- *Corresponding Author:
- Joyeeta Bhattacharya
Department of Pharmaceutical Technology,
Adamas University,
Kolkata,
West Bengal 700126,
India
E-mail: joyitabhatacharya@gmail.com
| Date of Received | 24 November 2020 |
| Date of Revision | 24 September 2021 |
| Date of Acceptance | 18 May 2022 |
| Indian J Pharm Sci 2022;84(3):631-641 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The primary focus of the research was to evaluate the antidiabetic activity of the ethanolic and aqueous extract of Vitis pedata in alloxan induced rats. The entire study was divided into two phases: Phase 1 and phase 2. The phase 1 is initiated with the collection and authentication of the plant, followed by extraction with ethanol and water and finally screening of the phytochemical constituents of the extracts. The phase 2 is more inclined on the therapeutic effect of the plant extracts on experimentally induced diabetic rats. Several parameters such as estimating the body weight and liver weight, blood glucose level, total proteins, hemoglobin, serum albumin, serum urea, serum cholesterol levels were examined in the diabetic rats. The histopathological changes in the pancreas of diabetic rats were also studied. The aqueous extract of Vitis pedata showed a significant reduction in the blood glucose levels, lipid profile and serum biomarkers in diabetic rats, quite similar to the standard treatment of glibenclamide, whereas the ether extract showed a less effect compared to the other two extracts. The plant extracts also highlighted an improvement in the beta cell mass in islets of pancreas. Thus, the study of aqueous and ethanolic extract of Vitis pedata indicated a promising antihyperglycemic activity in alloxan induced diabetic rats. Furthermore, the study also opens the door for extended research to explicate the mechanism of action of the plant extracts.
Keywords
Diabetes mellitus, phytochemical, Vitis pedata, alloxan, antihyperglycemic activity
Diabetes mellitus has become a real issue of public health in most of the developing countries, where its prevalence is moving to a higher surge particularly in low and middle-income countries. According to World Health Organization (WHO), in 2016, diabetes was solely responsible for 1.6 million of deaths globally[1]. The ever-increasing growth of diabetic mellitus is attributed to several factors such as age, obesity, sedentary lifestyles and unhealthy eating habits[2]. In such economically under-developed countries, adequate treatment is either expensive or unavailable. The genre of medical science has witnessed a huge number of advancements in the development of synthetic pharmaceutical medicaments to combat the disease. Examples of these drugs can be classified into major chemical groups such as biguanides, sulphonylureas, thiazolidinediones, alpha (α)-glucosidase inhibitors and also insulin analogues. Each of these drugs has different mechanism as an antidiabetic agent. The management of diabetes mellitus by the existing therapeutic agents is failing to arrest the pathogenesis of the disorder. Although they possess hypoglycemic effect, the long term usage of these drugs is associated with many adverse effects such as gastrointestinal disturbances, nephrological complications, brain atrophy and hepatic disorders. The treatment of diabetes mellitus with insulin analogues also shows detrimental effects in the long term use[3,4]. The shooting price of the medicaments and the lack of availability of up-to-date treatment procedures are still not accessible to a major proportion of rural population especially in the developing countries. Thus, there is an urge amongst the researchers to develop alternating approaches to treat this disease with respect to the present therapeutic approach of diabetes mellitus[5]. The evolution of human civilization has witnessed the significance of plants and its parts as a remedy for several ailments. The paradigm shift from the synthetic drugs to the herbal medicines for the treatment of the diabetes mellitus, without any unwanted effects, appealed the scientists around the globe. The ethno botanical information reports state that huge number of plants may possess antidiabetic potential[6]. A report of the clinical trials conducted on human patients during the past few years affirmed the glycemic control properties of several medicinal plants such as Scoparia dulcis[7], Cinnamomum cassia[8], Ficus racemosa bark and Portulaca oleracea[9]. Most of the plants have been found to contain substances like glycosides, alkaloids, terpenoids, flavonoids etc., that are frequently implicated as having a specific mode of action of these plant drug or herbal formulation used for treating diabetes[10].
Vitis pedata (V. pedata) (also known as Cayratia pedata (C. pedata))[11] is a woody climber, sometimes vines, rarely small succulent trees, hermaphroditic or polygamomonoecious to polygamodioecious. It belongs to the family Vitaceae, which comprises about 14 genera and 900 species and it is distributed worldwide, but mostly in tropical and subtropical regions. It is locally known as Goalilata[12,13]. The ethno pharmacological claims for V. pedata included the utilization of its leaves as an anti-inflammatory agent[14]. The leaves of the plant is having astringent and haemostatic properties, which are being used in the treatment of diarrhea, hemorrhage, varicose veins, hemorrhoids, inflammatory disorder, pain, hepatitis and free radical related diseases and also as a wound healer on external use[15]. A number of research work on the plant extract have also highlighted its antimicrobial[16], anti-nociceptive[17], anti-arthritic and anti-oxidant properties[18].
To understand the rationalization of choosing this plant species for the research, we need to throw some light on the association of inflammation and its biomarkers on the pathogenesis of diabetes mellitus. Insulin resistance which is a key component of type 2 diabetes is majorly attributed with steatosis (accumulation of fats in liver) which is a resultant of obesity. This in turn causes decreased hepatic insulin sensitivity followed by increased fasting hyperglycemia. Extensive researches on the obesity has also reported the activation of two inflammatory pathways, namely stress-activated c-Jun N-Terminal Kinase (JNK) and the transcription factor Nuclear Factor kappa B (NF-κB). These inflammatory pathways are responsible for augmented adipokinesis through production of several cytokines in obese people thus making them more prone to develop metabolic diseases like diabetes mellitus[19].
Intensive researches have also suggested the role of Interleukin-1 (IL-1) in auto inflammatory process leading to destruction of beta cells in the islets of pancreas[20].
Pradhan et al. carried out a nested case control study among 27 628 women where he evaluated the levels of inflammatory biomarkers such as IL-6 and C-reactive proteins. The results highlighted the elevated levels of inflammatory biomarkers, thus confirming the possible role of inflammation in the onset of diabetes[21].
Several studies on V. pedata confirmed its anti-inflammatory studies. Rajmohanan et al. investigated the anti-inflammatory properties of C. pedata leaf extract in both in vivo and in vitro models[22]. Alcoholic extract of C. pedata (250 mg/kg and 500 mg/kg body weight (b.w.), orally (p.o.)) were efficient in reducing the formation of granuloma thus inhibiting the inflammation. The study also explored the mechanism of action of the extract which demonstrated the inhibitory action of cyclooxygenase in lipopolysaccharide macrophages and also extensively prevent the action of proteinase.
Furthermore, Rajendran et al. in his study confirmed the anti-inflammatory properties of aqueous and alcoholic extracts of V. pedata [17].
The significant correlation between inflammation and diabetes mellitus discussed above became the hallmark of our research. Since V. pedata, had been a traditional anti-inflammatory agent, we thought to investigate its anti-diabetic properties, which have not been conducted till date. The main objective of the proposed work was to screen the ethanolic and aqueous extract of the plant V. pedata and to evaluate of its anti-diabetic activity in alloxan induced rats.
Materials and Methods
Materials:
Alloxan (Spectrachem Pvt. Ltd. Company), sodium Tripolyphosphate (TPP) was procured from Sigma- Aldrich (Missouri (MO), United States of America (USA)) while water of High Performance Liquid Chromatography (HPLC) grade and acetic acid was obtained from Spectrochem (Mumbai, India), albumin estimation kit, cholesterol estimation kit, urea estimation kit, High-Density Lipoprotein Cholesterol (HDL-C) estimation kit were purchased from Span Diagnostics, glibenclamide (Darwin Formulations). Windows Excel (version 2003; Redmond, Washington), Statistical Package for Social Sciences (SPSS)/10.0 (SPSS, USA), SigmaPlot (version 6.0; Zendal Scientific, USA) softwares were used for data analysis.
Plant material:
The whole plant of V. pedata was collected from Howrah district (West Bengal) and authenticated by Botanist Dr. Jagadeesh Singh, Principal, East Point College of Pharmacy, Bangalore, Karnataka. The voucher specimen (No: Vitis pedata/02/EPCP/2013-2014) was preserved in the Department of Pharmacology laboratory of East Point College of Pharmacy for future reference. The plant was processed, powdered coarsely and coarse plant materials were used for extraction.
Preparation of plant material:
The collected plant material was cut into small pieces and shade dried. The dried material was then powdered by a mechanical grinder. The resulting powder was then processed for extraction with ethanol and water. The Ethanolic Extract of V. pedata (EEVP) and the Aqueous Extract of V. pedata (AEVP) were concentrated under reduced pressure and stored in desiccator.
Preliminary phytochemical analysis of EEVP and AEVP[19]:
Phytochemical analysis was carried out by using the standard procedures. Alkaloids, carbohydrates, flavonoids, glycosides, phytosterols/terpenes, proteins, tannins, saponins and lipids were qualitatively analyzed.
Alkaloids: EEVP and AEVP were separately dissolved in dilute Sulphuric acid (H2SO4) and filtered. The filtrate was treated with Dragendorff’s, Hager’s, Mayer’s and Wagner’s reagent separately. Appearance of reddish brown, yellow, orange brown and cream coloured precipitates in response to the above reagents respectively indicate the presence of alkaloids.
Carbohydrates: EEVP and AEVP were separately treated with Benedict’s, Fehling’s, Molisch’s and Barfoed’s reagents under suitable conditions. Appearance of reddish brown, purple ring at junction and brick red colour in response to the above reagents respectively indicates the presence of carbohydrates.
Flavonoids: EEVP and AEVP were separately treated with few ml of alcohol and were heated with magnesium ribbon and concentrated Hydrochloric acid (HCl) under cooling. Appearance of magenta red colour indicates the presence of flavonoids. A few ml of both the extracts were treated with Ferric chloride (FeCl3), an appearance of intense green colour was observed. The extracts were again treated with few ml of aqueous Sodium hydroxide (NaOH), appearance of yellow colour and changes to colourless with HCl indicate the presence of flavonoids. EEVP and AEVP were treated with lead acetate (10 %), formation of yellow precipitates indicates presence of flavonoids.
Glycosides: Under suitable conditions, small quantity of the EEVP and AEVP were subjected to Baljet’s, Borntrager’s, Keller-Killiani, Legal and Modified Borntrager’s test respectively. Appearance of yellow-orange, pink-violet brown, lower layer reddish brown and upper layer bluish green, pinkred and rose pink-cherry red colour in response to the above tests respectively indicates the presence of glycosides.
Phytosterol/Terpenes: The EEVP and AEVP were treated with Lieberman-Burchard, Salkowski’s and Zak’s tests respectively under suitable condition. Appearance of brown ring at junctions and upper layer turns green, lower layer turns red-yellow and purple-olive green colour in response to the above tests respectively indicate the presence of phytosterols/terpenes.
Proteins: The EEVP and AEVP were respectively subjected to Biuret, Million’s, Xanthoproteic and Ninhydrin tests. Appearance of blue colour, yellow stain, yellow precipitate and blue colour, in response to the above reactions respectively indicates the presence of proteins.
Tannins: Small quantity of EEVP and AEVP were dissolved in water and to that FeCl3 (5 %) or gelatine solution (1 %) or lead acetate solution (10 %) was added. Appearance of dark blue colour with FeCl3 or precipitation with other reagent indicates the presence of tannins and phenols.
Saponins: Small quantity of EEVP and AEVP were mixed with water in a test tube and shaken well for 15 min. Foam was observed, it indicates the presence of saponins.
Lipids: Few drops of 0.5 N alcoholic NaOH was added to small quantity of EEVP and AEVP respectively with a few drops of phenolphthalein. The mixture is heated on water bath for 1-2 h, formation of soap or partial neutralisation of alkali was observed, it indicates the presence of lipids.
Experimental animals:
Albino Wistar rats weighing 150-200 g were procured from Ganesh animal supplier, Vijayanagar, Bangalore. They were maintained in the animal house of East Point College of Pharmacy for experimental purpose. Animals were kept under controlled conditions of temperature at 27°±2° and 12 h light-dark cycles for 1 w. They were housed in polypropylene cages with paddy husk as bedding. The animals were fed with commercially available rat pellet diet. Water was allowed ad libitum under strict hygienic conditions. All the studies conducted were approved by the Institutional Animal Ethical Committee (IAEC) of East Point College of Pharmacy, Bangalore (REF-EPCP/IAEC/02/2013- 14) according to prescribed guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Oral Glucose Tolerance Test (OGTT) in normal rats[12]:
The OGTT was performed in rats weighing 150-200 g. The selected animals were fasted for 16 h before the commencement of experiment. However, they were allowed for free access to water. These rats were categorized into four groups having six in each group. Rats of all groups were administered with glucose 2 g/kg, p.o. 30 min after drug administration. Blood samples were collected from the tail vein prior to drug administration and at 30, 90 and 150 min of glucose administration.
Group-I: Saline was supplied and serves as control.
Group-II: Animals received a dose of 5 mg/kg b.w., p.o. of standard drug (Glibenclamide).
Group-III: Animals received a dose of 400 mg/kg b.w., p.o. of AEVP.
Group-IV: Animals received a dose of 400 mg/kg b.w., p.o. of EEVP.
Experimentally induced diabetes mellitus[13]:
The Wistar albino rats 150-200 g of either sex were allowed to fast for 24 h prior to the experiment. The diabetes was induced by injection of single dose of alloxan 120 mg/kg of b.w. in 0.3 % sodium Carboxymethyl Cellulose (CMC) by intraperitoneal (i.p) route. After 1 h of alloxanisation the animals were given feed ad libitum and 5 % dextrose solution for a day to avoid early hypoglycemic phase. The blood glucose was monitored after every 24 h of alloxanisation. The diabetic condition was observed at 48 h and 72 h of alloxan injection. The diabetic rats (glucose level>300 mg/dl) were separated and used for the study. The animals were divided into five groups.
Group-I: Animals received saline and served as normal control.
Group-II: Animal received saline+Alloxan (120 mg/kg b.w.) and served as diabetic control.
Group-III: Animals received a dose of 5 mg/kg b.w. of glibenclamide (p.o)+Alloxan (120 mg/kg b.w.).
Group-IV: Animals received a dose of 400 mg/kg b.w. p.o. of EEVP+Alloxan (120 mg/kg b.w.).
Group-V: Animals received a dose of 400 mg/kg b.w., p.o. of AEVP+Alloxan (120 mg/kg b.w.).
The study was conducted for 21 d. On d 0, before the administration of the extracts, the fasting blood glucose levels were recorded. Following this, the extract along with the standard drug (Glibenclamide) were administered daily for a continuous of 21 d.
The blood glucose levels were monitored on 0, 7, 14 and 21 d of treatment period. Blood was collected from the rat tail. Blood glucose levels were measured by using the glucometer. The b.w. of the all animals in each group was noted on the 0, 7, 14 and 21 d of the experiment period. The differences in weight were calculated. At the end of the experiment, blood was collected by cardiac puncture from each rat under mild ether anaesthesia. The blood samples were used for the estimation of haemoglobin levels and remaining was allowed to clot for 30 min at room temperature and they were centrifuged at 3000 rpm for 10 min. The serum was used for the estimation of serum albumin levels[23,24], serum urea[25,26], serum total proteins[27,28], serum HDL-C[29,30], haemoglobin levels[31]. The liver was isolated and washed with saline. The weights were determined by using an electronic balance. The liver weights were expressed with respect to its b.w. i.e. g/100 g.
Ultrastructural studies[32]:
The pancreas of each animal was isolated and was cut into small pieces and preserved and fixed in 10 % formalin for 2 d. Following this, the pancreas pieces were washed in running water for 12 h followed by dehydration with isopropyl alcohol of increasing strength (70 %, 80 % and 90 %) for 12 h each. Then the final dehydration is done using absolute alcohol with about three changes for 12 h each. The clearing was done by using chloroform with two changes for 15 to 20 min each. After clearing the pancreas pieces were subjected to paraffin infiltration in automatic tissue processing unit. The pancreas pieces were washed with running water to remove formalin completely. To remove the water, alcohol of increasing strength were used since it is a dehydrating agent. Further alcohol was removed by using chloroform and chloroform was removed by paraffin infiltration.
Statistical analysis:
The values were expressed as mean±Standard Error of the Mean (SEM). The data was analysed by using one way Analysis of Variance (ANOVA) followed by Dunnett’s test using Graph pad prism software. Statistical significance was set at p≤0.05.
Results and Discussion
The phytochemical analysis revealed the presence of alkaloids, carbohydrates, steroids, flavones and flavonoids in ether extract, whereas aqueous extract exhibited presence of carbohydrates, alkaloids, phenols, flavones and flavonoids, tannins (Table 1).
| S. No | Test | Ethanol extract | Aqueous extract |
|---|---|---|---|
| 1 | Carbohydrates | ||
| Molisch’s test | + | + | |
| Fehling’s test | + | + | |
| 2 | Proteins and amino acids | ||
| Ninhydrin test | _ | _ | |
| Biuret test | _ | _ | |
| 3 | Alkaloids | ||
| Mayer’s test | + | + | |
| Wagner’s test | + | + | |
| 4 | Glycosides | ||
| Borntrager’s test | _ | _ | |
| Legal’s test | _ | _ | |
| 5 | Steroids | ||
| Lieberman-Burchard test | + | _ | |
| Salkowski’s test | + | _ | |
| 6 | Triterpenoids | ||
| Tin+thionyl chloride | + | _ | |
| 7 | Phenolics and tannins | ||
| Ferric chloride test | _ | + | |
| Gelatine test | _ | + | |
| Lead acetate test | + | + | |
| Alkaline reagent test | + | + | |
| Dilute Nitric acid (HNO3) test | + | + | |
| 8 | Saponins | ||
| Foam test | + | + | |
| Haemolysis test | + | + | |
| 9 | Flavones and flavonoids | ||
| Caddy’s test | + | + | |
| Shinoda test | + | + |
Table 1: Preliminary Phytochemical Screening of Extracts.
The effect of AEVP and EEVP on OGTT was tabulated in the Table 2. AEVP of 400 mg/kg, p.o. did not show significant reduction in blood glucose levels at 0, 30, 90 min and it shows the significant effect at 150 min (p<0.01), whereas, EEVP of 400 mg/kg show a significant decrease in blood glucose levels, when administered 30 min before glucose loading. It showed a significant activity at the time intervals of 90 min and 150 min (p<0.01). Significant reduction was more at 150 min when compared with the 90 min. Glibenclamide showed its potent antidiabetic activity in normal rats, it bring backs the elevated blood glucose levels to normal levels compared to normal control group at 90 min (p<0.001).
| Groups | Treatment | Blood glucose levels (mg/dl) and time in min | |||
|---|---|---|---|---|---|
| 0th min | 30th min | 90th min | 150th min | ||
| Group-I | Saline | 87.33±2.81 | 132.70±1.11 | 111.50±1.40 | 96.67±3.13 |
| Group-II | Glibenclamide (5 mg/kg) | 81.83±1.79 ns | 102.00±2.88*** | 84.83±2.38*** | 60.00±1.71*** |
| Group-III | AEVP (400 mg/kg) | 93.33±2.459 ns | 109.50±2.952 ns | 87.33±2.996 ns | 74.33±4.161** |
| Group-IV | EEVP (400 mg/kg) | 93.00±4.844 ns | 115.8±4.347* | 102.7±4.072*** | 77.67±3.981*** |
Note: Values are represented as mean±SEM (n=6), one way ANOVA followed by Dunnett’s test. Where, ***p<0.001; **p<0.01; *p<0.05 and ns: Not significant
Table 2: Effect of V. Pedata Plant Extract on Blood Glucose Levels on Ogtt in Normal Rats.
The study of 21 d was done in alloxan induced diabetic rats with plant extracts of V. pedata and the results of blood glucose levels are tabulated in the Table 3. On d 0, there was no much variation in the blood glucose levels within the group. Immediately after the administration of alloxan, the diabetes control rates (Group II) highlighted a substantial rise in the blood glucose level from (320.6±17.39 mg/dl) at d 0 to (371.5±23.43 mg/dl) and (395.23±23.85 mg/dl) on d 7 and d 21 respectively. In case of the diabetic rats treated with AEVP (400 mg/kg) (Group V), a significant decrease of the blood glucose levels were observed from 331.32±20.04 to 106.89±9.28 (p<0.001) from d 0 to d 21. On the other hand, the blood glucose levels in the Group IV animals receiving EEVP of 400 mg/ kg, gradually decreased from 316.84±24.47 on d 0 to 178.78±13.58 (p<0.001) on d 21. Being the standard drug glibenclamide showed its potency and reduced the blood glucose levels of diabetic rats to the level significantly (332.5 3±20.38 to 108.23±6.83) at d 21.
| Groups | Treatment | Blood glucose levels (mg/dl) | |||
|---|---|---|---|---|---|
| 0 d | 7 d | 14 d | 21 d | ||
| Group-I | Saline | 80.10±5.08 | 83.15±5.27*** | 79.36±3.88** | 83.37±3.67*** |
| Group-II | Saline+alloxan (120 mg/kg) | 320.6±17.39 | 371.5±23.43 | 372.6±22.49 | 395.23±23.85 |
| Group-III | Glibenclamide (5 mg/kg)+alloxan (120 mg/kg) | 332.5 3±20.38 | 257.73±11.62*** | 181.34±20.20*** | 108.23±6.83*** |
| Group-IV | EEVP (400 mg/kg)+alloxan (120 mg/kg) | 316.84±24.47 | 292.56±11.40** | 249.77±13.04*** | 178.78±13.58*** |
| Group-V | AEVP (400 mg/kg)+alloxan (120 mg/kg) | 331.32±20.04 | 282..83±10.89*** | 176.86±15.25*** | 106.89±9.28*** |
Note: Values are represented as mean±SEM (n=6) one way ANOVA followed by Dunnett’s test. Where, ***p<0.001 and **p<0.01. All values are compared with diabetic control
Table 3: Effect of V. Pedata Plant Extracts on Blood Glucose Levels in Alloxan Induced Diabetic Rats.
There was a dynamic modification seen in the b.w. of animals after the treatment with the plant extract. The decreased b.w. of the animals was seen to regain when compared with the diabetic control animals after treatment for 21 d. The b.w of normal control group was significantly increased as compared to initial b.w. The changes in the b.w. of animals during 0, 7, 14 and 21 d were tabulated in the Table 4. Rats treated with alloxan showed a decrease in the liver weight of untreated diabetic rats, whereas in treated rats there was a significant restoration of wet liver weight which was near to the normal levels. The values of the wet liver weight were tabulated in the Table 4.
| Groups | Treatment | Body weight (g) | Liver Weight | ||||
|---|---|---|---|---|---|---|---|
| 0 d | 7 d | 14 d | 21 d | Wet liver weight | Weight/100 g body weight | ||
| Group-I | Saline | 180.3±5.11 | 185.4±4.49*** | 188.3±4.89*** | 191.3±4.58*** | 5.60±0.27*** | 2.98±0.14** |
| Group-II | Saline+alloxan (120 mg/kg) | 171.5±4.74 | 158.24±1.93 | 153.72±3.25 | 151.02±2.490 | 3.89±0.14 | 2.07±0.21 |
| Group-III | Glibenclamide (5 mg/kg)+alloxan (120 mg/kg) | 185.3±4.78 | 182.5±2.59*** | 187.5±2.67*** | 191.8±2.42*** | 5.38±0.16*** | 2.98±0.09** |
| Group-IV | EEVP (400 mg/kg)+alloxan (120 mg/kg) | 189.5±5.06 | 177.7±3.67* | 180.7±3.67*** | 184.7±3.99*** | 4.99±0.14* | 2.69±0.13 ns |
| Group-V | AEVP (400 mg/kg)+alloxan (120 mg/kg) | 183.4±4.99 | 181.5±3.66** | 185.6±3.53*** | 188.9±3.99*** | 5.29±0.27** | 2.79±0.16* |
Note: Values are represented as mean±SEM (n=6) one way ANOVA followed by Dunnett’s test. Where, ***p<0.001; **p<0.01; *p<0.05 and ns: Not significant. All values are compared with diabetic control
Table 4: Effect of V. Pedata Plant Extracts on Body Weight and Wet Liver Weight.
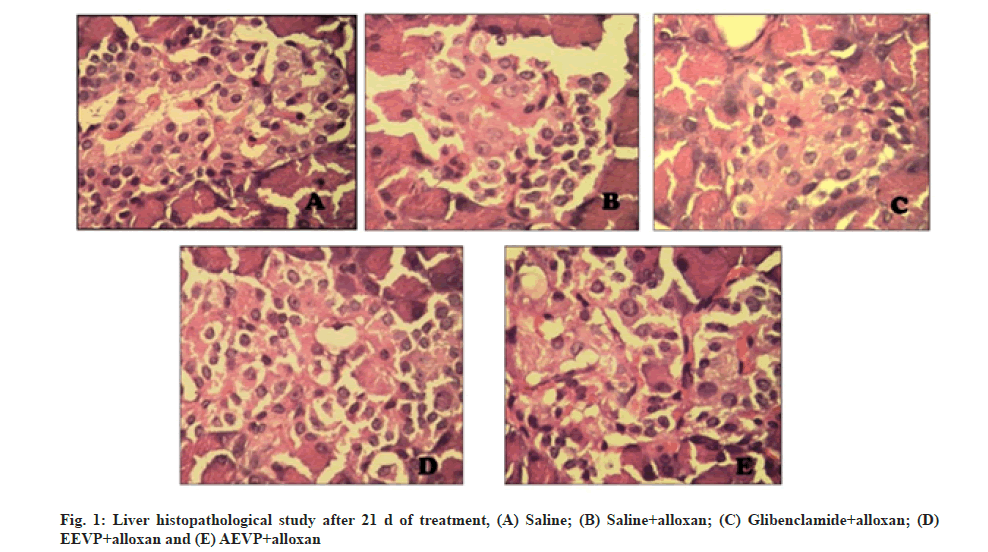
The alloxan diabetic animals showed a significant increase in the serum urea, Triglycerides (TGs), Total Cholesterol (TC), Low-Density Lipoprotein Cholesterol (LDL-C) and Very Low-Density Lipoprotein Cholesterol (VLDL-C) levels whereas a suppression in serum albumin, serum protein, hemoglobin and HDL-C levels. However after the treatment with the extracts the serum albumin, serum protein, hemoglobin and HDL-C levels were increased and serum urea, TGs, TC, LDL-C and VLDL-C levels were decreased and almost similar to the normal group, which has been depicted from Table 5-Table 9. The histopathological studies of the tissues from each group have been illustrated in fig. 1.
| Groups | Treatment | Serum albumin levels (g/dl) |
|---|---|---|
| Group-I | Saline | 3.56±0.09*** |
| Group-II | Saline+alloxan (120 mg/kg) | 1.64±0.11 |
| Group-III | Glibenclamide (5 mg/kg)+alloxan (120 mg/kg) | 3.16 ± 0.11*** |
| Group-IV | EEVP (400 mg/kg)+alloxan (120 mg/kg) | 2.46 ± 0.10** |
| Group- V | AEVP (400 mg/kg)+alloxan (120 mg/kg) | 3.07 ± 0.22*** |
Note: Values are mean±SEM (n=6) one way ANOVA followed by Dunnett’s test. Where, ***p<0.001 and **p<0.01. All values are compared with diabetic control
Table 5: Effect of V. Pedata Plant Extracts on Serum Albumin Levels in Alloxan Induced Diabetic Rats.Table 5: Effect of V. Pedata Plant Extracts on Serum Albumin Levels in Alloxan Induced Diabetic Rats.
| Groups | Treatment | Serum urea levels (mg/dl) |
|---|---|---|
| Group-I | Saline | 25.16±0.76*** |
| Group-II | Saline+alloxan (120 mg/kg) | 42.17±1.22 |
| Group-III | Glibenclamide (5 mg/kg)+alloxan (120 mg/kg) | 28.47±0.90*** |
| Group-IV | EEVP (400 mg/kg)+alloxan (120 mg/kg) | 37.25±0.66* |
| Group- V | AEVP (400 mg/kg)+alloxan (120 mg/kg) | 33.98±1.96*** |
Note: Values are mean±SEM (n=6) one way ANOVA followed by Dunnett’s test. Where, ***p<0.001 and *p<0.05. All values are compared with diabetic control
Table 6: Effect of V. Pedata Plant Extracts on Serum Urea Levels in Alloxan Induced Diabetic Rats.
| Groups | Treatment | Serum total protein levels (mg/dl) |
|---|---|---|
| Group-I | Saline | 8.65±0.31*** |
| Group-II | Saline+alloxan (120 mg/kg) | 5.37±0.32 |
| Group-III | Glibenclamide (5 mg/kg)+alloxan (120 mg/kg) | 8.28±0.25*** |
| Group-IV | EEVP (400 mg/kg)+alloxan (120 mg/kg) | 6.99±0.21** |
| Group- V | AEVP (400 mg/kg)+alloxan (120 mg/kg) | 7.05±0.25** |
Note: Values are mean±SEM (n=6) one way ANOVA followed by Dunnett’s test. Where, ***p<0.001 and **p<0.01. All values are compared with diabetic control
Table 7: Effect of V. Pedata Plant Extract on Serum Total Protein Levels in Alloxan Induced Diabetic Rats.
| Groups | Treatment | Haemoglobin (mg/dl) |
|---|---|---|
| Group-I | Saline | 12.07±0.55*** |
| Group-II | Saline+alloxan (120 mg/kg) | 8.98±0.49 |
| Group-III | Glibenclamide (5 mg/kg)+alloxan (120 mg/kg) | 12.47±0.64*** |
| Group-IV | EEVP (400 mg/kg)+alloxan (120 mg/kg) | 11.09±0.44* |
| Group- V | AEVP (400 mg/kg)+alloxan (120 mg/kg) | 12.34±0.42*** |
Note: Values are mean±SEM (n=6) one way ANOVA followed by Dunnett’s test. Where, ***p<0.001 and *p<0.05 and ns: Not significant. All values are compared with diabetic control
Table 8: Effect of V. Pedata Plant Extracts on Haemoglobin Levels in Alloxan Induced Diabetic Rats.
| Groups | Treatment | Serum lipid profile (mg/dl) | ||||
|---|---|---|---|---|---|---|
| TC | TG | HDL-C | LDL-C | VLDL-C | ||
| Group-I | Saline | 62.94±1.08*** | 74.25±4.69*** | 23.46±0.42*** | 24.66±0.90*** | 14.86±0.92*** |
| Group-II | Saline+alloxan (120 mg/kg) | 120.5±3.15 | 139.86±10.03 | 15.78±0.72 | 76.65±3.28 | 27.96±2.00 |
| Group-III | Glibenclamide (5 mg/kg)+alloxan (120 mg/kg) | 74.86±0.81*** | 75.28±4.91*** | 22.23±0.82*** | 37.53±1.31*** | 15.05±0.98*** |
| Group-IV | EEVP (400 mg/kg)+alloxan (120 mg/kg) | 96.76±2.48*** | 87.47±6.51*** | 18.16±0.27* | 61.54±2.23*** | 17.19±1.42*** |
| Group- V | AEVP (400 mg/kg)+alloxan (120 mg/kg) | 81.24±2.71*** | 85.25±2.04*** | 19.55±0.47*** | 44.56±3.00*** | 17.03±0.41*** |
Note: Values are represented as mean±SEM (n=6) one way ANOVA followed by Dunnett’s test. Where, ***p<0.001 and *p<0.05. All values are compared with diabetic control
Table 9: Effect of V. Pedata Plant Extracts on Serum Lipid Profile of Alloxan Induced Diabetic Rats.
The rising trends of diabetes mellitus associated with its complications have manifested it to an epidemic level especially in the developing countries. Bringing in modifications in the lifestyle pattern such as weight loss, cutting out sedentary regime and adopting physical exercise with staple diet are considered as the first line treatment in controlling the diabetes mellitus. However, combatting the complications associated with it, does require support of medications[2]. Apart from the standard treatment with synthetic drugs, the medical team around the world are focusing on the therapeutic impact of phytochemicals owing to its improved patient acceptability and reduced systemic toxicity[33]. Right from the birth of humankind, medicinal plants have always been a key resource to alleviate several diseases and that tradition is religiously followed till present era. In fact, the modern pharmacopoeia also incorporates 25 % of plant-derived drugs[34]. The demand of plant extracts are increasing in the modern society for the management of diabetes mellitus and its complications[4].
In the present study, the phytochemical studies revealed the presence of alkaloids, carbohydrates, steroids, flavones and flavonoids in ether extract and the presence of carbohydrates, alkaloids, flavones and flavonoids, phenols and tannins in the aqueous extract. Several studies have highlighted the antihyperglycemic properties of some of these phytochemicals such as flavones, alkaloids, tannins etc. For example, the antihyperglycemic properties of flavones and flavonoids are accredited to its capability to modulate the cell signaling process and also showcasing the anti-oxidant potency. In another study, Sharma et al.[35] have demonstrated the possible antidiabetic mechanism of alkaloids through the significant improvement in the glucokinase, Glucose Transporter type 4 (GLUT4) and Peroxisome Proliferator-Activated Receptor gamma (PPAR-γ) activities. The study also reported the decrease in TC and TGs.
One of the well-established facts in controlling the post-prandial hyperglycemia is to arrest the absorption of glucose which is mainly achieved by inhibiting α-glucosidase enzyme. Kunyanga et al. in his work reported that tannins, which are polyphenolic molecules, exhibit the antidiabetic properties possibly by its inhibitory effect of α-amylase and α-glucosidase enzymes[36,37]. Although we can find the presence of alkaloids and flavonoids in EEVP, we hypothesized AEVP to be a better candidate having anti-diabetic property since it contains alkaloids, flavonoids, as well as phenols and tannins. This fact was evidenced from the results of OGTT in normal rats and experimentally induced in alloxan induced diabetic rats.
The in vivo study was initiated with an OGTT, mainly to evaluate the glucose homeostasis in normal and identify the pre-diabetic conditions if any. The animals having normal glucose homeostasis were selected for further studies. Alloxan, a pyrimidine derivative was used for diabetes induction of insulin by partially damaging the beta (β)-cells in pancreas, thereby compromising the production of sufficient insulin which further increases the blood glucose levels[13]. A continuous treatment of the diabetic rats with our plant extracts EEVP (400 mg/kg), AEVP (400 mg/kg) were able to markedly reduce the blood glucose level as compared with that of the untreated rats. The probable reason for the hypoglycemic property of V. pedata, may be linked to the stimulation and restoration of the surviving β-cells to release insulin. Additionally, there was a decrease in the b.w. of the animals due to the induction of alloxan was mainly due to the wasting of the tissue protein[38].
Diabetic rats which received the EEVP and AEVP showcased an improved result in comparison to the normal diabetic control. The probable reasons behind this could be the protective effect of plants extracts in controlling the muscle wasting, thus reversing the process of gluconeogenesis. The study highlighted a marked decrease in the liver weight of diabetic control animals, probably as a result of insufficient insulin release that prompted a diminution in the storage of glucose as glycogen in liver. Nevertheless, when AEVP 400 mg/kg was administered for 21 d, it improved the liver weight more efficiently than EEVP 400 mg/kg. The presence of albumin in the urine is a key indicator of diabetic induced nephropathy[39]. In the present study, a marked reduction in albumin and total protein was observed in the diabetic rats. Consequently, the treatment of the diabetic rats with AEVP (400 mg/ kg) and glibenclamide showed a significant increase in the levels of albumin and protein levels compared to the normal untreated diabetic rats. Urea, being a metabolic product of protein is mostly excreted through the kidneys. The increased levels of serum urea levels are also an indicative to diabetic induced kidney damage[40]. An increased serum urea levels was found in the diabetic rats when compared with the respective control group rats. While after the treatment with extracts of V. pedata, the levels were significantly diminished. Hyperlipidaemia is triggered as a result of diabetes, mainly due to the increased action of lipase in breaking down of TGs into fatty acids which are then easily available in the circulation. Following to this the excess fatty acids are converted into phospholipids and cholesterol in the liver, which is then released as lipoproteins in the blood circulation[41]. Diabetic rats treated with the AEVP (400 mg/kg) and glibenclamide has shown a significant decrease in the levels of TG, TC, LDL-C and VLDL-C, where as it increases the levels of HDL-C when compared to the normal diabetic control rats. In EEVP treated rats HDL-C levels is less significant. The histological evidence showing the authenticated injury caused by alloxan and the protection offered by AEVP (400 mg/kg) and EEVP (400 mg/kg) and glibenclamide in pancreatic cells are shown. The haemoglobin levels of the diabetic group of rats were found to be reduced significantly as against the normal haemoglobin levels of the normal group of rats; which is possibly due to the fact that hyperglycemia promoted hemoglobin aggregation and thus may subsequently decrease the level of hemoglobin[42].
Histopathological examination demonstrated a cell necrosis with inflammatory collections in the central zone in alloxan induced diabetic rats. Results proved that V. pedata has the capacity to increase islet cell mass and restore the hepatic tissue architecture. Maximum homeostasis was observed in AEVP treated group which is similar to other reports.
The present study indicated that administration of AEVP at doses of 400 mg/kg and EEVP at a dose of 400 mg/kg, produced significant antihyperglycemic activity in alloxan induced diabetic rats. The acute toxicity study indicated that the extracts are devoid of major toxic effects. Besides this the drug treated to alloxan induced diabetic rats showed a significant reduction in blood glucose levels and the other serum biomarker levels and also increases the hemoglobin levels. The reports of histopathology study concluded that there is an increased mass of β-cells in the pancreatic islets. The result showed in AEVP of 400 mg/ kg, which is more similar to glibenclamide treated group was used as reference standard. In overall we observed significant activity may be due to presence of active constituents present in leaves extract of V. pedata. These observations concluded that the plant extracts of the plant V. pedata possess antidiabetic as well as hypolipidemic property. Further, the work could be extended to evaluate the effectiveness of the marker compounds for the treatment of diabetes at its cellular level to elucidate its exact mechanism for the traditional claim.
Acknowledgements:
The authors are dedicated to Chancellor, Adamas University and Principal of East Point College of Pharmacy for providing the support and necessary facilities for execution of this research work.
Conflict of interests:
The authors declare that they have no conflict of interest in the publication.
References
- World Health Organization. Newsroom/Fact sheets/Detail/Diabetes; 2022.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14(2):88-98.
- Jacob B, Narendhirakannan RT. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech 2019;9(1):1-7.
- Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J Tradit Complement Med 2018;8(3):361-76.
- Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron Physician 2016;8(1):1832-42.
- Sharmila S, Kalaichelvi K, Dhivya SM, Premamalini P, Abirami P, Jayanthi G. Pharmacognostic assesment of the endemic and vulnerable medicinal climber-Cayratiapedata (Lam.) Gagnep. var. glabra Gamble and its antibacterial activity. Pharmacognosy Res 2017;9(1):27.
- Senadheera SP, Ekanayake S, Wanigatunge C. Anti-hyperglycaemic effects of herbal porridge made of Scoparia dulcis leaf extract in diabetics-A randomized crossover clinical trial. BMC Complement Altern Med 2015;15(1):1-9.
- Anderson RA, Zhan Z, Luo R, Guo X, Guo Q, Zhou J, et al. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J Tradit Complement Med 2016;6(4):332-6.
- Karim S, Khurhsid R, Saeed-ul-Hassan S, Tariq I, Sultana M, Rashid AJ, et al. Hypoglycemic activity of Ficus racemosa bark in combination with oral hypoglycemic drug in diabetic human. Acta Pol Pharm 2013;70(6):1045-9.
- Kokate CK, Khandelwal KR, Pawar AP, Gohale SB. Practical Pharmacognosy. 18th ed. Pune: Nirali Prakashan; 2017. p. 137-9.
- Trias-Blasi A, Parnell JA, Watson MF. Nomenclatural notes on species of Asian Vitaceae. Taxon 2017;66(3):718-33.
- Sornalakshmi V, Tresina SP, Paulpriya K, Packia LM, Mohan VR. Oral glucose tolerance test (OGTT) in normal control and glucose induced hyperglycemic rats with Hedyotis leschenaultiana DC. Int J Toxicol Pharmacol Res 2016;8(1);59-62.
- Macdonald Ighodaro O, Mohammed Adeosun A, Adeboye Akinloye O. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 2017;53(6):365-74.
- Aswathy TR, Gayathri E, Praveen J, Achuthsankar SN, Sugunan VS. Phytoprofiling of medicinal plant Cayratia pedata by qualitative and quantitative method. J Pharmacogn Phytochem 2019;8(2):1637-42.
- Sharmila S, Kalaichelvi K, Dhivya SM. Pharmacognostical and phytochemical analysis of Cayratia pedata var. glabra-A vitaceae member. Int J Pharm Sci Res 2018;9(1):218-26.
- Nayak K, Lazar J. Antimicrobial efficacy of leaf extract of Cayratia pedata Lam. Vitaceae. Int J Chemtech Res 2014;6(14):5721-5.
- Rajendran V, Rathinambal V, Gopal V. A preliminary study on anti-inflammatory activity of Cayratia pedata leaves on Wister albino rats. Der Pharm Lett 2011;3(2):433-7.
- Selvarani K, Bai GV. Anti-arthritic activity of Cayratia pedata leaf extract in Freund’s adjuvant induced arthritic rats. Int J Res Pharm Sci 2014;4(2):55-9.
- Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: Current concepts and future perspectives. Eur Cardiol 2019;14(1):50-9.
- Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med 2008;14(3):222-31.
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6 and risk of developing type 2 diabetes mellitus. JAMA 2001;286(3):327-34.
- Rajmohanan, TP, Nair S. Evaluation of anti-inflammatory activity of Cayratia pedata (Lam.) Juss. leaf extract in some in vivo and in vitro experimental models. Int J Pharm Res Scholars 2015;4(3):163-75.
- Chaney AL, Marbach EP. Modified reagents for determination of urea and ammonia. Clin Chem 1962;8(2):130-2.
- Kollar A. Proteins. In: Kaplan LA, Pesce AJ, editors. Clinical chemistry: Theory, analysis and correlation. Toranto: Mosby Elsevier; 1984. p. 1268-327.
- Doumas BT, Bayse DD, Carter RJ, Peters Jr T, Schaffer R. A candidate reference method for determination of total protein in serum. I. development and validation. Clin Chem 1981;27(10):1642-50.
- Herbert K. Lipids. In: Kaplan LA, Pesce AJ, editors. Clinical chemistry: Theory, analysis and correlation. Toranto: Mosby Elsevier; 1984. p. 1182-230.
- Izzo C, Grillo F, Murador E. Improved method for determination of high-density-lipoprotein cholesterol I. Isolation of high-density lipoproteins by use of polyethylene glycol 6000. Clin Chem 1981;27(3):371-4.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499-502.
- Adjene JO, Avbunudiogba JA, Igbigbi PS. Oxidative stress induced by chronic administration of efavirenz on the intracranial visual relay centers of adult Wistar rats. Biol Med 2011;3(5):16-24.
- Patel PM, Patel NM, Goyal RK. Holistic classification of herbal antidiabetics: A review. PharmaTimes 2006;38(5):19-25.
- Salahuddin M, Jalalpure SS. Evaluation of antidiabetic activity of Cassia glauca Lam. leaf in streptozotocin induced diabetic rats. Int J Pharm Technol 2010;9(1):29-33.
- Abdul-Hamid M, Moustafa N. Protective effect of curcumin on histopathology and ultrastructure of pancreas in the alloxan treated rats for induction of diabetes. J Basic Appl Zool 2013;66(4):169-79.
- Teoh SL, Das S. Phytochemicals and their effective role in the treatment of diabetes mellitus: A short review. Phytochem Rev 2018;17(5):1111-28.
- Surya S, Salam AD, Tomy DV, Carla B, Kumar RA, Sunil C. Diabetes mellitus and medicinal plants-A review. Asian Pac J Trop Dis 2014;4(5):337-47.
- Sharma B, Salunke R, Balomajumder C, Daniel S, Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J Ethnopharmacol 2010;127(2):457-62.
- Kunyanga CN, Imungi JK, Okoth M, Momanyi C, Biesalski HK, Vadivel V. Antioxidant and antidiabetic properties of condensed tannins in acetonic extract of selected raw and processed indigenous food ingredients from Kenya. J Food Sci 2011;76(4):560-7.
- You Q, Chen F, Wang X, Jiang Y, Lin S. Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT Food Sci Technol 2012;46(1):164-8.
- Swanston-Flatt SK, Day C, Bailey CJ, Flatt PR. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia 1990;33(8):462-4.
- Al Hroob AM, Abukhalil MH, Alghonmeen RD, Mahmoud AM. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed Pharmacother 2018;106:381-9.
- Dollah MA, Parhizkar S, Izwan M. Effect of Nigella sativa on the kidney function in rats. Avicenna J Phytomed 2013;3(2):152.
- Banda M, Nyirenda J, Muzandu K, Sijumbila G, Mudenda S. Antihyperglycemic and antihyperlipidemic effects of aqueous extracts of Lannea edulis in alloxan-induced diabetic rats. Front Pharmacol 2018;9:1099.
- Ye S, Ruan P, Yong J, Shen H, Liao Z, Dong X. The impact of the HbA1c level of type 2 diabetics on the structure of haemoglobin. Sci Rep 2016;6(1):1-8.