- *Corresponding Author:
- Y. Srinivasa Rao
Department of Pharmaceutics, Vignan Institute of Pharmaceutical Technology, Beside VSEZ, Duvvada, Visakhapatnam -530 049, India
E-mail: ysrvignan@gmail.com
| Date of Received | 21 June 2020 |
| Date of Revision | 20 September 2020 |
| Date of Acceptance | 24 December 2020 |
| Indian J Pharm Sci 2020;82(6):1044-1049 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Microcapsules ethylene vinyl acetate with nifedipine as the core were prepared by the method of emulsion solvent evaporation and studied. Microcapsules were free flowing discrete, spherical, types of multi nuclear matrix and monolithic. The efficiency of microencapsulation was of the order of 93-99 %. Differential calorimetry studies scanning and infrared spectroscopy indicates that there is no interaction between the coating, vinyl acetate and ethylene and the base nifedipine. The release rate of nifedipine from the microcapsules was very slow and depends on the coat: core ratio, the wall thickness and the size of the microcapsules. Release was controlled by diffusion mechanism and followed first order kinetics. A good linear relationship was observed between the wall thickness of the microcapsules and the drug release rate and the 50 % release time value. The amount of nifedipine release in microcapsule MC 1 (Size 20/35) was close to the required theoretical sustained release amount and also meets the official release rate specification of nifedipine sustained release tablets (United States Pharmacopoeia XXIV). Nifedipine soft capsules were quickly absorbed after administration. The absorption of nifedipine in ethylene vinyl acetate microcapsules was slower and lasts for up to 8-10 h. For ethylene vinyl acetate microcapsules, the serum nifedipine concentration was stable and maintained in a narrow range (28-43 ng/ml) for a longer period of time (1-10 h). Using ethylene vinyl acetate microcapsules, the average residence time increased from 3.34 h for soft capsules to 7.05 h. Compared with soft capsules (100 %), the relative bioavailability of ethylene vinyl acetate microcapsules was 91.5 %.
Keywords
The standard text[1,2] specifies the microencapsulation and application of different polymers. Ethylene vinyl acetate copolymer (EVA) turned into a copolymer of ethylene and vinyl acetate. Although EVA has properly having good film forming properties[3,4] its potential applications in microencapsulation has not been studied yet. We reported earlier[5] the preparation of EVA microcapsules by emulsion solvent evaporation method. In this work, nifedipine was microencapsulated by EVA and the effects of controlled release of the microcapsules obtained were evaluated by in vitro and in vivo methods.
Nifedipine was used to treat angina pectoris and control high blood pressure and has a short biological half-life of 2-3 h, which can be rapidly cleared and the antihypertensive effect lasts for a few hours[6]. Therefore, nifedipine needs to extend its time of action and improve patient compliance, thus requiring a sustained release (SR) product. The SR nifedipine product also prevents adverse reactions associated with vasodilators, such as hot flashes and palpitations. Nifedipine SR tablets were officially used in United States Pharmacopoeia (USP) XXIV.
Materials and Methods
Nifedipine was a sample gift from M/s Cipla Ltd. in Mumbai. Ethylene vinyl acetate (grade 1408) was purchased from M/s Polyesters Industries Ltd. of Bombay. Chloroform GR (Merck), sodium lauryl sulfate and sodium carboxymethyl cellulose (sodium CMC, 1 % w/v solution viscosity of 1500-3000 cps, 25° Loba Chemie) were purchased from commercial sources. All experiments were performed in low light to prevent nifedipine photo degradation.
Preparation of microcapsule:
EVA copolymer (0.5 g) was mixed with warm chloroform (25 ml) to form a homogeneous polymer solution. Add the nifedipine (0.8 g) of the core material to the polymer solution (10 ml) and mix well. The providing mixture was introduced in a dilute stream into 200 ml of aqueous CMC (0.5 %) sodium solution contained in a 450 ml beaker with stirring at 1000 rpm to emulsify the adjusted dispersion, use a Remi Medium Duty Stirrer (RQT 124) with a speedometer for mixing. The chloroform solvent was then removed by continuous stirring for 3 h at room temperature (28°) to produce spherical microcapsules. The microcapsules were collected by vacuum filtration and washed several times with water. The product was then air dried to obtain microcapsules. Different proportions of the core for coating the substance were used to prepare microcapsules with different thickness of the coating, namely 9:1 (MC 1), 8:2 (MC 2), 7:3 (MC3) and 6:4 (MC4).
Estimation of nifedipine:
Based on the measurement of Ultraviolet (UV) absorbance at 238 nm in a pH 6.8 phosphate buffer, the content of nifedipine in the microcapsules was estimated by UV spectrophotometry. The linearity, accuracy and accuracy of the method are verified. The method complies with Beer’s law in the concentration range of 1-10 μg/ml. When repeating the determination of the standard drug solution (n=6), the average error (accuracy) and relative standard deviation (precision) were found to be 0.6 % and 1.5 % respectively.
Characterization of microcapsules:
Use the following formula to calculate the microencapsulation efficacy, microencapsulation efficacy= (percent of estimated drug content/percent of theoretical drug content)×100. For analysis of the particle size distribution, the different batch sizes were separated by sieving on standard sieves. Weigh the amount retained on the different sieves. The wall thickness of the microcapsules was determined using the method of Luu et al[7]. Using the equation, h=r̄(1-p) d1/3[pd2+(1-p)d1], where h is the wall thickness, r̄ is the radius of the microcapsule arithmetic mean, d1 is the core material density, d2 is the coating material density and p is the drug proportion in the microcapsules.
Drug release studies:
Nifedipine release from 20/35 and 35/60 size EVAcoated microcapsules in phosphate buffer pH 6.8 containing 1 % Sodium lauryl sulfate (SLS) (900 ml) was studied using a speed test apparatus. Eight station dissolution rate (model Disso2000, M/s Lab India) with a paddle stirrer at 50 rpm and 37±0.50 as prescribed for nifedipine extended release tablets in USP XXIV[8]. A sample of microcapsules equivalent to 20 mg of nifedipine has been used in the test. The samples were drawn through a filter (0.45 μm) at different time intervals and the double beam spectrophotometer Shimadzu UV-150 was used to determine the level of nifedipine to 238 nm. Drug release experiments were performed in three times.
Differential scanning calorimetry (DSC):
Differential scanning calorimetry (DSC) was performed on nifedipine, EVA and EVA nifedipine using model DSC Sieko (Japan) 220C. Samples were sealed in aluminum pans and the DSC thermograms were recorded at a heating rate of 10°/min from 30 to 300°.
Infrared spectra:
Perkin Elmer 841 infrared spectrophotometer was used to obtain the infrared spectrum of nifedipine and EVA microcapsules containing nifedipine. The infrared spectrum was recorded by preparing a film of the preparation dispersed in Nujol.
Pharmacokinetic evaluation:
Pharmacokinetic evaluation was done on conventional soft gelatin capsules of nifedipine (10 mg) as the reference standard and EVA microcapsules, MC 1 (Size 20/35) equivalent to 20 mg of nifedipine in healthy human subjects as per a crossover randomized block design (n=4).
Four human subjects were healthy males between the age range 24-28 y (average weight 605 kg) were included in this study. All subjects were admitted to the study received oral and written explanation of the purpose of the trial and the properties of the drugs given. Each subject gave written informed consent, confirmed that participation is voluntary. They were ordered not to take drugs during the study period. Before the study started, the approval of the ethics committee was obtained. Each subject was given a single product once a month. If it is a soft capsule, was given at a dose of 10 mg of nifedipine; if it is a microcapsule EVA, it is given at a dose of 20 mg of nifedipine. After fasting overnight, the product is taken orally in the morning. Within 4 h after application of the product, no food or liquids are allowed except water.
After collecting blood samples 0 h (blank), the product under investigation were taken orally with a glass of water. Blood samples (5 ml) were collected 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, 10.0 and 12.0 h after administration. The blood samples were centrifuged at 5000 rpm and the serum was separated collected in a dry tube and all samples were stored in refrigerated conditions and subsequently measured. Serum concentrations of nifedipine were determined by Highperformance liquid chromatography (HPLC) method known[9], as described below.
Add methanol (100 μl) and acetonitrile (1 ml) to 0.5 ml of serum and stirred for 5 min with a mixer circulation. After centrifugation at 5000 rpm for 5 min, transfer 1 ml of the supernatant to a tube stopper, and then add 5 ml of a mixture of chloroform-acetone (2:1 v/v). The mixture was stirred for 15 min and then centrifuged at 5000 rpm for 5 min. A portion of the organic layer (4.5 ml) was transferred to a boiling tube and evaporated to dryness at 40° under reduced pressure. The residue was dissolved in 0.5 ml mobile phase and 20 μl of the solution was injected into an HPLC column (Shimadzu) (C-18 RP 250x4.6 mm internal diameter; particle size: 5 m).
From the time vs. serum concentration curves peak concentration (Cmax), time at which peak occurred (Tmax), area under the curve (AUC) were recorded. The elimination rate constant (Kel), biological half-life (t1/2), percent absorbed to various times and absorption rate constant (Ka) were calculated by applying known methods[10,11] to time vs. concentration data.
Results and Discussion
Nifedipine EVA microcapsules were prepared by the solvent emulsion evaporation method, wherein chloroform was the solvent of the polymer. It has been found that the microcapsules were discrete free flowing, spherical and multi-core and integral type. The size can be separated and a microcapsule more uniform size range can be easily obtained. Analysis of the size of the individual microcapsules shows that about 64 % and 23 % of the size of the meshes were in the range of -20+35 (670 μm) and -35+60 mesh ((375 μm) respectively. A log-normal size distribution of the microcapsules in all batches was prepared and it was observed.
A low coefficient of variation (CV) percent drug content (<2.79 %) indicates that the drug content in each batch of microcapsules was uniform (Table 1). The effectiveness of microencapsulation for various products was in the range of 93-99 %. The drug content in the microcapsules was found to be the same at the different sieves. As the microcapsules were spherical, the wall thickness of the microcapsules was calculated according to the method of Luu et al.[7]. It was found that the microcapsules prepared with different ratios of the coat: core had different wall thicknesses (Table 1).
| Microcapsules (size) | Nifedipine content (%) of microcapsules | Microencapsulation efficiency (%) | Wall thickness (mm) | |
|---|---|---|---|---|
| Theoretical | Practical | |||
| MC1 (20/35) | 90 | 84.21 (0.43)* | 93.56 | 27.27 |
| MC1 (35/60) | 90 | 85.42 (0.39) | 94.91 | 14.27 |
| MC2 (20/35) | 80 | 78.34 (1.15) | 97.92 | 38.14 |
| MC2 (35/60) | 80 | 76.03 (2.30) | 95.03 | 25.52 |
| MC3 (20/35) | 70 | 68.84 (1.02) | 98.34 | 55.16 |
| MC3 (35/60) | 70 | 66.48 (2.52) | 94.97 | 37.51 |
| MC4 (20/35) | 60 | 59.12 (0.43) | 98.53 | 75.89 |
| MC4 (35/60) | 60 | 58.22 (1.33) | 97.15 | 43.23 |
*Fig in parentheses are coefficient of variation (CV) values
Table 1: Drug Content, Microencapsulation Efficiency and Wall Thickness of Eva Microcapsules
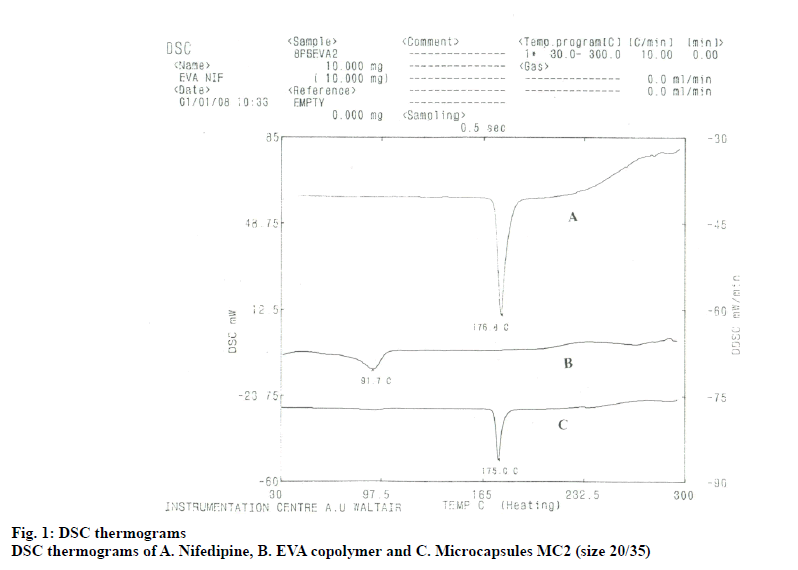
The DSC thermogram of nifedipine (fig. 1) shows a fine endothermic peak at 176.8°, corresponding its melting point. DSC thermogram of nifedipine EVA microcapsules also showed an endothermic melting peak at 175°, indicating that between the coat (EVA) and the drug core (nifedipine) there was no interaction.
The infrared spectra of nifedipine and its EVA microcapsules were the same. The main absorption infrared peaks of nifedipine were at 1121cm-1 (-C-Oester), 1380 cm-1 (-C-CH3), 1530 cm-1 (NO2), 1620 cm-1 (–C=C-aromatic) and 1689 cm-1 (C=O ester) was observed in the spectra of nifedipine and its microcapsules.
SR tablet as specified in the test release rate of nifedipine in the USP XXIV, nifedipine release microcapsule was studied in a pH 6.8 phosphate buffer containing 1 % SLS . Nifedipine slowly releases from the microcapsules and extends over a long period of time (Table 2). Release follows a first order kinetics (r>0.98) and depends on the coat: core ratio and size of the microcapsules. As more the ratio of layer, the release of nifedipine decreases.
| Micro capsule | Percent Nifedipine Released at 5 Times (h, x±SD) | T50 (h) | k1✕102 (h-1) | ||||
|---|---|---|---|---|---|---|---|
| 1.0 | 2.0 | 4.0 | 8.0 | 12.0 | |||
| Size -20/35 | |||||||
| MC1 | 13.36±0.89 | 22.59±1.52 | 41.26±0.89 | 68.74±0.69 | 90.00±2.56 | 5.0 | 17.33 |
| MC2 | 14.65±2.10 | 21.96±1.59 | 36.98±0.89 | 57.76±0.23 | 76.48±0.29 | 6.9 | 10.59 |
| MC3 | 15.68±1.06 | 21.72±1.25 | 35.25±0.09 | 51.26±0.96 | 70.24±0.89 | 7.8 | 8.98 |
| MC4 | 13.65±0.96 | 20.16±1.69 | 33.45±1.69 | 46.68±0.79 | 58.21±2.10 | 8.5 | 6.90 |
| Size -35/60 | |||||||
| MC1 | 21.12±1.25 | 30.27±0.59 | 56.26±1.23 | 80.29±0.29 | 90.09±1.21 | 3.2 | 20.00 |
| MC2 | 21.68±2.06 | 29.45±1.26 | 44.68±0.56 | 63.74±1.23 | 83.29±0.23 | 5.3 | 13.61 |
| MC3 | 19.68±0.67 | 27.68±1.24 | 44.95±1.29 | 54.36±0.65 | 77.28±0.35 | 6.5 | 10.24 |
| MC4 | 15.68±1.32 | 27.26±1.25 | 39.08±0.67 | 51.29±0.75 | 63.84±1.2 | 7.3 | 7.76 |
T50 is time for 50 % release and k1 is first order release rate constant
Table 2: Release Characteristics of Eva Microcapsules of Nifedipine
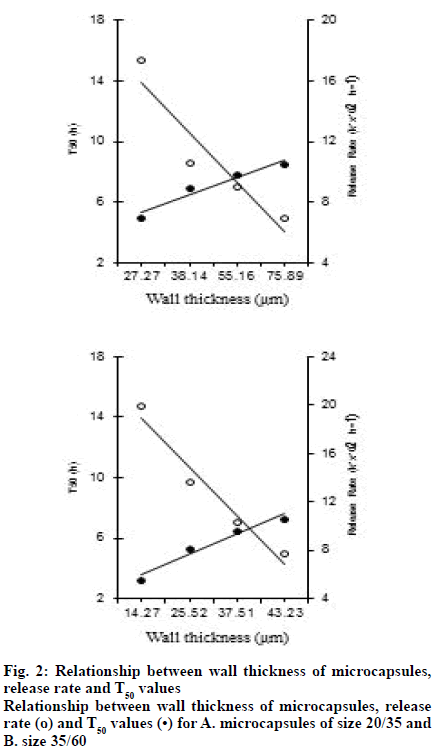
As the size of the microcapsules decreases, it increases the release. A good linear relationship between the wall thickness of the microcapsules and the release rate of the drug (k1) and T50 (50 % release time) values (fig. 2) was observed. The release mechanism of the drug from the microcapsules was diffusion controlled because it was found that the ratio of release and the square root of time to be linear (r>0.98).
The release of nifedipine from EVA microcapsules was compared with the theoretical SR required based on its pharmacokinetics. According to the recommendations of Wagner[12], the theoretical SR rate (ko) and the required dose were calculated based upon the nifedipine pharmacokinetic parameters. The SR product of nifedipine should have contain a total dose of 20 mg and should release 5 mg within the 1 h and the remaining rest should be released at a rate of 1.38 mg/h.
Based on this rate, the theoretical SR curve required for the SR product of nifedipine was calculated. Nifedipine SR tablets were official in USP XXIV, which stipulates that these tablets release 10-30 % within 3 h, 40-65 % within 6 h and no less than 80 % within 12 h. The release of nifedipine from the microcapsule MC1 with a size of 20/35 was close to the theoretical SR required for nifedipine. These microcapsules also met the official (USP XXIV) release rate test specification for nifedipine SR tablets.
In the pharmacokinetic evaluation, the elimination rate constant (Kel) of nifedipine after oral administration of nifedipine was 0.259±0.021 h-1 and the corresponding biological half-life (t1/2) was 2.68±0.21 h soft capsule. The t1/2 value of nifedipine obtained in this work was consistent with the earlier reported[13] value. The average residence time (MRT) was found to be 3.34±0.54 h. These t1/2 and MRT values indicate that nifedipine was rapidly eliminated in the body when administered in the form of soft capsules. All absorption parameters, namely Cmax, Tmax, Ka and absorption percentages (Table 3) at 0.5 h and 1.0 h (Table 3) indicate that nifedipine was rapidly absorbed after oral administration as a soft capsule. The rapid absorption of nifedipine from the soft capsule was due to the presence of nifedipine in solution in the soft capsule.
| Product | Cmax (ng/ml) | Tmax (h) | AUC (ng.h/ml) | Relative Bio-availability (%) |
MRT(h) |
% absorbed in (h) | Ka (h-1) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 2.0 | 8.0 | |||||||
| Nifedipine soft capsules | 70.70±11.60 | 1.0 | 260.4 | 100 | 3.34 | 75.43 | 98.6 | - | - | 4.32± 0.08 |
| EVA Micro- capsules MC1 (Size 20/35) |
42.70±15.94 | 3.0 | 476.9 | 91.5 | 7.05 | -- | 20.17 | 40.61 | 84.72 | 0.263±0.02 |
Pharmacokinetic parameters of oral nifedipine soft capsules and EVA microcapsules MC 1 of size 20/35. Cmax is maximum concentration reached, Tmax is time required to reach maximum concentration, AUC is area under curve from zero to infinity, MRT is mean residence time and Ka is absorption rate constant
Table 3: Pharmacokinetic Parameters.
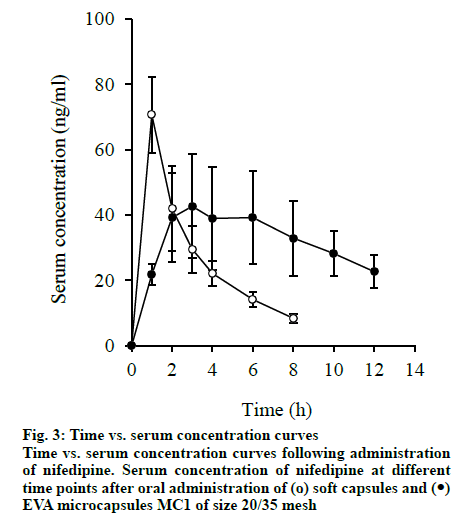
The slow absorption of nifedipine was observed from EVA microcapsules. After administering the EVA microcapsule MC1, absorption lasts up to 8-10 h. It is found that the absorption rate constant (Ka) of the microcapsules was 0.263±0.02 h-1. The Ka of the soft capsule was 4.32×0.08 h. In the case of EVA microcapsules, the serum concentration of nifedipine stabilizes and maintains within a narrow range of 28-43 ng/ml within 1 to 10 h for a longer time. In the case of soft capsules, the serum concentration remains within this range only for 0.5 to 3 h. MRT increased from 3.34 h for soft capsules to 7.05 h.
Microcapsules showed that when administered in the form of EVA microcapsules, the residence time of the drug in the body was longer. Compared with soft capsules (100 %), the relative bioavailability of EVA microcapsules is 91.5 %. Therefore, the pharmacokinetic evaluation showed that nifedipine in EVA microcapsules (fig. 3) was released over a longer period of time and absorbed slowly.
Therefore, EVA spherical microcapsules with nifedipine as the core may be prepared by the emulsion solvent evaporation method. Microcapsules were discrete, spherical, free-flowing, multi core and monolithic matrix types. The range of microencapsulation efficiency was 93-99 %. DSC and Infrared (IR) spectroscopy studies show that there was no interaction between EVA and nifedipine. The rate of nifedipine release from the microcapsules was very slow and depends on the coating: core ratio, wall thickness and size of the microcapsules.
The release was controlled by diffusion and follows first order kinetics. A good linear relationship was observed between the wall thickness of the microcapsules and the drug release rate and T50 value. The release amount of nifedipine in microcapsule MC 1 (Size 20/35) was close to the required theoretical SR amount and also meets the official release rate specification of nifedipine SR tablets (USP XXIV). These microcapsules also showed good SR characteristics in the evaluation of in vivo pharmacokinetics.
Acknowledgements
The authors thank Dr. L. Rathaiah, Chairman, Vignan Group of Institutions for providing necessary facilities to carry out research work.
Conflict of interests
The authors declared no conflicts of interest.
References
- Kondo A. Microcapsule processing and technology. Marcel Dekker 1979:18.
- Gutcho MH. Microcapsules and Microencapsulation Techniques. 1976:236.
- Shin SC, Byun SY. Controlled release of ethinylestradiol from ethylene-vinyl acetate membrane. Int J Pharm 1996;137:95-102.
- Chen SX, Lostritto RT. Diffusion of benzocaine in poly (ethylene-vinyl acetate) membranes: effects of vehicle ethanol concentration and membrane vinyl acetate content. J Control Release 1996;38:185-91.
- Chowdary KR, Babu JS. Characterization and drug release studies on ethylene vinyl acetate copolymer microcapsules. Indian J Pharm Sci 2001;63(6):500-3.
- Reynolds JEF. Martindale-The Extra Pharmacopoeia, XXIX, London: The Pharmaceutical Press 1989:1510.
- Si-Nang L, Carlier PF, Delort P, Gazzola J, Lafont D. Determination of coating thickness of microcapsules and influence upon diffusion. J Pharm Sci 1973;62(3):452-5.
- The United States Pharmacopoeia, XXIV, Rockville, MD: United States Pharmacopeial Convention, Inc 2000:2644.
- Miyazaki K, Kohri N, Arita T, Shimono H, Katoh K, Nomura A, et al. High-performance liquid chromatographic determination of nifedipine in plasma. J Chromatogr B Biomed Sci Appl 1984;310:219-22.
- Wagner, JG, Nelson E. Kinetic analysis of blood levels and urinary excretion in the absorptive phase after single doses of drug. J Pharm Sci1964;53(11):1392-403.
- Banker GS, Rhodes CT. Modern Pharmaceutics. 3rd Ed. New York: Marcel Dekker, Inc.; 1996:75.
- Wagner JG. Biopharmaceutics and Relevant Pharmacokinetics, 1st ed., Washington DC: Drug Intelligences Publications 1964:150.
- Syed LA. Nifedipine In: Kluas F. Analytical Profiles of Drug Substances, New York: Academic Press Inc., 1990:273.