- *Corresponding Author:
- Rongfeng Liao
Department of Ophthalmology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui Province 230022, China
E-mail: suyu123666@163.com
| This article was originally published in a special issue,“Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “403-410” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Strabismus in children is a common condition that can lead to significant visual disturbances and social stigma if not effectively managed. In this study, we explored the use of atropine sulfate for its potential to stabilize ocular alignment following corrective interventions in pediatric strabismus. We conducted a retrospective analysis of 80 pediatric patients with congenital strabismus, divided into two groups; one receiving daily atropine eye drops (n=40) and a control group (n=40) that did not receive atropine. Over 3 y, we assessed the stability of ocular alignment and the necessity for additional interventions at intervals of 1 mo, 6 mo, and annually. The atropine group demonstrated significantly better ocular alignment stability over the study period. Initial mean deviations were 25.0 prism diopters in the atropine group and 24.5 prism diopters in the control group. By the end of the study, these figures had improved to 6.0 prism diopters and 10.0 prism diopters, respectively, with a statistically significant difference (F-value=32.5, p<0.0001). Furthermore, the need for additional interventions was dramatically lower in the atropine group (17.5 %) compared to the control group (50 %), with statistical significance (χ2=13.44, p=0.0002). Adverse effects such as pupillary dilation, photophobia, blurry vision and allergic reactions were slightly more prevalent in the atropine group but did not reach statistical significance. Our findings support the use of atropine as a beneficial adjunct in the management of pediatric strabismus, promoting improved stability of ocular alignment with fewer additional interventions. Atropine could thus be considered an effective treatment option in the comprehensive care of children with strabismus.
Keywords
Pediatric strabismus, atropine, ocular alignment, stability, vision
Strabismus commonly referred to as eye misalignment, affects approximately 2 %-4 % of children worldwide. This condition disrupts the ability of the eyes to maintain parallel alignment, leading to significant visual impairments such as amblyopia (lazy eye), compromised binocular vision, and issues with depth perception[1]. Effective management of pediatric strabismus is essential, not only for the development of visual function but also for enhancing psychosocial interactions, as noticeable eye misalignment can negatively affect a child’s self-esteem and social interactions[2,3].
Traditionally, the surgical correction of strabismus focuses on realigning the eyes to improve both visual function and cosmetic appearance[4]. Although the efficacy of these surgical interventions at various points has been extensively documented, the debate over the optimal timing for such surgeries, especially in young children, remains unresolved[5,6]. It is hypothesized that early surgical intervention may be more beneficial in younger individuals due to the greater neural plasticity in this age group, potentially leading to better sensory motor integration and a higher likelihood of achieving stable binocular vision[7].
However, the long-term stability of ocular alignment following these early surgical interventions is still not well understood. Stability is critical for the lasting success of the surgery, as recurrent misalignments may necessitate additional surgical procedures and could result in a progressive loss of visual function. Clinical studies have shown mixed results, with some reporting stable outcomes following early surgery, while others observe frequent reoccurrences of misalignment[8-10].
In addition, the existing literature often consists of retrospective studies or those with limited sample sizes that do not adequately control for confounding factors such as the type of strabismus, the presence of syndromic associations, and baseline visual acuity[8-11]. These limitations highlight the need for well-designed prospective studies to address these critical issues. Therefore, our study aims to address these gaps by exploring the impact of atropine treatment on the stability of ocular alignment over time in a pediatric cohort.
Materials and Methods
Study design:
This was a retrospective study conducted to evaluate the effectiveness of atropine on ocular alignment stability in children diagnosed with strabismus. The study spanned 3 y, from January 2020 to December 2022. The protocol received approval from the Institutional Review Board of our affiliated hospital, and informed consent was obtained from the guardians of all participating children.
General information:
Children aged between 6 mo and 6 y, diagnosed with congenital strabismus were included in this study. Inclusion criteria were a confirmed diagnosis of either esotropia or exotropia. Exclusion criteria exclude previous ocular surgeries, presence of any neurodevelopmental disorders, or systemic conditions that might affect ocular health. Based on their treatment plans, children were categorized into two groups; those who received atropine treatment and those who did not, with 40 children in each group.
Treatment protocol:
The children in the atropine group received atropine eye drops, administered according to a standardized protocol that involved daily application. The specific concentration and dosing schedule were based on the severity of strabismus as determined at the initial assessment. The children in the control group received standard care, which included routine ocular exercises and regular monitoring without the administration of atropine. This approach is typical in managing pediatric strabismus where pharmacological intervention is not utilized.
Follow-up care:
Follow-up for all participants included regular checkups scheduled at 1 mo, 6 mo and annually up to 3 y. During these visits, ocular alignment was assessed using standard clinical methods, and any adverse effects of the treatment were recorded.
Outcome measures:
The primary outcome measure was the stability of ocular alignment, quantitatively assessed as the angle of deviation in Prism Diopters (PD) at each followup visit. Stability was defined as a deviation of <10 PD from the baseline measurement. Secondary outcomes included the incidence of adverse reactions to atropine and the need for additional interventions.
Sample size calculation:
The sample size calculation was based on an expected 30 % improvement in alignment stability in the group treated with atropine[12]. Using a twosided significance level (alpha) of 0.05 and a desired power of 80 % (beta=0.20), the required sample size was determined using the formula for comparing two proportions in independent groups.
Using a two-sided significance level (alpha) of 0.05 and a power of 80 % (beta=0.20) to detect a minimum significant difference, the required sample size was determined using the formula for comparing two proportions in independent groups
n=2(p1-p2)2(Zα/2+Zβ) 2(p1(1-p1)+p2(1-p2))
Where, Zα/2 is the critical value of the normal distribution at α/2α/2 (1.96 for α=0.05); Zβ is the critical value of the normal distribution at β (0.84 for a power of 80 %) and p1 and p2 are the expected proportions in the early and delayed surgery groups, respectively.
Assuming p1=0.65 (65 % stability in the early surgery group) and p2=0.35 (35 % stability in the delayed surgery group), the calculation yielded an estimated sample size of approximately 37 participants per group. To account for potential dropouts and loss to follow-up, the sample size was rounded up to 40 participants per group, yielding a total of 80 participants for the study.
Statistical analysis:
Statistical analysis was conducted using Statistical Package for the Social Sciences (SPSS) software, version 26.0. Baseline characteristics of the two groups were compared using the Chi-square (χ2) test for categorical variables and the student’s t-test for continuous variables. The primary and secondary outcomes were analyzed using the Chi-square test and repeated measures Analysis of Variance (ANOVA), respectively. A p<0.05 was considered statistically significant.
Results and Discussion
The demographic analysis (Table 1) showed no significant differences between groups regarding age, gender, type of strabismus, pre-operative deviation, and previous ocular conditions. The age and preoperative deviation were closely matched between the two groups, suggesting a well-balanced baseline for comparing surgical outcomes.
| Demographic | Atropine group (n=40) | Control group (n=40) | p |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 3.2 (1.5) | 3.5 (1.4) | 0.42 |
| Gender | |||
| Male | 22 (55 %) | 20 (50 %) | 0.73 |
| Female | 18 (45 %) | 20 (50 %) | |
| Type of strabismus | |||
| Esotropia | 25 (62.5 %) | 24 (60 %) | 0.81 |
| Exotropia | 15 (37.5 %) | 16 (40 %) | |
| Pre-operative deviation | |||
| Mean (SD) in PD | 25.0 (10.2) | 24.5 (9.8) | 0.88 |
| Previous ocular conditions | |||
| Yes | 5 (12.5 %) | 4 (10 %) | 0.74 |
| No | 35 (87.5 %) | 36 (90 %) | |
Table 1: Demographic Variables Assessed Across the Sample Size
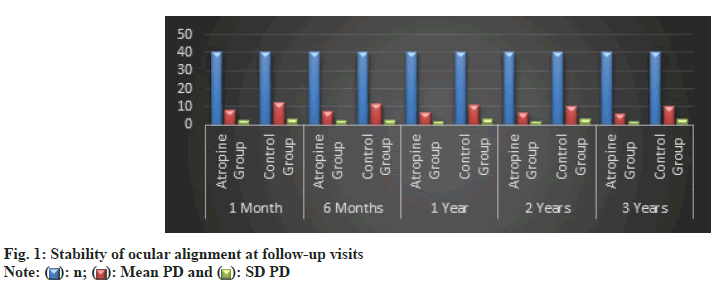
The follow-up data revealed a consistently better outcome in the atropine group regarding the stability of ocular alignment (fig. 1). At each time point from 1 mo to 3 y, the atropine group exhibited significantly smaller mean deviations in PD compared to the delayed surgery group. This trend suggested that atropine led to more stable and enduring alignment corrections.
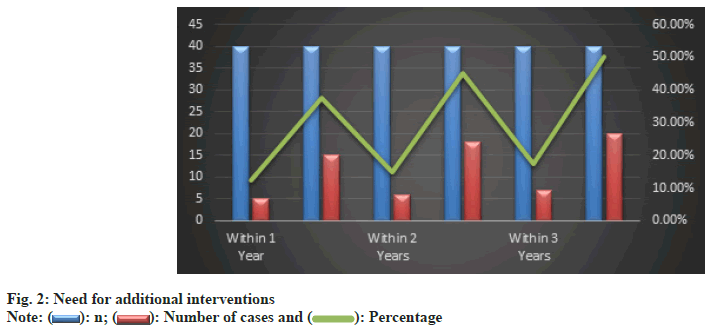
Furthermore, the need for additional interventions was significantly lower in the atropine group (fig. 2). By the end of the 3rd y, only 17.5 % of the atropine group required further interventions compared to 50 % in the control group. This substantial difference highlighted the effectiveness of atropine in achieving satisfactory alignment with fewer additional interventions.
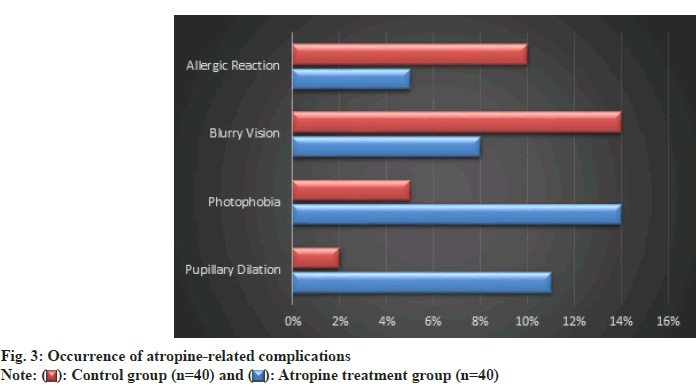
Among the 40 patients treated with atropine, pupillary dilation was observed in 10 %, photophobia in 12.5 %, blurry vision in 7.5 %, and allergic reactions in 5 % (fig. 3). In the control group, these percentages were lower for pupillary dilation and photophobia, at 2.5 % and 5 % respectively, but higher for blurry vision and allergic reactions, at 15 % and 10 % respectively. None of the comparisons reached statistical significance, as indicated by p>0.05 for all complication types.
By the 3 y mark, the mean deviation in PD for the atropine treatment group had reduced from an initial 25.0 PD to 6.0 PD, while the control group reduced from 24.5 PD to 10.0 PD (Table 2). This difference was statistically significant with an F-value of 32.5 and a p-value of 0.0001, indicating a more stable ocular alignment in the atropine group over time.
| Time point | Atropine treatment group mean deviation (SD) (PD) | Control group mean deviation (SD) (PD) | F | p |
|---|---|---|---|---|
| Baseline | 25.0 (10.2) | 24.5 (9.8) | - | - |
| 1 mo | 8.0 (2.5) | 12.0 (3.0) | - | - |
| 6 mo | 7.5 (2.2) | 11.5 (2.8) | - | - |
| 1 y | 7.0 (2.1) | 11.0 (3.1) | - | - |
| 2 y | 6.5 (1.9) | 10.5 (3.2) | - | - |
| 3 y | 6.0 (1.8) | 10.0 (2.9) | 32.5 | 0.0001 |
Table 2: Stability of Ocular Alignment over Time
At different time points, a higher percentage of patients in the atropine group maintained stability compared to the control group (Table 3). Specifically, by the 3rd y, 100 % of the atropine group was stable, compared to 45 % in the control group. Each time point from 1 mo onward showed statistically significant differences, with p-values decreasing over time, reflecting increasing differences between the two groups.
| Time point | Atropine treatment group stable (%) | Control group stable (%) | χ2 | p |
|---|---|---|---|---|
| 1 mo | 35 (87.5 %) | 25 (62.5 %) | 8.16 | 0.004 |
| 6 mo | 36 (90 %) | 24 (60 %) | 10.24 | 0.001 |
| 1 y | 38 (95 %) | 22 (55 %) | 14.88 | <0.001 |
| 2 y | 39 (97.5 %) | 20 (50 %) | 22.36 | <0.001 |
| 3 y | 40 (100 %) | 18 (45 %) | 30.25 | <0.001 |
Table 3: Proportion of Patients Achieving Stability of Ocular Alignment
As elucidated through Table 4, fewer patients in the atropine group required additional interventions; 30 patients required no additional intervention compared to 15 in the control group. The need for one additional intervention was significantly higher in the control group (20 vs. 7 in the treatment group). The difference in patients requiring two additional interventions was not statistically significant.
| Intervention type | Atropine treatment group (n) | Control group (n) | χ2 | p |
|---|---|---|---|---|
| None | 30 | 15 | 13.44 | 0.0002 |
| One additional | 7 | 20 | 12.58 | 0.0004 |
| Two additional | 3 | 5 | 1.22 | 0.269 |
Table 4: Frequency and Types of Additional Interventions Over 3 Y
Our data indicates that the atropine group consistently maintained better ocular alignment with significantly smaller mean deviations in PD at each followup point compared to the delayed surgery group. Specifically, the mean deviation in the atropine treatment group decreased from an initial 25.0 PD to 6.0 PD by the end of the 3rd y, demonstrating a statistically significant improvement in stability of ocular alignment over time. Furthermore, the need for additional interventions was markedly lower in the atropine group. By the end of the study period, only 17.5 % of patients in the atropine group required further interventions compared to 50 % in the control group. This substantial reduction in the necessity for additional treatments underscores the potential of atropine not only to correct but also to sustain alignment with fewer medical or surgical interventions. These findings hold considerable implications for the future management of pediatric strabismus. The ability of atropine to maintain stable ocular alignment with fewer interventions could make it a preferable first-line treatment or adjunct in managing specific types of strabismus, potentially reducing the need for surgical interventions which are typically more invasive and carry greater risks.
The Oculocardiac Reflex (OCR) is defined by a decline in heart rate of >20 % from baseline or the development of arrhythmias due to ocular pressure or manipulation of the extraocular muscles, potentially leading to cardiac rhythm disturbances like sinus bradycardia, atrioventricular block, and in severe cases, ventricular fibrillation or asystole[11,13]. Usually, OCR episodes are mild and short-lived, with cardiac rhythm normalizing quickly after the cessation of muscle traction[14]. In cases where the reflex is more pronounced, the administration of anticholinergics such as atropine (7 μg/kg) or glycopyrrolate (1 μg/ kg) may be required[15].
OCR is notably prevalent during pediatric strabismus surgeries, observed in up to 86 % of such procedures[11,16]. The likelihood of experiencing OCR is heightened by factors such as elevated carbondioxide levels, reduced oxygen saturation[12,14], light anesthesia levels (bispectral index >50)[17], and the strength and duration of the ocular stimulus[13]. The reflex is more acute with sudden . gradual traction on the extraocular muscles and tends to decrease with repeated stimulation, although the medial rectus muscle shows significant resistance to this fatigue effect[14].
The type of anesthesia used can also affect the occurrence of OCR. For instance, the utilization of sevoflurane in general anesthesia is linked with a lower rate of OCR compared to halothane[18], with no marked difference between sevoflurane and desflurane concerning OCR incidence[19]. Ketamine, when administered intraoperatively at doses of 1.0-1.5 mg/kg, has been effective in mitigating the incidence of OCR, albeit potentially increasing the risk of postoperative nausea and extending the duration of recovery[12,13]. The combined use of ophthalmic regional block and general anesthesia has significantly lowered OCR rates from 94 % to 13 % in pediatric cases[20]. Furthermore, maintaining general anesthesia with volatile agents rather than propofol has been shown to more effectively reduce OCR rates in pediatric strabismus surgeries (22 % vs. 49 %)[21]. Additionally, the intraoperative application of rapidacting opioids such as fentanyl, sufentanil[22], and remifentanil[23], or the use of dexmedetomidine[24], has been noted to influence the occurrence of OCR during these procedures.
Glycopyrrolate, another muscarinic antagonist, does not permeate the blood-brain barrier, distinguishing it from atropine. Studies have documented a decrease in OCR occurrences when atropine (5-15 μg/kg IV/intramuscular)[25-27] and glycopyrrolate (5–10 μg/kg IV/intramuscular)[28] are administered pre-operatively. Both substances are more potent in preventing OCR when delivered intravenously in higher dosages. However, their application is constrained by potential side effects, including the induction of other types of arrhythmias, which may lead to prolonged episodes and more severe hemodynamic instability than those induced by OCR[27-29].
In comparison to the findings that we observed, the study by Farsani et al.[29] focused on the immediate perioperative effects of atropine in preventing the OCR during strabismus surgery. They reported a reduced incidence of OCR with atropine in the cutting phase of surgery, highlighting atropine’s effectiveness in managing intraoperative physiological reflexes. While Farsani et al.[30] concentrated on acute intraoperative outcomes, our research extends into the long-term postoperative benefits of atropine, offering a broader perspective on its therapeutic utility in strabismus management.
Hunsley et al.[30] examined the dose-related effects of atropine and glycopyrrolate on OCR prevention, noting that neither drug completely prevented heart rate reductions, although they improved protection against OCR. This study, similar to that of Farsani et al.[29], emphasizes the immediate perioperative benefits of atropine but does not address long-term ocular alignment, which was the primary focus of our study. Their findings support the safety of atropine used in specific doses, which complements our observations of atropine’s long-term utility by confirming its immediate safety and efficacy.
Klockgether et al.[31] investigated the effect of atropine on OCR and postoperative vomiting in pediatric patients undergoing strabismus surgery. Their results showed a significant reduction in the incidence of OCR with the preoperative administration of atropine, aligning with our observations regarding atropine’s effectiveness. However, they did not find a relationship between atropine use and the reduction of postoperative vomiting, which was outside the scope of our study. Their focus on the immediate surgical setting provides valuable insights into atropine’s role in managing specific surgical complications, while our study extends the understanding of atropine’s benefits into long-term postoperative care.
Our findings further indicate a potent therapeutic role of atropine in pediatric strabismus with a manageable safety profile, despite minor complications like pupillary dilation and photophobia. Comparatively, the study by Araki et al.[32] explored the effects of 1 % atropine on choroidal structures in children with hyperopic anisometropic amblyopia, revealing significant increases in choroidal thickness in the fellow eyes but not in the amblyopic eyes. This study’s focus on anatomical changes in the choroid highlights a different aspect of atropine’s effects, unrelated to the primary outcomes of our study. However, both studies underscore atropine’s significant impact on ocular characteristics, though in different contexts and with different outcomes.
The findings from Wang et al.[33] provided insights into the efficacy of Combined Atropine and Patching Therapy (CAPT) vs. patching alone for severe amblyopia. They reported significant improvements in visual acuity with CAPT over patching alone. Our study and Wang et al.[33] support the efficacy of atropine in treating ocular disorders in children, albeit with different primary outcomes (ocular alignment in our study vs. visual acuity improvement in theirs). Both studies, however, contribute to a broader understanding of atropine’s versatility in pediatric ophthalmology.
The study by Breliant et al.[34] evaluated the effects of varying concentrations of atropine on visual acuity, pupil size, binocular vision, and accommodation. Their results, showing significant differences in pupil size but no impact on visual acuity or binocular vision functions, provide a detailed look at atropine’s pharmacodynamics. While our study and Breliant et al.[34] both report on atropine’s safety and side effects, our study suggests more clinically beneficial outcomes concerning strabismus management.
In the course of our study, several limitations were identified that could influence the interpretation of our findings regarding the use of atropine in pediatric strabismus management. First, the retrospective nature of the study inherently limits our ability to control for confounding variables that may have impacted the outcomes, such as variations in the baseline severity of strabismus, the precise timing and consistency of atropine application, and adherence to treatment protocols by patients and caregivers. Another limitation is the absence of randomization, which introduces potential selection bias. The assignment of patients to either the atropine or control group was not randomized, which might have resulted in unequal distribution of patient characteristics that could influence treatment outcomes. Additionally, while significant statistical differences were observed in ocular alignment and the need for further interventions, the clinical significance of these findings remains to be fully elucidated. For example, while a reduction in mean deviation and fewer interventions are statistically favorable, the real-world impact on the patient’s visual functionality and quality of life was not directly assessed.
Based on our assessments from the follow-up data, we recommend the consideration of atropine as a viable initial treatment option for maintaining ocular alignment in pediatric patients with strabismus. The demonstrated efficacy of atropine in stabilizing ocular alignment while reducing the frequency and necessity for additional interventions suggests that it could serve as a potential alternative to more invasive surgical procedures, particularly in cases where less severe misalignments are present. Given the generally well-tolerated nature of atropine, as indicated by the minor and statistically insignificant side effects observed, atropine can be safely integrated into treatment plans. However, it is crucial to monitor patients for any adverse effects, particularly those related to pupillary dilation, photophobia, blurry vision, and allergic reactions. Furthermore, the results obtained from this study underscore the importance of conducting further research. Future studies should aim to confirm these findings through larger scale, randomized controlled trials to fully establish the long-term efficacy and safety profile of atropine in different subtypes of strabismus. Additionally, exploring the underlying mechanisms by which atropine stabilizes ocular alignment could enhance understanding and guide the refinement of treatment protocols. As per our findings,
As per our findings, atropine treatment was found to significantly enhance and maintain ocular alignment over a 3 y period in pediatric patients with strabismus compared to a control group receiving delayed surgery. This was evidenced by consistently smaller mean deviations in PD observed in the atropine group at each follow-up interval. Additionally, the need for additional interventions was substantially lower in the atropine group, indicating its effectiveness in achieving stable alignment with fewer treatments. Side effects related to atropine were noted but were not statistically significant, suggesting an acceptable safety profile, suggesting that atropine may be a beneficial, less invasive treatment option for managing certain cases of pediatric strabismus, warranting further investigation across larger controlled trials.
Conflict of interests:
The authors declared no conflict of interests.
References
- Uchiyama M, Demura S. The role of eye movement in upright postural control. Sport Sci Health 2009;5:21-7.
- Mettler A, Chinn L, Saliba SA, McKeon PO, Hertel J. Balance training and center-of-pressure location in participants with chronic ankle instability. J Athl Train 2015;50(4):343-9.
[Crossref] [Google Scholar] [PubMed]
- Farassat N, Böhringer D, Küchlin S, Molnár FE, Schwietering A, Seger D, et al. Low-dose Atropine for Myopia Control in Children (AIM): Protocol for a randomised, controlled, double-blind, multicentre, clinical trial with two parallel arms. BMJ Open 2023;13(4):e068822.
[Crossref] [Google Scholar] [PubMed]
- Dembinski RL, Collins ME, Kraus CL. Outcomes following surgery for horizontal strabismus in children of lower socioeconomic backgrounds. Strabismus 2019;27(2):47-53.
[Crossref] [Google Scholar] [PubMed]
- Heikl KA, Fatma EA, Mona R, Yasser M. Comparative study between atropine and ketamine for prevention of oculocardiac reflex in children undergoing strabismus surgery. Med J Cairo Univ 2018;86:311-7.
- Chen X, Fu Z, Yu J, Ding H, Bai J, Chen J, et al. Prevalence of amblyopia and strabismus in Eastern China: Results from screening of preschool children aged 36-72 months. Br J Ophthalmol 2016;100(4):515-9.
[Crossref] [Google Scholar] [PubMed]
- Gunton KB, Wasserman BN, deBenedictis C. Strabismus. Prim Care 2015;42(3):393-407.
[Crossref] [Google Scholar] [PubMed]
- Li J, Ye H, Shen W, Chen Q, Lin Y, Gan X. Retrospective analysis of risk factors of postoperative nausea and vomiting in patients undergoing ambulatory strabismus surgery via general anaesthesia. Indian J Anaesth 2020;64(5):375-82.
[Crossref] [Google Scholar] [PubMed]
- Aksoy M, ?nce ?, Ah?skal?o?lu A, Kele? S, Doymu? O. Effect of intravenous preoperative vs. postoperative paracetamol on postoperative nausea and vomiting in patients undergoing strabismus surgery: A prospective randomized study. Agri 2018;30(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Jiang J, Long W, Hu Y, Zhao F, Zhao W, Zheng B, et al. Accommodation and vergence function in children using atropine combined with orthokeratology. Cont Lens Anterior Eye 2023;46(1):101704.
[Crossref] [Google Scholar] [PubMed]
- Espahbodi E, Sanatkar M, Sadrossadat H, Vafsi ME, Azarshahin M, Shoroughi M. Ketamine or atropine: Which one better prevents oculocardiac reflex during eye surgery? A prospective randomized clinical trial. Acta Med Iran 2015:158-61.
[Google Scholar] [PubMed]
- Blanc VF, Hardy JF, Milot J, Jacob JL. The oculocardiac reflex: A graphic and statistical analysis in infants and children. Can Anaesth Soc J 1983;30:360-9.
[Crossref] [Google Scholar] [PubMed]
- Min SW, Hwang JM. The incidence of asystole in patients undergoing strabismus surgery. Eye 2009;23(4):864-6.
[Crossref] [Google Scholar] [PubMed]
- van Noord BA, Zhao M. Asystole during vitrectomy secondary to increasing intraocular infusion pressure transmitted via sclerotomy infusion cannula. J Anesth Clin Res 2012;3(3):198.
- Ravishankar S, Jaichandran VV, Prasanna L. Anaesthesia for strabismus surgery. The principles and practice of ophthalmic anaesthesia. New Delhi: Jaypee Brothers Medical Publishers; 2017. p. 274-85.
- Allen LE, Sudesh S, Sandramouli S, Cooper G, McFarlane D, Willshaw HE. The association between the oculocardiac reflex and post-operative vomiting in children undergoing strabismus surgery. Eye 1998;12(2):193-6.
[Crossref] [Google Scholar] [PubMed]
- Karaman T, Demir S, Dogru S, ?ahin A, Tapar H, Karaman S, et al. The effect of anesthesia depth on the oculocardiac reflex in strabismus surgery. J Clin Monit Comput 2016;30:889-93.
[Crossref] [Google Scholar] [PubMed]
- Allison CE, de Lange JJ, Koole FD, Zuurmond WW, Ros HH, van Schagen NT. A comparison of the incidence of the oculocardiac and oculorespiratory reflexes during sevoflurane or halothane anesthesia for strabismus surgery in children. Anesth Analg 2000;90(2):306-10.
[Crossref] [Google Scholar] [PubMed]
- Oh AY, Yun MJ, Kim HJ, Kim H. Comparison of desflurane with sevoflurane for the incidence of oculocardiac reflex in children undergoing strabismus surgery. Br J Anaesth 2007;99(2):262-5.
[Crossref] [Google Scholar] [PubMed]
- Gupta N, Kumar R, Kumar S, Sehgal R, Sharma KR. A prospective randomised double blind study to evaluate the effect of peribulbar block or topical application of local anaesthesia combined with general anaesthesia on intra-operative and postoperative complications during paediatric strabismus surgery. Anaesth 2007;62(11):1110-3.
[Crossref] [Google Scholar] [PubMed]
- Tramer M, Moore A, McQuay H. Prevention of vomiting after paediatric strabismus surgery: A systematic review using the numbers-needed-to-treat method. Br J Anaesth 1995;75(5):556-61.
[Crossref] [Google Scholar] [PubMed]
- Arnold RW, Jensen PA, Kovtoun TA, Maurer SA, Schultz JA. The profound augmentation of the oculocardiac reflex by fast acting opioids. Binocul Vis Strabismus Q 2004;19(4):215-22.
[Google Scholar] [PubMed]
- Chung CJ, Lee JM, Choi SR, Lee SC, Lee JH. Effect of remifentanil on oculocardiac reflex in paediatric strabismus surgery. Acta Anaesthesiol Scand 2008;52(9):1273-7.
[Crossref] [Google Scholar] [PubMed]
- Arnold RW, Biggs RE, Beerle BJ. Intravenous dexmedetomidine augments the oculocardiac reflex. J AAPOS 2018;22(3):211-3.
[Crossref] [Google Scholar] [PubMed]
- Espahbodi E, Sanatkar M, Sadrossadat H, Vafsi ME, Azarshahin M, et al. Ketamine or atropine: which one better prevents oculocardiac reflex during eye surgery? A prospective randomized clinical trial. Acta Med Iran 2015:158-61.
[Google Scholar] [PubMed]
- Juan I, Lin M, Greenberg M, Robbins SL. Surgical and anesthetic influences of the oculocardiac reflex in adults and children during strabismus surgery. Surv Ophthalmol 2023;68(5):977-84.
[Crossref] [Google Scholar] [PubMed]
- Arnold RW. Surgical and anesthetic influences of the oculocardiac reflex in adults and children during strabismus surgery. Surv Ophthalmol 2024;69(2):295.
- Robbins SL, Greenberg M, Juan I. Surgical and anesthetic influences of the oculocardiac reflex in adults and children during strabismus surgery. Surv Ophthalmol 2024;69(2):296-7.
[Crossref] [Google Scholar] [PubMed]
- Farsani DM, Shakerinia SE. Comparing effectiveness and safety of intravenous atropine with topical tetracaine in preventing and relieving oculocardiac reflex in patients undergoing strabismus surgery: A randomized clinical trial. Adv Biomed Res 2024;13:8.
[Crossref] [Google Scholar] [PubMed]
- Hunsley JE, Bush GH, Jones CJ. A study of glycopyrrolate and atropine in the suppression of the oculocardiac reflex during strabismus surgery in children. Br J Anaesth 1982;54(4):459-64.
[Crossref] [Google Scholar] [PubMed]
- Klockgether-Radke A, Demmel C, Braun U, Mühlendyck H. Emesis and the oculocardiac reflex. Drug prophylaxis with droperidol and atropine in children undergoing strabismus surgery. Anaesthesist 1993;42(6):356-60.
[Google Scholar] [PubMed]
- Araki S, Miki A, Goto K, Fujiwara A, Yamashita T, Yoneda T, et al. Changes in choroidal thickness and structure induced by 1 % atropine instillation in children with hyperopic anisometropic amblyopia. J Pediatr Ophthalmol Strabismus 2023;60(1):39-45.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Wen W, Zhu W, Liu Y, Zou L, Tian T, et al. Effect of combined atropine and patching vs. patching alone for treatment of severe amblyopia in children aged 3 to 12 years: A randomized clinical trial. JAMA Ophthalmol 2021;139(9):990-6.
[Crossref] [Google Scholar] [PubMed]
- Breliant RE, Pang Y, Bandstra A, Kattouf V. Effect of low-dose atropine on binocular vision and accommodation in children aged 6 to 17 years. Optometry Vision Sci 2023;100(8):550-6.
[Crossref] [Google Scholar] [PubMed]