- *Corresponding Author:
- A. V. Shitole
Department of Plant Pathology, Dr. Panjabrao Deshmukh Krishi Vidyapeeth, Akola-444 104, India
E-mail: amolsht@gmail.com
| Date of Submission | 11 June 2016 |
| Date of Revision | 02 January 2017 |
| Date of Acceptance | 13 April 2017 |
| Indian J Pharm Sci 2017; 79(3):377-384 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study dealt with the assessment of antifungal activities of acetone, ethanol, methanol and chloroform leaf and fruit extracts of Aegle marmelos, Syzygium cumini and Pongamia pinnata against the soil borne fungi, Pythium debaryanum. Maximum inhibition of fungal growth was found with methanol extract of A. marmelos leaves and fruits. At 1 ml concentration (C4), complete inhibition of mycelial growth of P. debaryanum was observed with extract S3P1 (100%), followed by S3P3 (97.63%) and S2P1 (95.21%). Lowest inhibition was oberved with S4P2 (75.40%). Pot culture study revealed that tomato seed treatment with Pseudomonas fluorescens (10 g/kg)+Trichoderma viride (4 g/kg)+methanol extract of A. marmelos 4% was effective to control pre and post emergence damping off caused by P. debaryanum. The methanol extract revealed strongest antifungal activity against P. debaryanum, followed by ethanol extract and lowest antifungal activity was found in chloroform extract.
Keywords
Extracts, antifungal activity, Aegle marmelos, Syzyium cumini, Pongamia pinnata, Pythium debaryanum
Traditional fungicides have been used for many centuries by a substantial proportion of populations of many countries. The use of medicinal plants as a source of secondary metabolites has increased worldwide. Medicinal plant extracts were continuously explored for identifying new active compounds with potential to act against multi-drug resistant bacteria[1]. The complete potential of higher plants as a source of new drugs is still largely unexplored[2]. The last decade has also witnessed exploration of new biomolecules from plants for plant disease management. In India also, among the entire medicinal plant diversity only a small percent of plants have been investigated for antimicrobial activity against plant pathogens.
Plants serve as a rich storehouse of biochemicals, which have the potential to play an important role in the defence against several diseases. These phytochemicals have been exploited as natural pesticides, flavouring agents, fragrances, medicinal compounds, fibres, beverages and food metabolites and were identified by nuclear magnetic resonance (NMR), mass spectrometry (MS) and X-ray analysis techniques[3].
Phytochemical analysis of Aegle marmelos showed presence of alkaloids, saponins, tannins, flavonoids and furanocoumarins[4]. Analysis of Syzygium cumini indicated the presence of gallic acid and quercetin in the methanol extract[5]. Phytochemical analysis of methanol and ethanol extracts of S. cumini seeds revealed the presence of phenols, alkaloids, flavonoids, saponins, tannins and triterpenoids[6,7]. Recent studies on Pongamia pinnata methanol extracts[8] reported alkaloids, steroids, flavonoids, glycosides, saponins and tannins.
Soil borne diseases in general are difficult to control. Crop rotations, breeding with resistant plant varieties and the application of pesticides have been found insufficient to control such diseases. However, the use of fungicides could be hazardous to human health and might directly increase environment pollution. In addition, some fungicides may not readily be biodegradable and tend to persist for years in the environment[9]. Tahira and Sharma[10] reported the antifungal activity of crude aqueous, 50% hydroalcoholic and alcoholic leaf extracts of A. marmelos, P. pinnata, Polyalthia longifolia and Terminallia arjuna against Pythium aphanidermatum and Pythium myriotylum. Therefore, the present investigation was undertaken to screen the antifungal potential of crude extracts of some medicinal plants against the damping-off caused by the soil fungus P. debaryanum.
Materials and Methods
P. debaryanum was tested on tomato by soil inoculation technique[11]. The Pathogenicity of the fungus was tested on the basis of percent pre and post-damping-off in artificial sick soil. The field soil was sterilized for three successive days at 120°. Soil sickness was developed by adding 100 g mass inoculum of P. debaryanum in 1 kg of soil. Plastic pots of 15 cm diameter were filled with the above mixture. The pots were watered and incubated for 6 d to multiply the pathogen in soil. Fresh leaves of A. marmelos, S. cumini and P. pinnata were collected from various places within 5 sq. km area around the headquarter in October 2014. Identification and authentication of the plants was carried out at the Nagarjun Medicinal Plants Garden, Dr. Panjabrao Deshmukh Krishi Vidyapeeth Akola, India. Leaves were thoroughly washed under tap water followed by distilled water to remove dust and other impurities and then shade-dried separately with occasional shifting for about 3 to 4 w. The dried leaves were coarsely powdered with a sample grinder and stored in airtight container until further use[12].
Acetone, ethanol, methanol and chloroform were used for preparing leaf extracts. Forty grams powder of each leaf was soaked separately in 200 ml of acetone, ethanol, methanol and chloroform in 500 ml conical flasks and then plugged tightly with cotton and wrapped with paper. All conical flasks were kept on a rotary shaker for 4 d and then allowed to stand for 5 h to settle the leaf material. Supernatant from each flask was filtered separately through Whatman No. 1 filter paper and evaporated at room temperature. Residual portion of leaves were repeatedly extracted three times to harvest maximum metabolites from leaves. Air dried extracts were weighed separately, transferred into small vials and kept in refrigerator at 5° until further use and the percent yield of extraction was calculated[13].
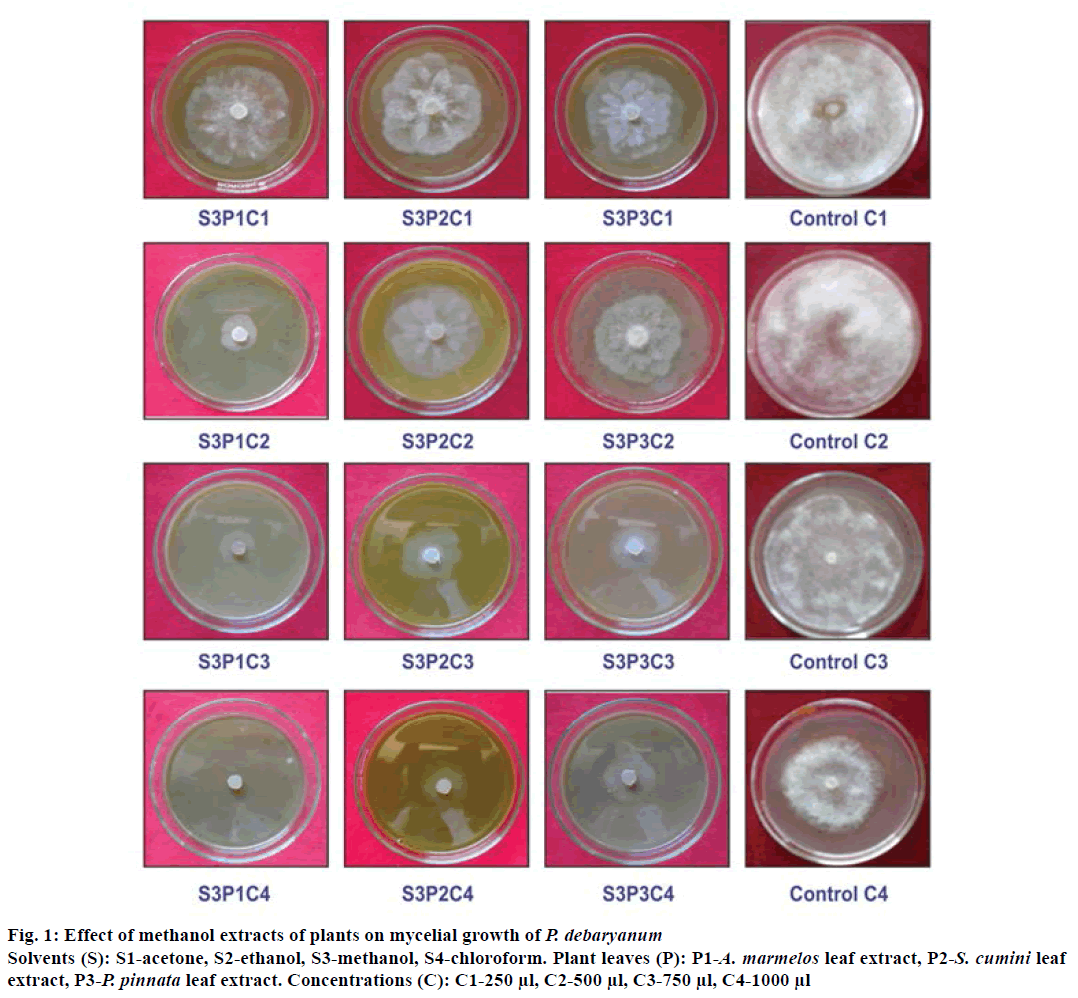
The effects of acetone, ethanol, methanol and chloroform extracts of A. marmelos, S. cumini and P. pinnata at 250, 500, 750 and 1000 μl concentrations were tested against P. debaryanum under in vitro conditions by following the poisoned food technique[14] (Figure 1). One gram crude leaf extracts of all the plants extracted with acetone, ethanol, methanol and chloroform were diluted in 10 ml dimethyl sulphoxide (DMSO) separately and from this 250, 500, 750 and 1000 μl suspensions were poured separately in conical flasks, which contained 60 ml sterilized melted potato dextrose agar (PDA) medium. The conical flasks were shaken well for uniform mixing of plant extract with the media, which was poured into plates and allowed to solidify. For each treatment, 3 replicates (plates) were used and 250, 500, 750 and 1000 μl of DMSO served as a vehicle control. All the plates were inoculated individually with 5 mm diameter discs of the test fungal cultures and incubated at 28±2°, until the control plates reached full growth. Percent growth inhibition (I) of test fungus was calculated[15].
Figure 1: Effect of methanol extracts of plants on mycelial growth of P. debaryanum
Solvents (S): S1-acetone, S2-ethanol, S3-methanol, S4-chloroform. Plant leaves (P): P1-A. marmelos leaf extract, P2-S. cumini leaf extract, P3-P. pinnata leaf extract. Concentrations (C): C1-250 μl, C2-500 μl, C3-750 μl, C4-1000 μl
Antagonistic activity of bioagents, Pseudomonas fluorescens and Trichoderma viride on growth of P. debaryanum was studied by dual culture technique on PDA plates. Inoculated plates were incubated at 22±2° for 3 d. Antagonistic effect of all plant extracts against the test pathogen was recorded on day 3 after inoculation and growth inhibition was calculated[15].
Pot culture experiments were carried out for studying antagonistic activity of P. fluorescence, T. viride and A. marmelos methanol extract alone or in combination as seed treatment against P. debaryanum causing damping-off of tomato. Each treatment was performed in triplicate using a factorial completely randomized design (FCRD) in a glasshouse. Incidence of dampingoff of tomato was recorded at 7, 14 and 21 d after sowing.
The plant extracts were given the following code names, S1P1 (acetone extract of A. marmelos), S1P2 (acetone extract of S. cumini), S1P3 (acetone extract of P. pinnata), S2P1 (ethanol extract of A. marmelos), S2P2 (ethanol extract of S. cumini), S2P3 (ethanol extract of P. pinnata), S3P1 (methanol extract of A. marmelos), S3P2 (methanol extract of S. cumini), S3P3 (methanol extract of P. pinnata), S4P1 (chloroform extract of A. marmelos), S4P2 (chloroform extract of S. cumini) and S4P3 (chloroform extract of P. pinnata).
In vitro effect of the plant extracts on test pathogen was studied using FCRD with three factors having four levels in each factor. Pot culture studies were carried out using completely randomized design and each treatment was applied in triplicate. Statistical analysis of the data was performed according to the method of Panse and Sukhatme[16]. ‘F’ test of significance was used to know whether observed treatment effects were real or not from the data in which the treatment effects were significant. The standard error (SE) and critical difference (CD) at 1% level of probability were calculated.
Result and Discussion
The results revealed that all tested plant extracts inhibited growth of P. debaryanum at all concentrations tested (Table 3). The rate of inhibition of growth exerted by the plant extracts correlated well with the concentrations studied. Employing medicinal plant waste as the raw material for plant-derived fungicides, it would be possible to manage plant fungal diseases while creating an environmental friendly use for the unwanted waste material.
P. debaryanum was isolated on Vaartaza’s medium from infected tomato plants collected from vegetable research unit, Dr. Panjabrao Deshmukh Krishi Vidyapeeth Akola. Colonies appeared on medium were cottony with areal mycelium. Sporangia were smooth-walled, sub spherical, terminal or intercalary. Ramamoorthy et al.[17] isolated P. aphanidermatum from naturally infected tomato plants. The results obtained in this investigation were quite similar to those reported earlier by Braun[18]. The pathogenicity of P. debaryanum was tested on a local variety of tomato by the soil inoculation technique that was already described. The symptoms were seen from 7-21 d after inoculation along with some seed decay observed as the pre-emergence damping-off. Stem lesions appeared somewhat water soaked and post-emergence dampingoff included top rot or top damping-off and root rot.
The results in Table 1 indicated that the extraction yield depended significantly on the solvent used for extraction. This was in principal depended on the polarity of the solvent and its ability to extract soluble substances. Methanol has been extensively used to extract a great number of constituents from plants with higher yields and in the current investigation the yields obtained were A. marmelos, 7.44%, S. cumini, 8.52% and P. pinnata, 7.21%.
| Sample and local name | Extraction yield (%) in solvents | |||
|---|---|---|---|---|
| Acetone | Ethanol | Methanol | Chloroform | |
| A. marmelos(Beal) | 1.82 | 2.01 | 7.44 | 1.81 |
| S. cumini (Jamun) | 3.42 | 3.22 | 8.52 | 2.01 |
| P. pinnata(Karanj) | 3.03 | 4.21 | 7.21 | 1.85 |
Table 1: Effect of Different Solvents on Percent Extraction Yield from Dry Weight of Leaves
Comparison was made between the 2 bioagents for their ability to control mycelial growth of P. debaryanum employing dual culture technique. All the extracts were effective in inhibiting fungal growth. Among the bioagents tested, maximum inhibition of mycelial growth was noticed with Trichoderma viride (51.11%), which was found to be significantly more compared to other treatments (Table 2). Similar results were observed by Muthukumar et al.[19] in which they reported 53.8 to 71.5% and 57.8 to 76.7% inhibition with Trichoderma and Pseudomonas isolates, respectively. Pseudomonads were more efficient in inhibiting various Pythium isolates than bacilli[20-22]. Muthukumar et al.[23] evaluated different isolates of T. viride for their antifungal activity against P. aphanidermatum and reported that all the isolates significantly inhibited mycelial growth of fungus.
| Treatment | Radial mycelial growth (mm) P. debaryanum |
Percent inhibition P. debaryanum |
|---|---|---|
| T. viride | 44.00 | 51.11 (45.64)* |
| P. fluorescens | 46.71 | 48.10 (43.90) |
| Control | 90.00 | 0.00 (0.00) |
| F test | Sig | Sig |
| SEM± | 0.90 | 0.59 |
| C.D. at (P=0.01) | 3.53 | 2.30 |
Table 2: Efficacies of Bioagents on Mycelial Growth of Pythium Debaryanum
Interaction of solvents, plant extracts and concentrations were evaluated for their effectiveness against test fungus. There was varied degree of fungal toxicity among all the interactions. At 250 μl concentration (C1), maximum 18.89% inhibition was observed in the interaction S3P3, which was significantly greater than all the tested interactions at C1 concentration. Lowest inhibition was recorded in interactions S4P2 followed by 10.96% inhibition in S1P2, S4P1, which are at par with each other. At 500 μl concentration (C2), maximum 86.35% inhibition of test fungus occurred in S3P1 followed by interaction S2P1, which recorded 57.63 and 48.84% mycelial inhibition of P. debaryanum, respectively. At 750 μl concentration (C3), 90.91% inhibition of test fungus occurred in S3P1, followed by 86.41% in interaction S2P1 and S3P3. Lowest (43.01%) inhibition was recorded in interaction S4P2 of test fungus. Results in Table 3 represented that, at 1000 μl concentration (C4), complete inhibition of mycelial growth of test fungus was recorded in S3P1, followed by S3P3, S2P1. Lowest inhibition (75.40%) was recorded in S4P2 (Table 3). Earlier study by Al-Rahmah et al.[14] reported that T. vulgaris extract was most effective in suppressing growth of P. aphanidermatum due to presence of phenolic compounds as thymol and carvacrol, which played vital role in growth inhibition of phytopathogenic fungi.
| S×P×C (Solvent×plant×concentration) |
% Inhibition over control | |||||
|---|---|---|---|---|---|---|
| C1 (250 µl) | C2 (500 µl) | C3 (750 µl) | C4 (1000 µl) | |||
| S1P1 | 13.6 (21.68)* | 43.04 (41.00) | 79.69 (63.21) | 86.57 (68.30) | ||
| S1P2 | 10.96 (19.33) | 29.98 (33.19) | 50.98 (45.56) | 81.05(64.19) | ||
| S1P3 | 14.45 (22.34) | 41.68 (40.21) | 68.78 (56.03) | 88.86 (70.50) | ||
| S2P1 | 15.86 (23.47) | 57.63 (49.39) | 86.41 (68.37) | 95.21 (77.36) | ||
| S2P2 | 12.16(20.41) | 33.33 (35.26) | 57.76 (49.46) | 85.43 (67.56) | ||
| S2P3 | 16.64 (24.07) | 45.50 (42.42) | 74.45 (59.63) | 91.04 (72.59) | ||
| S3P1 | 16.99 (24.34) | 86.35 (68.31) | 90.91 (72.45) | 100.00 (89.76) | ||
| S3P2 | 14.40 (22.30) | 37.67 (37.86) | 63.22 (52.66) | 88.73 (70.39) | ||
| S3P3 | 18.89 (25.76) | 48.84 (44.33) | 80.01 (63.44) | 97.63(81.15) | ||
| S4P1 | 10.96 (33.11) | 29.85 (33.11) | 74.11 (59.41) | 77.64 (61.78) | ||
| S4P2 | 8.83 (28.88) | 23.33 (28.88) | 43.01 (40.98) | 75.40(60.26) | ||
| S4P3 | 13.31 (38.57) | 38.88 (38.57) | 62.18 (52.02) | 77.73(61.84) | ||
| Control | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | ||
| Source | S.E (M)± | C.D. at (P=0.01) | ||||
| Solvent (S) | 0.04 | 0.18 | ||||
| Plants (P) | 0.04 | 0.18 | ||||
| Concentrations (C) | 0.04 | 0.18 | ||||
| Solvent×plants (S×P) | 0.08 | 0.31 | ||||
| Solvent×concentrations (S×C) | 0.09 | 0.36 | ||||
| Plants×concentrations (P×C) | 0.08 | 0.31 | ||||
| Solvent×plants×concentrations (S×P×C) | 0.14 | 0.62 | ||||
Table 3: Effect of Interaction Means of Solvents×Plants×Concentrations (S×P×C)
Methanol extract of A. marmelos, P. fluorescens and T. viride alone and in combination in pot culture experiment were screened against P. debaryanum causing damping-off in tomato. Observations on percent seed germination, pre and post emergence damping-off at 7, 14 and 21st d after sowing were recorded and results were presented in Table 4. Significant differences were observed in the treatments for pre and post emergence damping-off in tomato.
| S. No. | Treatments | Seed emergence (%) | Damping-off (%) | Total reduction over control (%) | |
|---|---|---|---|---|---|
| Pre | Post | ||||
| T1 | P. fluorescensalone (10 g/kg) |
71.33 (57.63)* | 14.63 (22.49)* | 32.67 (33.54)* | 49.27 |
| T2 | T. viridealone (4 g/kg) |
74.89 (59.93) | 10.37 (18.79) | 33.33 (33.01) | 55.01 |
| T3 | Methanol extract of A. marmelosalone 2% | 61.56 (51.68) | 26.33 (30.87) | 38.33 (40.11) | 23.79 |
| T4 | Methanol extract of A. marmelosalone 3% | 65.11 (53.80) | 22.07 (28.02) | 37.00 (37.99) | 32.63 |
| T5 | Methanol extract of A. marmelosalone 4% | 70.00 (56.79) | 16.22 (23.75) | 34.67 (35.07) | 44.69 |
| T6 | P. fluorescens(10 g/kg)+T. viride (4 g/kg) | 77.11 (61.42) | 7.71 (16.12) | 30.33 (30.81) | 61.87 |
| T7 | P. fluorescens(10 g/kg)+methanol extract of A. marmelos4% | 74.67 (59.78) | 10.64 (19.03) | 29.33 (30.79) | 58.62 |
| T8 | T. harzianum(4 g/kg)+methanol extract of A. marmelos4% | 76.67 (61.12) | 8.25 (16.69) | 26.33 (28.59) | 65.01 |
| T9 | P. fluorescens(10 g/kg)+T. viride(4 g/kg)+methanol extract of A. marmelos4% | 79.11 (62.80) | 5.32 (13.33) | 27.33 (28.68) | 68.16 |
| T10 | Control (pathogen inoculated ) | 53.56 (47.04) | 35.90 (36.81) | 42.67 (46.78) | 0.00 |
| T11 | Soil control | 85.12 (52.91) | - | - | - |
| F test | Sig | Sig | Sig | - | |
| SE (M)± | 1.16 | 0.27 | 0.59 | - | |
| CD at (P=0.01) | 4.301 | 1.02 | 2.20 | - | |
Table 4: Effects of P. Fluorescens, T. Viride and methanol extract of A. Marmelos alone and in combination on damping-off of tomato caused by P. Debaryanum
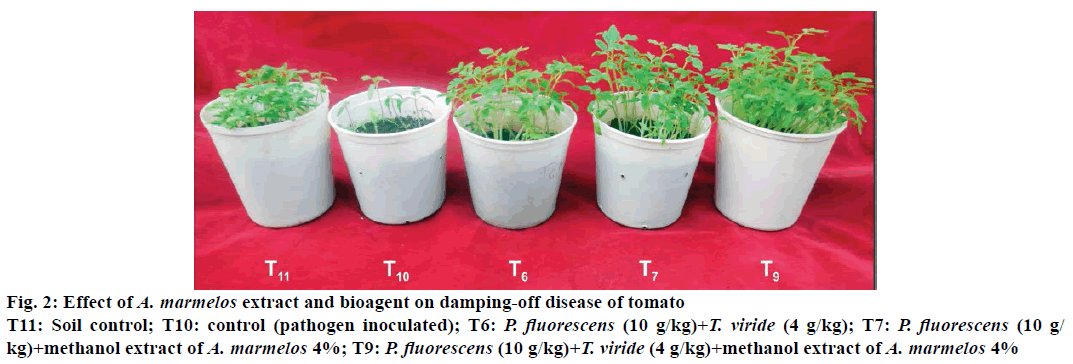
Two potential biocontrol agents and methanol crud extract of A. marmelos with three different concentrations alone and in combination as seed treatment were screened against damping-off of tomato caused by P. debaryanum (Figure 2). Results from Table 4 revealed that, among all the treatments, maximum 79.11% germination was observed in treatment T9 (P. fluorescens 10 g/kg seed+T. viride 4 g/kg seed+methanol extract of A. marmelos 4%) followed by 76.67% in T8 (T. viride 4 g/kg seed+methanol extract of A. marmelos 4%) and both these treatments were at par with each other. Lowest germination was reported in control T10 (53.56%).
Figure 2: Effect of A. marmelos extract and bioagent on damping-off disease of tomato
T11: Soil control; T10: control (pathogen inoculated); T6: P. fluorescens (10 g/kg)+T. viride (4 g/kg); T7: P. fluorescens (10 g/kg)+methanol extract of A. marmelos 4%; T9: P. fluorescens (10 g/kg)+T. viride (4g/kg)+methanol extract of A. marmelos 4%
Significant differences in all the 11 treatments were observed for pre emergence damping-off in tomato. Minimum (5.32%) pre emergence damping-off was recorded in the treatment T9 (P. fluorescens 10 g/kg seed+T. viride 4 g/kg seed+methanol extract of A. marmelos 4%) and was significantly superior over control and other treatments. Treatment T8 (T. viride 4 g/kg seed+methanolic extract of A. marmelos 4%) and T7 (P. fluorescence 10 g/kg seed+methanol extract of A. marmelos 4%) showed 8.25 and 10.10% pre emergence damping-off in tomato, respectively and these treatments were at par with each other. Maximum 35.90% pre emergence damping-off was recorded in control (Table 4).
It was observed from the results of current study, that maximum efficacy was recorded in combined application of both the bioagents and methanol extract of A. marmelos. It was due to the synergetic activity of their components. These results are in agreement with Muthukumar et al.[23] who reported that T. viride, P. fluorescens and zimmu leaf extract significantly reduced damping-off incidence. Inducing a plant’s own defence mechanism by prior application of a biological agent is a novel strategy in plant disease management. It has been reported that application of P. fluorescens triggers/activates plants’ latent defence mechanisms in response to infection by pathogen[17,20,21].
Bioagents and methanol extract of A. marmelos were evaluated alone and in combination against P. debaryanum. Significant differences for post emergence damping-off in tomato caused by P. debaryanum were observed between treatments. Data from Table 4, showed that minimum post emergence damping-off (26.33) was recorded in T8 (T. viride 4 g/kg seed+methanol extract of A. marmelos 4%) and 27.33 in T9 (P. fluorescens 10 g/kg seed+T. viride 4 g/ kg seed+methanol extract of A. marmelos 4%), which are at par with each other. Maximum post emergence damping-off (42.67) was exhibited in T10 control. The broad spectrum antifungal activity of plant species was observed to be related to the presence of saponins, tannins and alkaloids[24]. Similar results were also observed by Muthukumar et al.[23].
Eleven treatments were screened to see the comparative effect of methanol extract of A. marmelos and bioagents for reducing damping-off in tomato. All the treatments were effective to reduce damping-off. There were significant differences among all the treatments for reducing damping-off. Maximum reduction in tomato damping-off (68.16%) was observed under pot experiment in treatment T9 (P. fluorescens 10 g/ kg seed+T. viride 4 g/kg seed+methanol extract of A. marmelos 4%), followed by 65.01% in treatment T8 (T. viride 4 g/kg seed+methanol extract of A. marmelos 4%) and both these treatments were found to be at par with each other. Results indicated a gradual decrease in damping-off with corresponding increase in concentration of methanol extract of A. marmelos when integrated with bioagents. Earlier it was reported by Muthukumar et al.[23] that T. viride, P. fluorescens and zimmu leaf extract significantly reduced dampingoff incidence, which corroborated the present findings.
In the present study combined application of bacterial and fungal bioagents with methanolic extract of A. marmelos resulted in maximum activity against P. debaryanum than individual application. It may be due to synergetic effect of combined treatment. As above stated bioagents have promising effect due to presence of different antifungal constituents. This may go long way in providing better alternative to the over dependency on synthetic fungicides. The integrated fungi management could reduce over reliance on one source of agricultural chemical to the farmers, as well as cut down the cost of production[25]. The plant leaves used in the present study are readily available at no or low cost and with easy method of extraction, which can be exploited in the control of damping-off of tomato. Further field experiments are suggested to investigate the in vivo effects of these extracts as compared to some chemical fungicides for the management of dampingoff of tomato.
The current status of research suggests that there are indeed alternatives to replace the synthetic fungicides for management of this notorious soil as well as seed borne fungi: Pythium, which causes loss of multimillion dollars. The use of biofungicides will not leave any ill effect in the soil, water as well as in the environment. It is possible that by combining these approaches (use of plant extracts, antagonistic microorganisms and plants solvent extract) an economically viable alternative for crop production system can be developed. So the use of bio fungicides proved to be economical alternative that can be implemented at the farm level. For the effective production of crops, formulation protocols as well as its using methods should be provided to the farmers. P. debaryanum was pathogenic to tomato. Methanol was found effective to give maximum extraction yield of selected plants. Methanol was the best solvent for extraction of antifungal constituents from tested medicinal plant leaves. Methanol extract of A. marmelos was proved for promising activity under in vitro against P. debaryanum. T. viride and P. fluorescens were found effective. In pot culture study combined application of T. viride and P. fluorescens and methanol extract of A. marmelos as seed treatment was found effective. Further field experiments are suggested to investigate in vivo effects of these medicinal plant extracts in comparison with some chemical fungicides for the management of collar rot of chickpea.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Mothana RA, Lindequist U. Antimicrobial activity of some medicinal plants of the Island qotra. J Ethnopharmacol 2005;96:177-81.
- Dubey SC, Suresh M, Singh B. Evaluation of Trichoderma species against Fusarium oxysporum f. sp. cicerifor integrated management of chickpea wilt. Biol Cont 2007;40:118-27.
- Yazdani D, Tan YH, Abidin MA, Jaganath IB. A review on bioactive compounds isolated from plants against plant pathogenic fungi. J Med Plants Res 2011;5:6584-9.
- Rani MS, Dayanand CD, Shetty J, Vegi PK, Moideen Kutty AV. Evaluation of antibacterial activity of Pongamia pinnataLinn. on pathogens of clinical isolates. AJPCT 2013;1:645-51.
- Kothari V, Seshadri S, Mehta P. Fractionation of antibacterial extracts of Syzygium cumini(myrtaceae) seeds. Res Biotechnol 2011;2:53-63.
- Kumar VK, Raju SK, Reddy MS, Kloepper JW, Lawrence KS, Groth DE, et al. Evaluation of commercially available PGPR for control of rice sheath blight caused by Rhizoctonia solani. J Pure Appl Microbiol 2009;3:485-8.
- Sagrawat H, Mann A, Kharya M. Pharmacological potential of Eugenia jambolana: A review Pharmacog Mag 2002;2:96-105.
- Dahikar SB, Arote SR, Yeole PG. Phytochemical screening and antibacterial properties of leaves of Pongamia pinnatalinn. (Fabaceae) from India. Continental J Microbiol 2008;2:5-10.
- Brady NC. The Nature and properties of soils. New York: MacMillan Publishing Company; 1984. p. 528.
- Tahira P, Sharma K. Phytochemical Profiling of leaves and stem bark of Terminalia arjunaand Tecomella undulata. Int J Pharma Bio Sci 2014;1:1-7.
- Mythukumar A. Trichoderma virideand Pseudomnasfluorescens formulations against Pythiumaphanidermatuminin vitro. Indian J Plant Prot 2009;37:204-06.
- Thenmozhi M, Bhavya, PK, Sivaraj R. Compounds identification using HPLC and FTIR in Eclipta alba and Emilia sonchifolia. Int J Engin Sci Technol 2011;3:292-8.
- Khan ZS, Shinde VN, Bhosale NP, Sahera N. Chemical composition and antimicrobial activity of angiospermic plants. Middle-East J Scientific Res 2010;6:56-61.
- Al-Rahmah AN, Mostafa AA, Abdel-Megeed A, Yakout SM, Hussein SA. Fungicidal activities of certain methanolic plant extracts against tomato phytopathogenic fungi. Afr J Microbiol Res 2013;7:517-24.
- Vincent JH. Distortion of fungal hyphae in the presence of certain inhibitors. Nature 1947;15:850.
- Panse VG, Sukhatme PV. Statistical methods for Agricultural Workers. New Delhi: ICAR Publication; 1978.
- Ramamoorthy V, Raguchander T, Samiyappan R. Enhancing resistance of tomato and hot pepper to Pythium diseases by seed treatment with Fluorescent pseudomonads. Eur J Plant Pathol 2002;108:429-41.
- Braun H. Comparative studies of Pythium debaryanumtwo related species from Geranium. J Agric Res 1925;11:1043-62.
- Muthukumar A, Eswaran A, Sangeetha G. Induction of systemic resistance by mixtures of fungal and endophytic bacterial isolates against Pythium aphanidermatum. Acta Physiol Plant 2011;33:1933-44.
- Georgakopoulos DG, Fiddaman P Leifert C, Malathrakis NE. Biological control of cucumber and sugar beet damping-off caused by Pythiumultimumwith bacterial and fungal antagonists. J Appl Microbiol 2002;92:1078-86.
- Shitole AV, Gade RM, Archana Zalte, Bandgar MS. Utilization of spent mushroom substrate for management of tomato damping off. J Plant Dis Sci 2013;8:196-9.
- Archana Zalte, Gade RM, Shitole AV, Belkar YK. Management of tomato damping off by using plant growth promoting microorganisms. J Plant Dis Sci 2013;8:200-03.
- Muthukumar A, Eswaran A, Nakkeeran S, Sangeetha G. Efficacy of plant extracts and biocontrol agents against Pythium aphanidermatuminciting chilli damping-off. Crop Prot 2010;29:1483-8.
- Ndukwe KC, Okeke IN, Lamikanra A, Adesina SK, Aboderin O. Antibacterial activity of aqueous extracts of selected chewing sticks. J Contemp Dent Pract 2005;6:86-94.
- Oja BA, Olufolaji DB. Evaluation of the efficacy of crude Neem bark extracts in enhancing germination and seedling establishment of Anthracnose diseased seeds. Niger J Plant Prot 2005;22:132-8.