS extract on Vero cells. The LC50 value was 63.06 μg/ml. The extract was toxic to the Vero cell lines and the pathogens with SI values ranging from 0.01 to 0.04 (Table 3). With SI values of less than 10 against all the bacteria, it appears that the observed antibacterial activity may be a result of general metabolic toxicity.
Figure 3: Effect of DCS extract on cell proliferation of Vero cells
Vero cells at a density of or 1×104 cells/ml were treated with
25, 50, 100, 200 or 400 μg/ml DCS extract or ethanol alone
(0 μg/ml DCS) as control for 48 h. The cells growth inhibition
was evaluated by MTT assay. Results were calculated as the
percentage of the control values. Data presented are the means
of three replicates (n=3)
Acknowledgements:
We would like to thank Duong Thi Phung Cac and Ngo Thi Phuong Dung (Ho Chi Minh City, Vietnam) for supporting materials using in this study.
References
- Hoang VS, KhamSeng N, Kessler PJA. Trees of Laos and Vietnam: a field guide to 100 economically or ecologically important species. Blumea 2004;49:201-349.
- Do HB, Dang QC, Bui XC, Nguyen TD, Do TD, Pham VH, et al. Medicine plant and animal in Viet Nam (Cây thuốc và động vật làm thuốc ở Việt Nam) Tập 1. Hanoi, Vietnam: Nhà xuất bản Khoa học và Kỹ thuật; 2006.
- Olajubu F, Akpan I, Ojo D, Oluwalana S. Antimicrobial potential of Dialium guineense (Wild.) stem bark on some clinical isolates in Nigeria. Int J Appl Basic Med Res 2012;2:58-62.
- Gideon IO, Joachim E, Ehinobu JM. Antioxidant and antimicrobial activities of Dialium guineense (Willd) leaf extract. Pharm Pharmacol Res 2013;1:1-7.
- Okeke NC, Udeani TK, Onyebuchi UL. Onyebuchi. Wound-healing and antimicrobial properties of dichloromethane fraction of Dialium guineense (Wild) fruit coat. Res Pharm Sci 2016;11(3):219-26.
- Adeleye AO, Ajiboye TO, Iliasu GA, Abdussalam FA, Balogun A, Ojewuyi OB, et al. Phenolic extract of Dialium guineense pulp enhances reactive oxygen species detoxification in aflatoxin B(1) hepatocarcinogenesis. J Med Food 2014;17(8):875-85.
- Ciulei I, Grigorescu E, S. U. Medicinal plants, phytochemistry and phytotherapy [Plante medicinale, fitochimie si fitoterapie, Vol. 1]. Bucharest, Romania: Medical ed.; 1993.
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2007;2(4):875-7.
- Nunzia C, Vincenzo L. The Influence of Initial Carbonate Concentration on the Folin-Ciocalteu Micro-Method for the Determination of Phenolics with Low Concentration in the Presence of Methanol: A Comparative Study of Real-Time Monitored Reactions. Am J Anal Chem 2011;2:840-8.
- Samatha T, Shyamsundarachary R, Srinivas P, Nanna RS. Quantification of total phenolic and total flavonoid contents in extracts of Oroxylum indicum L.Kurz. Asian J Pharm Clini Res 2012;5:177-9.
- Ghatak A, Nair S, Vajpayee A, Chaturvedi P, Samant S, Soley K, et al. Evaluation of antioxidant activity, total phenolic content, total flavonoids, and LC-MS characterization of Saraca asoca (Roxb.) De.Wilde. Int J Adv Res 2015;3(5):318-27.
- Jayanthi P, Lalitha P. Reducing power of the solvent extracts of Eichhornia crassipes (mart.) solms. Int J Pharm Pharm Sci 2011;3:126-8.
- Finn RK. Theory of Agar Diffusion Methods for Bioassay. Anal Chem 1959;31(6):975.
- Poonacha N, Nair S, Desai S, Tuppad D, Hiremath D, Mohan T, et al. Efficient Killing of Planktonic and Biofilm-Embedded Coagulase-Negative Staphylococci by Bactericidal Protein P128. Antimicrob Agents Chemother 2017;61(8):e00457-17.
- Rampersad SN. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012;12:12347-60.
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 2008;3:163-75.
- Thanh DM, Frentzel H, Fetsch A, Krause G, Appel B, Mader A. Tenacity of Bacillus cereus and Staphylococcus aureus in dried spices and herbs. Food Control 2018;83:75-84.
- Alhakmani F, Kumar S, Khan SA. Estimation of total phenolic content, in vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed 2013;3(8):623-7.
- Waqas MK, Saqib NU, Rashid SU, Shah PA, Akhtar N, Murtaza G. Screening of various botanical extracts for antioxidant activity using DPPH free radical method. African journal of traditional, complementary, and alternative medicines. Afr J Tradit Complement Altern Med 2013;10:452-55.
- Mehrotra S, Jamwal R, Shyam R, Meena DK, Mishra K, Patra R, et al. Antihelicobacter pylori and antioxidant properties of Emblica officinalis pulp extract: a potential source for therapeutic use against gastric ulcer. J Med Plants Res 2011;5:2577-583.
- Okoh SO, Asekun OT, Familoni OB, Afolayan AJ. Antioxidant and Free Radical Scavenging Capacity of Seed and Shell Essential Oils Extracted from Abrus precatorius (L). Antioxidants 2014;3:278-87.
- Loganayaki N, Siddhuraju P, Manian S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J Food Sci Technol 2013;50(4):687-95.
- Delcour AH. Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta 2009;1794:808-16.