- *Corresponding Author:
- P. Subramanian
Department of Botany, Kongunadu Arts and Science college, Coimbatore-641 029, India
E-mail: paulsami@yahoo.com
| Date of Submission | 05 Oct 2015 |
| Date of Revision | 02 Jun 2016 |
| Date of Acceptance | 14 Jun 2016 |
| Indian J Pharm Sci,2016;78(3):377-387 |
Abstract
Leaves of Solena amplexicaulis are traditionally used as a remedy for the treatment of inflammation related disorders in the form of decoction by the Paliyar tribal community of Theni district, Tamil Nadu, India. This study was designed to investigate the antiinflammatory and antioxidant potential of crude methanol leaf extract and the isolated compound, forskolin from S. amplexicaulis. Their effects were evaluated by carrageenan-induced paw edema in experimental rats for 4 h. In vivo antioxidant and histopathological studies were also performed. Therapeutic potential of forskolin was further substantiated using PASS software. The oral administration of forskolin (10 mg/kg) exerted potent antiinflammatory activity by reducing paw edema (87.79%) than the crude extract (150 and 300 mg/kg) and it was comparable with the standard drug, indomethacin (10 mg/kg, 93.89%). Consistent with these findings, antioxidant, histopathological architecture and PASS software also reflected its prominent curative property. The obtained results therefore suggest that the compound, forskolin obtained from S. amplexicaulis is considered to be a valuable source of remedy for inflammation.
Keywords
Solena amplexicaulis, forskolin, anti-inflammatory, antioxidant, histopathology
Introduction
Inflammatory diseases are major cause of morbidity and mortality in the world [1]. It is a complex mechanism in which leukocytes migrate from the vasculature into damaged tissues to destroy the agents that can potentially cause tissue injury [2]. At the site of inflammation, overproduction of reactive oxygen species (ROS) predominates subsequent oxidative stress and potentially cause damage to biomolecules such as DNA, lipids and proteins [3]. A plethora of evidence for herbal medications garnered wide interest as complementary and alternative therapeutics. Now a days, search for natural entities with anti-inflammatory activity with lesser side effects is vital [4]. Therefore, efforts are being continuously made to identify such agents and to validate their scientific authenticity.
Solena amplexicaulis (Lam.) Gandhi (Cucurbitaceae), is a perennial climber distributed in the dry deciduous forests of Southern India [5,6]. The healers of Paliyar tribal community in Theni district of Tamil Nadu, India who have long history of traditional medical practice prescribe the leaf extract for the treatment of inflammation related disorders [7-9] and also to cure jaundice [10]. Based on use value, information consensus factor and fidelity level, Venkatachalapathi et al. [11] reported that it is one among the five most important species in the treatment of inflammation and wound healing in Nilgiris of Western Ghats being prescribed by the Irula tribal community. In addition to this, it is known from the literature that the tubers are astringent, appetizer, carminative, cardiotonic, digestive, diuretic, expectorant, invigorating, purgative, stimulant, sour and thermogenic [12].Our early laboratory data also recorded several therapeutic properties as antimicrobial [13] and antioxidant activities [14] and phytochemical constituents [15,16] for this species. Terpenoids, the characteristic feature of the family, Cucurbitaceae [17,18] are attributed to various pharmacological claims including anti-inflammatory, anticancer, antimalarial, antiviral and antibacterial activities [19]. Literature data abounds in examples that terpenic compounds like cucurbitacin IIb and cucurbitacin E isolated from certain Cucurbitaceae members are promising candidates exhibiting antiinflammatory effects [20-22]. To the best of our knowledge, literature pertaining to the anti-inflammatory property of any terpenic compound isolated from S. amplexicaulis is still in lacunae. Therefore, the present study is addressed in search for novel bioactive entities with potential antiinflammatory and antioxidant activities in comparison with the crude leaf extract of this plant and to validate their therapeutic potential using PASS software.
Materials and Methods
The leaves of S. amplexicaulis were collected from the thorny scrub jungles of Madukkarai, Coimbatore, Tamil Nadu, India, during the month of April 2014 (fig. 1). The authenticity of the plant was confirmed by comparing with the reference specimen (Vide No: CPS 313) preserved at Botanical Survey of India, Southern Circle, Coimbatore. The leaf samples were cleaned, washed with copious amount of water, shade dried and coarsely powdered in a Willy Mill to 60 mesh size (Nippon Electricals, Chennai, India) for extraction.
Preparation of test samples
For qualitative and quantitative estimations, 50 g of powdered plant materials were sequentially extracted in a Soxhlet extractor using organic solvents viz. hexane, benzene, chloroform and methanol. The air dried residue was subjected to extraction with water at a controlled temperature. For characterization of compound and in vivo experimental studies, the coarsely powdered sample was exhaustively extracted with methanol after dewaxing with petroleum ether to yield a dark brown semisolid residue. The solvents were evaporated using rotary vacuum evaporator (RE300; Yamato, Japan) and lyophilized (4KBTXL-75; Vir Tis Benchtop K, New York, USA) to remove traces of water molecules and were stored at -20° until further use (extractive yield 18.60%).
Qualitative and quantitative estimations
The extracts were subjected to preliminary phytochemical analysis as described by Harborne [23], and Trease and Evans [24]. For quantification, the alkaloid content was gravimetrically determined and expressed as mg/g dry powder [23]. The total phenolic and tannin contents were estimated and expressed as gallic acid equivalents (GAE) (10 to 50 μg/ml; R2=0.996) mg/g extract according to the method described by Siddhuraju and Becker [25]. The total flavonoid content was determined spectrophotometrically using a standard curve rutin (RE) (10 to 200 μg/ ml; R2=0.991) as per the method of Zhishen et al [26]. Content of ascorbic acid was calculated on the basis of calibration curve of authentic L-ascorbic acid (5 to 25 μg/ml; R2=0.897) and the results were expressed as mg of ascorbic acid (AA) equivalent/g of extract, proposed by Klein and Perry [27]. Total saponin content was determined by the method described by Makkar et al [28]. with some modifications and their values were expressed as diosgenin equivalents (DE, 20 to 100 μg/ ml; R2=1) derived from a standard curve.
Isolation, purification and identification of the bioactive compound
Isolation was made using wet packing method of silica gel (60-120 mesh) column chromatography (5×80 cm) [29]. 15 g of methanol leaf extract was mixed with 15 g of silica gel (1:1) and allowed to dry to get free flow of admixture. At first, the slurry of adsorbent (silica gel) was added to the column approximately upto two third of its height, using 100% hexane and then the sample mixture was loaded at the top. It was eluted with solvent systems of increasing polarity such as hexane (100%), hexane:ethylacetate (95:5; 90:10; 85:15 and so on). Totally, 210 fractions (100 ml each) were eluted with 100% hexane. They were checked for their purity by analytical thin layer chromatography (TLC) in an appropriate solvent systems and the zone was detected with a UV lamp (366 nm) and iodine chamber (fig. 2). The fractions between 145 and 180 with similar Rf 0.46 (100% hexane), were pooled to provide a pale yellow amorphous powder (350 mg).
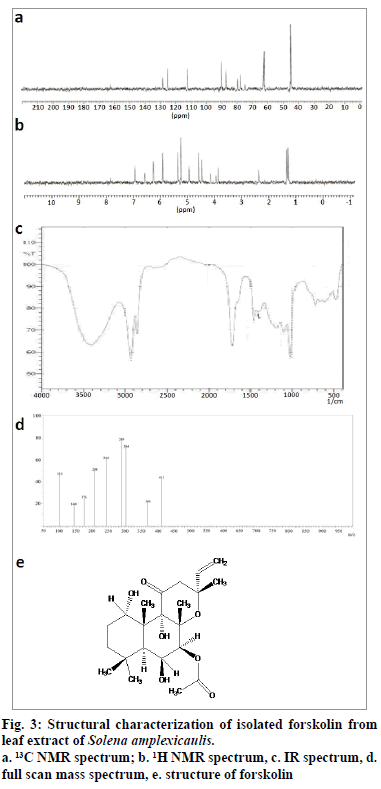
The melting point of the purified compound was 228~230°. In full scan mass spectrum (FSMS), molecular ion peak appeared at m/z 410 [M+H]+ which corresponds to the molecular formula of C22H34O7 (fig. 3d). The infrared (IR) spectrum of the isolated compound exhibited peaks at 3410.28 cm-1 which indicated the presence of hydroxyl group. The peak at 2929.97 cm-1 indicated the presence of olefinic carbon, and the peak registered at 1716.70 cm-1 showed the presence of carbonyl group (fig. 3c). In 13C NMR (nuclear magnetic resonance spectroscopy), signals between δ 45.21 and δ 90.55 confirmed the presence of aliphatic carbon. In addition, aromatic carbon signals appeared at δ 112.25. The signals at δ 155 and δ 165 revealed the presence of carbonyl carbon (fig. 3a). In 1H NMR, six methyl groups appeared as singlets in the region of δ 1.25-δ 1.36. The olefinic proton appeared at 5.36 ppm. Signals at 2.36 ppm established the presence of methylene protons (fig. 3b). The compound also displayed positive response to copper acetate test for the confirmation of diterpene. All our spectral data, tests and also earlier literature confirmed the chemical structure of the isolated compound, as forskolin, ((3R,4aR,5S,6S,6aS,10S,10aR,10bS)- 6,10,10b-Trihydroxy-3,4a,7,7,10a-pentamethyl-1- oxo-3-vinyldodecahydro-1H-benzo[f]chromen-5-yl acetate, fig. 3e).
Experimental animals
For acute toxicity screening, Swiss albino female mice (20-25 g) and for in vivo anti-inflammatory activity, Sprague Dawley female rats (150-200g) were procured from Small Animals Breeding Station, Mannuthy, Kerala and were acclimatized under standard environmental conditions (temperature 25±2°; humidity 35-60%; 14 h dark/10 h light cycles; air ventilation). They were fed with standard pellet diet (M/s. Hindustan Unilever Ltd., Mumbai, India) and fresh water ad libitum. The animals were housed for 10 days prior to experimental use in polyacrylic cages (38×23×10 cm) with not more than six per cage. The experiments conducted were approved by Institutional Animal Ethical Committee (Apprwith methanol leaf extract of S. amplexicaulis (150oval No: 659/02/a/CPCSEA).
in vivo acute toxicity studies
Acute oral toxicity study was carried out according to the Organisation for Economic Cooperation and Development (OECD) [30] guideline 423. The experimental animals were divided into eleven groups of six animals (n=6) each. First group served as control and was treated with normal water. Groups 2-8 were orally treated with the doses of 50, 150, 300, 500, 1000, 2000 and 3000 mg/kg, respectively of crude methanol leaf extract, whereas Groups 9-11 received oral doses of 10, 50 and 100 mg/kg of forskolin, respectively. Observations were made for mortality and clinical signs up to 14 days.
in vivo anti-inflammatory activity
Carrageenan-induced hind paw edema model was used for evaluating the anti-inflammatory activity [31]. The animals were fasted overnight prior to the experiment and were divided into 5 groups of six animals each as detailed below: Group I: normal control rats treated with water (normal); Group II: carrageenan (1% w/v in 0.9% saline) induced animals (negative control); Group III: carrageenan-induced animals pretreated with methanol leaf extract of S. amplexicaulis (150 mg/ kg, Leaf150); Group IV: carrageenan-induced animals pretreated with methanol leaf extract of S. amplexicaulis (300 mg/kg, Leaf300); Group V: carrageenan-induced animals pretreated with forskolin isolated from the methanol leaf extract of S. amplexicaulis (10 mg/kg, forskolin); Group VI: carrageenan-induced animals pretreated with the standard drug, indomethacin (10 mg/kg, indomethacin).
1 h after the oral administration of the test drug acute inflammation was induced in groups II to VI on the left hind paw by sub plantar injection of 0.1 ml carrageenan (1% w/v) in 0.9% saline. After the administration of carrageenan, paw volume was measured using Vernier caliper at every 30 min. The readings were recorded for the total of 4h and the percentage of paw edema inhibition was determined.
Collection of samples and preparation of homogenate
At the end of the experimental period, the animals were sacrificed by cervical dislocation under mild chloroform anesthesia. The blood samples were collected by cardiac puncture and used for separating the serum by centrifugation in a refrigerated centrifuge (4°) at 3000 rpm for 10 min. Spleen, thymus and left hind paw were excised immediately and washed with cold saline. The tissues were weighed and 10% (w/v) tissue homogenate was prepared with 0.1M Tris HCl buffer of pH 7.4 and centrifuged at 6000 rpm for 15 min at 4° for cytosolic separation. The resulting supernatant and serum were used to measure nitric oxide (NO), protein, lipid peroxide (LPO) and hydroperoxide (HPO) and for various in vivo antioxidant assays. A section of hind paw was preserved and used for histopathological observation.
Antioxidant Activity
NO and protein levels in serum samples were estimated by the method of Green et al [32] and Lowry et al [33], respectively. The enzymic antioxidant activities viz., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-s-transferase (GST) and glucose-6-phosphate dehydrogenase (G6PD), and non-enzymic antioxidant activities viz., total reduced glutathione (GSH) and ascorbic acid (Vit C) were determined in tissue homogenates of spleen, thymus and hind paw [34]. LPO and HPO levels were assayed both in serum and tissues [35].
Histopathological studies
The hind paw tissues were removed and fixed in 10% formal saline, dehydrated and processed for microscopic observation. Micrographs (Canon 10.1 MP digital camera, Japan) were captured using Axiostar plus microscope (Carl Zeiss, Germany).
Statistical analysis
Results were expressed as mean±standard deviation (SD, n=6). Statistical analysis was performed using one-way analysis of variance (ANOVA) by Tukey’s multiple comparison range test (GraphPad prism 4.0 software) to judge the difference between various groups. P-value of 0.05 (P<0.05) was considered to be significant.
Prediction of activity spectra for substances (PASS)
The computer software, PASS (version 9.1) predicts biological activity based on structural formula of chemical compounds [36].Thus by comparing the structure of anew substance with a well-known biologically active substances, it is possible to find out whether a new substance has a particular effect or not. PASS estimates the probabilities of a particular substance belonging to the active and inactive subsets of a substance from structure-activity relationships (SAR) base.
The result of the prediction was exhibited in a table containing the list of biological activity with appropriate probability values i.e., either revealed (Pa) or not revealed (Pi) for each activity type from the predicted biological activity spectrum and their values varied from 0.000 to 1.000. Only those activity types for which Pa>Pi are considered possible [37].
Results and Discussion
S. amplexicaulis leaves generally prescribed in the form of aqueous extract [9,10] are the common ingredients in many folkloric and herbal medicines and have been widely ascribed to cure human disorders including inflammation, jaundice and skin diseases. In the present investigation, sequential extraction of leaf samples were carried out using five different solvent systems for the separation of nonpolar to highly polar compounds. The calculated yield percentage for S. amplexicaulis ranged between 0.60 and 13.80% (expressed as w/w percentage). Among them, extraction with polar solvents (methanol and water) revealed the recovery of high amount of solids. In qualitative analysis also the methanol and water extracts recorded higher number of secondary metabolites with prevalent degree of precipitation (+++, Table 1). By quantitative estimations it was found that the dry powdered leaf sample of S. amplexicaulis contained appreciable amount of alkaloid content (9.92 mg/g dry powder). Similarly, the crude methanol and water extracts also displayed considerable amount of phytochemical constituents viz., phenolics, tannins, flavonoids, ascorbic acid and saponins depending upon the extractive capacity of the solvents (Table 2). The above findings were in concordance with the reports of Choe et al. [38] and Pirbalouti et al. [39] who found that the phenolic and flavonoid contents were generally higher in high polar solvents. Therefore, it is apparent that methanol was the most effective solvent in the extraction of bioactive compounds from the crude samples. According to the literature perusal pertaining to this study, it is insinuated that the favorable contents of flavonoids and phenolic compounds (Table 2) may be viewed as promising therapeutic entities against free radical-mediated pathologies [40].
| Solvent | Yield (%) | Phytoconstituents | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | CG | F | G | P | R | S | St | T | Te | Tr | ||
| Hexane | 9.00 | - | + | - | - | - | - | + | + | - | + | + |
| Benzene | 1.40 | - | + | - | - | - | + | + | - | - | - | - |
| Chloroform | 0.60 | + | - | - | - | - | - | + | - | - | - | + |
| Methanol | 13.80 | ++ | +++ | +++ | +++ | +++ | + | +++ | ++ | +++ | ++ | +++ |
| Water | 13.50 | + | - | +++ | +++ | +++ | - | - | ++ | +++ | ++ | + |
A-Alkaloids; CG–Cardiac glycosides; F-Flavonoids; G-Glycosides; P-Phenols; R-Resins; S-Saponins; St-Steroids; T-Tannins; Te-Terpenoids; Tr- Triterpenoids. +++: highly present, ++: moderately present, +: Low, -: absent.
Table 1: Extractive Yield And Preliminary Phytochemical Screening Of Various Solvent Extracts Of Solena amplexicaulis Leaf.
| Solvent | Total phenolics (mg GAE/g extract) |
Tannins (mg GAE/g extract) |
Total flavonoids (mg RE/g extract) | Ascorbic acid (mg AAE/g extract) |
Saponins (mg DE/g extract) |
|---|---|---|---|---|---|
| Hexane | 1.55±0.19b | 0.40±0.07c | - | 75.07±0.24b | 7.68±0.02a |
| Benzene | 0.28±0.01a | 0.03±0.03bc | - | 11.57±0.36ab | 10.28±0.09ab |
| Chloroform | 0.11±0.01a | 0.027±0.01a | - | 5.58±0.25a | 7.60±0.09a |
| Methanol | 4.41±0.27c | 1.46±0.11d | 18.46±0.20b | 117.00±0.73c | 11.97±0.08b |
| Water | 3.15±0.27bc | 1.14±0.08d | 2.94±0.40a | 117.33±0.36c | 9.66±0.03ab |
Values are mean±standard deviation (SD) of three independent experiments. Values not sharing a common letter in a column are significantly different (P<0.05). GAE–Gallic acid equivalent; RE–rutin equivalent; AAE–ascorbic acid equivalent; DE–diosgenin equivalent.
Table 2: Contents Of Secondary Metabolites In Different Solvent Extracts Of Solena amplexicaulisleaf
In acute toxicity study, oral administration of both the crude extract (up to 3000 mg/kg) and the isolated compound, forskolin (up to 100 mg/kg) of S. amplexicaulis leaf practically revealed its non-toxic nature. It did not induce any drug related toxicity or other mortality in animals at any of the doses tested. There were no visible signs of acute toxicity, net gain or loss in body weight and other gross behavioural changes (data not shown) till the end of the experiment (up to 14 days), indicating fairly high margins of safety. According to Loomis and Hayes [41], the substances with LD50 ranged between 5000 and 15 000 mg/kg are regarded practically non-toxic. Therefore, S. amplexicaulis leaf extract and forskolin can be considered as relatively safe.
In carrageenan-induced anti-inflammatory activity, preadministered groups of rats with crude extracts (150 and 300 mg/kg) and forskolin (10 mg/kg) evidenced weak inhibitory response during early phase of inflammation but was able to effectively inhibit the increase of paw volume during the late phase (4 h after carrageenan injection, Table 3). Based on the above findings and the biphasic nature of carrageenan-induced paw edema, it is possible to propose that the significant activity observed in the late phase of inflammation may be due to the ability of extracts to inhibit the release and/or activity of the late mediators involved in carrageenaninduced paw edema. In this study, it is noted that the methanol leaf extract generated a dose-dependent inhibition of edematous volume (150 and 300 mg/kg by 71.76 and 82.44%, respectively), which might perhaps be in part due to the synergetic action of phytochemical constituents present in it (Tables 1 and 2). Notably, the magnitude of anti-inflammatory activity was found to be more effective in forskolin (87.79%) than the crude extract. The prominent activity of the compound reported in this study might probably be related to its ability to prevent the production of proinflammatory mediators such as TNF-α, IL-Iβ, PGE2 and IL-b and suppressing the expression of COX-2 [42]. Some authors considered forskolin as a lead molecule for drug development as it trigger multiple biological activities including inflammation inhibition [43-45].
| Group | Paw volume (mm) at 30 min after carrageenan injection* | Increase in paw thickness (mm) | Inhibition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | |||
| Control | 4.13±0.14 | 4.13±0.14 | 4.13±0.19 | 4.13±0.19 | 4.13±0.13 | 4.13±0.20 | 4.13±0.26 | 4.13±0.14 | 4.13±0.12 | 0 | - |
| Negative control | 4.13±0.14 | 5.00±0.18a | 5.25±0.24a | 5.67±0.27a | 6.05±0.19a | 6.15±0.30a | 6.35±0.41a | 6.58±0.22a | 6.75±0.20a | 2.62 | - |
| Leaf 150 | 4.15±0.14 | 5.12±0.15ab | 5.82±0.27ab | 6.26±0.36ab | 6.53±0.33ab | 6.03±0.20ab | 5.85±0.18ab | 5.31±0.16ab | 4.89±0.19ab | 0.74 | 71.76 |
| Leaf 300 | 4.19±0.13 | 5.50±0.20ab | 5.95±0.24ab | 6.25±0.42ab | 6.63±0.33ab | 6.25±0.21ab | 5.81±0.18ab | 5.03±0.29ab | 4.65±0.18ab | 0.46 | 82.44 |
| Forskolin | 4.14±0.15 | 4.85±0.22ab | 5.31±0.27ab | 6.11±0.31ab | 6.53±0.26ab | 6.02±0.28ab | 5.41±0.25b | 4.84±0.21ab | 4.46±0.15b | 0.32 | 87.79 |
| Indomethacin | 4.19±0.18 | 4.65±0.14b | 5.12±0.20ab | 5.85±0.33ab | 5.95±0.24ab | 6.02±0.32ab | 5.55±0.21b | 5.02±0.19b | 4.35±0.13b | 0.16 | 93.89 |
*Values are represented as mean±SD (n=6). aP<0.05, as compared with Group I; bP<0.05, as compared with Group II.
Table 3: Anti-Inflammatory Activity Against Carrageenan-Induced Paw Edema In Rats
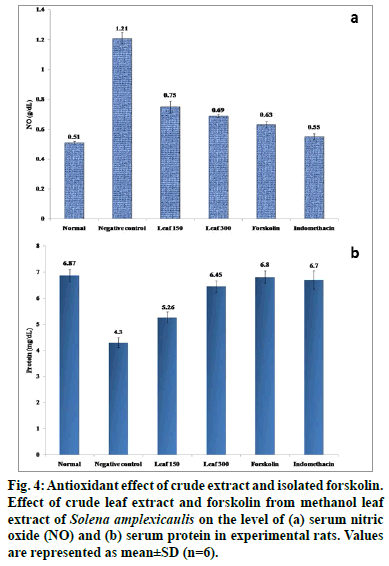
It is widely accepted that ROS may be important both as a triggering signal upon initial tissue injury and in the development of tissue damage. An increased ROS and insufficient antioxidant activity is associated with inflammation. A variety of antioxidants are collectively required for the complete removal of free radicals to protect the body from adverse effects of ROS, generated from inadvertent exposure to carrageenan challenge [46]. Carrageenan intoxicated group of rats evidenced significant climb (P<0.05) in serum NO and decreased protein levels when compared with normal control rats. Oral administration of S. amplexicaulis leaf extract (150 and 300 mg/kg) and forskolin (10 mg/ kg) however significantly attenuated (P<0.05) these serum marker levels towards normalcy (fig. 4). The compound, forskolin offered even better protection than the crude extracts.
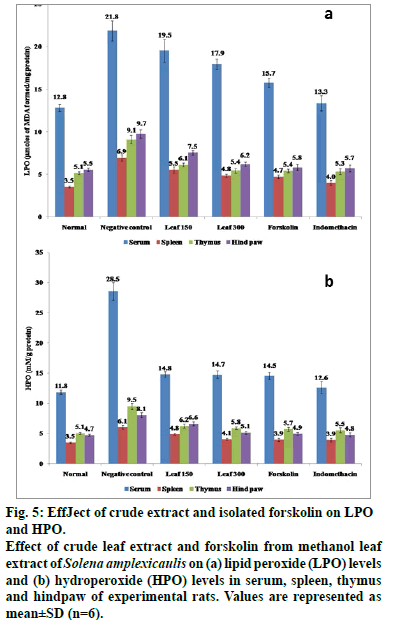
Carrageenan-induced group of rats evidenced higher content (P<0.05) of lipid peroxidation products and reduced levels of SOD, CAT, GPx, GST, G6PD, ascorbic acid and GSH in spleen, thymus and hind paw in comparison to normal control rats (Tables 4 and fig. 5). However, a significant restoration (P<0.05) of these parameters was observed when the herbal drugs were administrated. Consequently, forskolin (10 mg/kg) and the standard drug, indomethacin (10 mg/ kg) upregulated the enzyme activity more efficiently (P<0.05) than the crude drug. Antioxidants like phenolics and flovonoids present in the crude extract owe their antioxidant activity with their ability to trap peroxyl radicals and inactivate lipid peroxidation and also prevent cellular damage induced by carrageenan (Tables 1 and 2). Furthermore, the significant capacity of the compound, forskolin reported in this study is thought to arise from its protective effects by counteracting and neutralizing the ROS which might aid in maintaining the antioxidant armory [47]. It is noteworthy in mentioning that oral administration of forskolin (10 mg/kg) exerts its biological activity by stimulation of adenylate cyclase, thereby increasing cellular concentrations of the second messenger cyclic AMP [48]. Increased cellular cyclic AMP results in a broad range of physiological and biochemical effects mainly by reducing the release of one of the proinflammatory mediators, histamine [49].
| Enzymic/Non enzymic antioxidants# | Group | |||||
|---|---|---|---|---|---|---|
| Control | Negative control | Leaf150 | Leaf300 | Forskolin | Indomethacin | |
| Spleen | ||||||
| Enzymic | ||||||
| SOD | 5.10±0.16 | 2.50±0.10a | 3.15±0.09ab | 4.57±0.25ab | 4.62±0.14b | 4.92±0.24b |
| CAT | 22.96±0.75 | 12.47±0.63a | 15.45±0.66ab | 18.08±0.54ab | 21.06±0.32b | 22.00±1.32b |
| GPx | 10.09±0.31 | 8.90±0.50a | 9.27±0.54ab | 9.44±0.57ab | 9.58±0.28b | 9.85±0.39b |
| GST | 94.54±2.83 | 46.05±1.88a | 74.74±5.88ab | 81.97±5.00ab | 84.32±6.49b | 89.34±7.77b |
| G6PD | 1.39±0.05 | 0.67±0.02a | 0.80±0.05ab | 1.03±0.06ab | 1.15±0.06b | 1.22±0.09ab |
| Non enzymic | ||||||
| GST | 13.95±0.54 | 7.75±0.32a | 10.20±0.80ab | 11.50±0.58ab | 12.01±1.11ab | 13.06±1.08b |
| Vit C | 19.92±0.67 | 12.89±0.39a | 17.53±1.12ab | 18.40±1.30b | 18.99±1.25b | 19.61±1.31b |
| Thymus | ||||||
| Enzymic | ||||||
| SOD | 8.12±0.25 | 5.02±0.22a | 6.98±0.25ab | 7.54±0.41b | 7.61±0.44b | 7.90±0.45b |
| CAT | 25.57±0.79 | 17.73±1.02a | 20.92±1.10ab | 22.18±1.33ab | 22.97±1.16ab | 24.62±0.98b |
| GPx | 10.57±0.41 | 5.24±0.29a | 7.99±0.47ab | 9.08±0.54ab | 9.23±0.67ab | 9.95±0.79b |
| GST | 90.15±3.51 | 46.07±1.98a | 66.48±5.25ab | 70.01±4.83ab | 74.58±6.35ab | 85.35±7.42b |
| G6PD | 0.95±0.02 | 0.64±0.03a | 0.70±0.04a | 0.79±0.05ab | 0.83±0.04b | 0.90±0.07b |
| Non enzymic | ||||||
| GST | 12.52±0.38 | 5.61±0.23a | 8.75±0.69ab | 9.69±0.68ab | 10.01±0.71b | 11.82±0.74ab |
| Vit C | 15.73±0.48 | 11.32±0.57a | 14.58±0.93b | 14.97±0.91b | 15.01±1.14b | 15.39±1.03b |
| Hind paw | ||||||
| Enzymic | ||||||
| SOD | 11.80±0.41 | 6.41±0.35a | 7.50±0.35ab | 8.15±0.28ab | 8.85±0.69ab | 11.53±0.77b |
| CAT | 23.92±0.81 | 16.01±0.83a | 18.04±1.02a | 21.04±1.32ab | 21.86±1.25ab | 23.01±1.01b |
| GPx | 21.30±0.63 | 16.85±0.79a | 18.81±1.48a | 19.34±1.16b | 20.04±1.53b | 20.26±1.62b |
| GST | 104.33±3.54 | 62.03±2.91a | 95.34±7.53b | 95.54±5.82b | 97.34±4.67b | 101.62±3.43b |
| G6PD | 1.32±0.04 | 0.73±0.02a | 0.98±0.06ab | 1.01±0.06ab | 1.04±0.09ab | 1.27±0.10b |
| Non enzymic | ||||||
| GST | 26.83±1.01 | 15.82±0.64a | 18.30±1.35ab | 19.64±1.39ab | 20.75±1.68ab | 22.49±1.70b |
| Vit C | 25.62±0.77 | 12.47±0.67a | 16.35±1.11ab | 21.58±1.40ab | 22.79±1.35ab | 24.25±1.47b |
Pa – Probability of active; Pi – Probability of inactive.
Table 5: Predicted ‘Pa’ And ‘Pi’ Values By Pass Software
Figure 5: EffJect of crude extract and isolated forskolin on LPO and HPO. Effect of crude leaf extract and forskolin from methanol leaf extract of Solena amplexicaulis on (a) lipid peroxide (LPO) levels and (b) hydroperoxide (HPO) levels in serum, spleen, thymus and hindpaw of experimental rats. Values are represented as mean±SD (n=6).
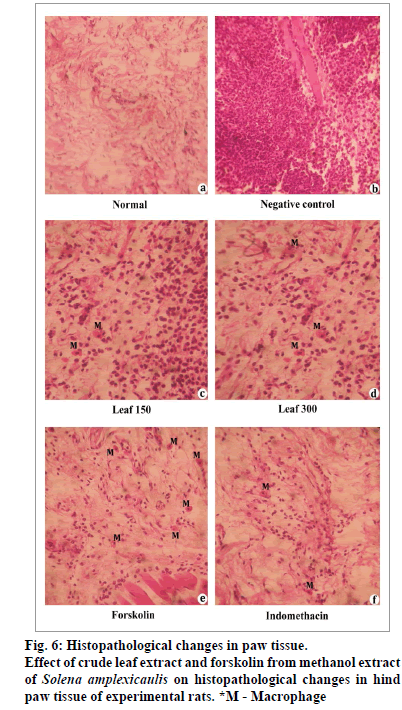
Histopathological examination of the paw biopsies revealed great number of neutrophils after carrageenan induction (fig. 6). In contrast, oral administration of crude methanol extract and the compound, forskolin significantly enhanced the histological architecture by inhibiting the neutrophil infiltration and occurrence of few macrophages, and their effects were often comparable with that of the control and the standard drug, indomethacin.
The computer software, PASS simultaneously predicts several hundreds of various biological activities of pharmacological interest such as mutagenicity, carcinogenicity and embryotoxicity which are based exclusively on the structural formula of the substance [37]. In the present study by PASS, the Pa and Pi values of the compound, forskolin ranged between 0.982 and 0.709, and 0.001 and 0.047, respectively and showed 11 different biological activities. The predicted values exerted the activities like ophthalmic (0.915 Pa, 0.003 Pi), antiglaucomic (0.890 Pa, 0.003 Pi), cardiotonic (0.834 Pa, 0.004 Pi) and others along with the functions of vasodilator (0.982 Pa, 0.001 Pi), and adenylate cyclase inhibitor (0.749 Pa, 0.000 Pi) might incredibly be involved in anti-inflammatory mechanism [50,51] (Table 5).
Our study clearly demonstrated that the oral administration of crude extract (150 and 300 mg/ kg) and the isolated compound, forskolin (10 mg/ kg) from S. amplexicaulis leaf markedly dropped the Carrageenan-induced oxidative stress by significantly boosting the antioxidant enzyme status. Interestingly, the presence of phytochemicals viz; phenolics, flavonoids, alkaloids, tannins and terpenoids may be attributed to the observed anti-inflammatory and antioxidant activities, thus exemplifying the pivotal role in acute phase of inflammation and treatment of various diseases emerging due to oxidative stress.Furthermore, PASS prediction and previous literature surveys strongly suggest that forskolin is a powerful lead molecular target used to eradicate dreadful diseases including inflammation. Giving the aforementioned evidence, it is tempting to speculate that forskolin represents an exciting scaffold to develop leads for the treatment of inflammation related disorders.
Financial support and sponsorship
The authors graciously acknowledge the financial support given by University Grants Commission, New Delhi (Grant No. F. 41-415/2012(SR)) to carry out the work.
Conflicts of interest
There are no conflicts of interest.
References
- Lucetti DL, Lucetti EC, Bandeira MA, Veras HN, Silva AH, Leal LK, et al.Antiinflammatory effects and possible mechanism of action of lupeol acetate isolated from Himatanthusdrasticus(Mart.) Plumel J Inflamm (Lond) 2010;7:60.
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006;8:S3.
- Chohan M, Naughton DP, Jones L, Opara EI. An investigation of the relationship between the anti-inflammatory activity, polyphenolic content, and antioxidant activity of cooked and in vitro digested culinary herbs. Oxid Med Cell Longev 2012;2014:627843.
- Ding Z, Dai Y, Hao H, Pan R, Yao X, Wang Z. Anti-inflammatory effects of scopoletin and underlying mechanisms. Pharm Bio 2008;46:854-60.
- Matthew KM. The flora of the Tamil Nadu Carnatic, Part I Polypetalae. Tiruchirapalli: The Rapinet Herbarium, St. Joseph’s College; 1983.
- Paulsamy S, Karthika K. Distribution and density of the climber, Solenaamplexicaulis(Lam.) Gandhi. in certain dry deciduous forests of southern Western Ghats, India. Int J EcolDev 2014;28:63-78.
- Dhananjay JD. A handbook of medicinal herbs: A source book of herbal remedies. Jodhpur: Agrobios; 2006.
- Ignacimuthu S, Ayyanar M, Sankarasivaraman K. Ethnobotanical study of medicinal plants used by Paliyartribals in Theni district of Tamil Nadu, India. Fitoterapia 2008;79:562-8.
- Jeyaprakash K, Ayyanar M, Geetha KN, Sekar, T. Traditional uses of medicinal plants among the tribal people in Theni District (Western Ghats), Southern India. Asian Pac J Trop Biomed 2011;1:S20-5.
- Mohammed R, Paritosh C, Alok Kumar P, Dilruba N, Rasheda A, Farhana J. A survey of preventive medicinal plants used by the Chakma residents of Hatimara (south) village of Rangamati district, Bangladesh. Am Eurasian J Sustain Agric 2011;5:92-6.
- Venkatachalapathi A, Sangeeth T, Paulsamy S. Ethnobotanicalinformations on the species of selected areas in Nilgiri Biosphere Reserve, the Western Ghats, India. J Res Biol 2015;5:43-57.
- Ghorbani A, Langenberger G, Feng L, Sauerborn J. Ethnobotanical study of medicinal plants utilised by Hani ethnicity in Naban River Watershed National Nature Reserve, Yunnan, China. J Ethnopharmacol 2011;134:651–67.
- Karthika K, Paulsamy S. Antimicrobial potential of tuber part of the traditional medicinal climber, Solenaamplexicaulis(Lam.) Gandhi. against certain human pathogens. J Drug Delivery Therap2014;4:113-7.
- Karthika K, Paulsamy S, Jamuna S. Evaluation of in vitroantioxidant potential of methanol leaf and stem extracts of Solenaamplexicaulis(Lam.) Gandhi. J Chem Pharm Res 2012;4:3254-8.
- Krishnamoorthy K, Subramaniam P. Phytochemical profiling of leaf, stem and tuber parts of Solenaamplexicaulis (Lam.) Gandhi. using GC-MS. IntSch Res Notices 2014;2014: 567409.
- Karthika K, Paulsamy S. TLC and HPTLC Fingerprints of various secondary metabolites in the stem of the traditional medicinal climber, Solenaamplexicaulis. Indian J Pharm Sci 2014;77:111-6.
- Kim HJ, Park JH, Kim JK. Cucurbitacin-I, a natural cell-permeable triterpenoid isolated from Cucurbitaceae, exerts potent anticancer effect in colon cancer. ChemBiol Interact 2014;219:1-8.
- Zhao GT, Liu JQ, Deng YY, Li HZ, Chen JC, Zhang ZR, et al.Cucurbitane – type triterpenoids from the stems and leaves of Momordicacharantia. Fitoterapia 2014;95:75-82.
- Mahato SB, Sen S. Advances in triterpenoid research, 1990-1994. Phytochem 1997;44:1185-236.
- Abdelwahab SI, Hassan LE, Sirat HM, Yagi SM, Koko WS, Mohan S, et al.Anti-inflammatory activity of cucurbitacin E isolated from Citrulluslanatusvar. citroides: role of reactive nitrogen species and cyclooxygenase enzyme inhibition. Fitoterapia 2011;82(8):1190-7.
- Qiao J, Xu LH, He J, Ouyang DY, He XH. Cucurbitacin E exhibits anti-inflammatory effect in RAW 264.7 cells via suppression of NF–kB nuclear translocation. Inflamm Res 2013;62:461-9.
- Wang Y, Zhao GX, Xu LH, Liu KP, Pan H, He J, et al.CucurbitacinIIb exhibits anti-inflammatory activity through modulating multiple cellular behaviors of mouse lymphocytes. PLoS one 2014;9:e89751.
- Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. London, New York: Chapman and Hall; 1998.
- Trease G, Evans SM. Pharmacognosy. London: Bailer Tindal; 2002.
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolics constituents from three different agroclimatic origins of drumstick tree (MoringaoleiferaLam.) leaves. J Agric Food Chem 2003;51:2144-55.
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoids contents in mulberry and their scavenging effects on super oxides radicals. Food Chem 1999;64:555-9.
- Klein BP, Perry AK. Ascorbic acid and Vitamin A activity in selected vegetables from different geographical areas of the United States. J Food Sci 1982;47:941-5.
- Makkar HPS, Siddhuraju P, Becker K. Methods in molecular biology: Plant secondary metabolites. Totowa, NJ: Humana Press. 2007.
- Reid RG, Sarker SD. Isolation of natural products by low-pressure column chromatography. Methods MolBiol 2012;864:155-87.
- OECD Guidelines for the testing of chemicals substances. Test Guideline 423: Acute oral toxicity organization for Economic Cooperation and Development, Paris; 2001.
- Winter CA, Risely EA, Nuss GN. Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. ProcSocExpBiol Med 1962;111:544-7.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 1982;126:131-8.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J BiolChem 1951;193:265-75.
- Panda SK. Assay guided comparison for enzymatic and non-enzymatic antioxidant activities with special reference to medicinal plants. In: El-Missiry MA, editor. Antioxidant Enzyme. Rijeka: InTech; 2012.
- Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem1992;202:384-9.
- Gloriozova TA, Filimonov DA, Lagunin AA, Poroikov VV. Evaluation of computer system for prediction of biological activity PASS on the set of new chemical compounds. Pharm Chem J 1998;32:658-64.
- Poroikov V, Akimov D, Shabelnikova E. Top 200 medicines: can new actions be discovered through computer-aided prediction? SAR QSAR Environ Res 2001;12:327-44.
- Choe KI, Kwon JH, Park KH, Oh MH, Kim MH, Kim HH, et al.The antioxidant and anti-inflammatory effects of phenolic compounds isolated from the root of RhodiolasachalinensisA. BOR. Molecules 2012;17:11484-94.
- Pirbalouti GA, Siahpoosh A, Setayesh M, Craker L. Antioxidant activity, total phenolic and flavonoid contents of some medicinal and aromatic plants used as herbal teas and condiments in Iran. J Med Food 2014;17:1151-7.
- Farasat M, Nejad RAK, Mohammad S, Namjooyan F. Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from Northern coasts of the Persian Gulf. Iran J Pharm Res 2014;13:163-70.
- Loomis TA, Hayes AW. Loomis’s Essentials of Toxicology. California: Academic press; 1996.
- Ownby SL, Fortuno LV, Au AY, Grzanna MW, Rashmir-Raven AM, Frondoza CG. Expression of pro-inflammatory mediators is inhibited by an avocado/soybean unsaponifiables and epigallocatechingallate combination. J Inflamm (Lond) 2014;11:8.
- Seamon KB, Pagett W, Daly JW. Forskolin: unique diterpene activator of adenylatecyclase in membranes and in intact cells. Proc Nat AcaSci USA 1981;78:3363-7.
- Ammon HP, Muller AB. Forskolin: from an ayurvedic remedy to a modern agent. Planta Med 1985;51:473-7.
- Hayashida N, Chihara S, Tayama E, Takaseya T, Enomoto N, Kawara T, et al.Anti-inflammatory effects of colforsindaropate hydrochloride, a novel water-soluble forskolin derivative. Ann ThoracSurg 2001;71:1931-8.
- de Lima VT, Vieira MC, Kassuya CA, Cardoso CA, Alves JM, Foglio MA, et al.Chemical composition and free radical-scavenging, anticancer and anti-inflammatory activities of the essential oil from Ocimumkilimandscharicum.Phytomedicine 2014;21:1298-308.
- Silva MR, Trujillo X, Hernandez BT, Pastor ES, Urzua Z, Mancilla E, et al.Effect of chronic administration of forskolin on glycemia and oxidative stress in rats with and without experimental diabetes. Int J Med Sci 2014;11:448-52.
- Pinto C, Papa D, Hubner M, Mou TC, Lushington GH, Seifert R. Activation and inhibition of adenylyl cyclase isoforms by forskolinanalogs. J PharmacolExpTher 2008;325:27-36.
- Moore AR, Willoughby DA. The role of cAMP regulation in controlling inflammation. ClinExpImmunol 1995;101:387-9.
- Raud J. Vasodilatation and inhibition of mediator release represent two distinct mechanisms for prostaglandin modulation of acute mast cell-dependent inflammation. Br J Pharmacol 1990;99:449-54.
- Mario D, Javier L, DoinaG.Vasoactive intestinal peptide and pituitary adenylatecyclase-activating polypeptide and pituitary adenylatecyclase-activating polypeptide activated microglia. J LeukocBiol 2003;73:155-64.