- *Corresponding Author:
- Manisha Mohapatra
Seed Bank and Seed Biology Division, Regional Plant Resource Centre R and D Institute of Forest and Environment Department, Government of Odisha-751015, India
E-mail: manishamohapatra7@gmail.com
| Date of Received | 26 October 2021 |
| Date of Revision | 08 September 2022 |
| Date of Acceptance | 02 May 2023 |
| Indian J Pharm Sci 2023;85(3):544-554 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Traditional and ethno-medicinal use of plants enforces a holistic approach towards human health by wittily utilizing the synergistic potency of the bioactive compounds. Quinones are unique molecules with several therapeutic properties that lead them as most vital compound in pharmaceutical system. They can easily undergo reduction reaction paving the path for many biological processing. This class of molecules helps in treatment of several chronic ailments. Many of the drugs in Ayurvedic formulations and/or modern medicinal sectors are having one or more types of quinone groups as a major bio-active compound. However these aforementioned properties of quinones make them unique and versatile. The biochemical knowledge of these compounds is necessary to understand their physiological and toxicological properties. Amongst all quinones found naturally, anthraquinones are one of its kinds due to wide spectrum utilization in several drug formulations. In this review a brief detail of six unique yet ethno-botanically and pharmacologically versatile quinone compounds are depicted with their natural resources, structural characterization and ethnopharmacological activities. The gathered information regarding the above mentioned bio-active compounds would be helpful in identification and isolation of these compounds from a wide range of natural sources with structural characterization and pharmacological potency. These data would be pivotal in their precise identification for further use in both Ayurvedic and modern drug formulation sectors thereby lessening the threat status of the frequently used rare, endangered and threatened plants.
Keywords
Anthraquinones, plant secondary metabolites, bio-active compound, therapeutic use
The plant based compounds are widely used as complementary alternative medicines for regulating several chronic ailments. Natural bio-active compounds, mostly the plant secondary metabolites, play a key role in preparation of several drug formulations[1,2]. Medicinal plants contain several natural bio-active compounds having therapeutic potency that can act as precursors in synthesis of several drug formulations[3]. These plant components are mostly utilized in its own defence mechanism and due to their toxic nature[4], used for protection against infections or infestations[5-7]. Ethno-botanical knowledge has given an adequate basis for further investigation of medicinal properties in traditionally used plants which lead to far-flung accelerated use of natural compound[8]. Many of the bio-active compounds are still unknown to us. Only very few have been identified, isolated and some of their medicinal properties have been studied. With the current decline in the number of new molecular entities, novel bio-active compounds are being sought from medicinal plants of natural origin.
Over exploitation of specific bio-active compound to mitigate market need of drug formulation has literally created havoc and to surpass that many adulterants are being utilized as substitutes to the concerned medicinal flora with less or sometimes no efficacy. This lead to major chaos in clinical organization and diverted the concern of scientists to discover more such novel natural compounds or to produce several synthetic analogue compounds with higher efficacy. Development in medicinal fraternity has paved the path in discovery of many such natural products with greater therapeutic value[9]. Natural products are being used as precursor of drugs since ancient times. Diverse structural formation of each compound, make them unique from each other and it also has a vital role in each one’s diverse biological functioning[10]. However identification and isolation of these compounds still remains a critical factor to known their efficacy[11].
Amongst various natural bioactive compounds available worldwide, Quinones are ubiquitous in nature and are one of the largest class of oxidized derivatives of particularly aromatic compounds[12]. The biochemistry of quinones is of particular interest because of its involvement in the electron and hydrogen transfer reactions in various biological energy conversion reactions. Some of the natural plant-derived quinones are emodin, juglone, shikonin, thymoquinone, embelin, rapanone, alizarin, chloranil, plumbagin, lapachol, ubiquinone, Thymoquinone, 5-Ipdoisatin etc[13-16]. A detailed structural elucidation along with the information regarding the source plant and their ethno-pharamacological activities are delineated here.
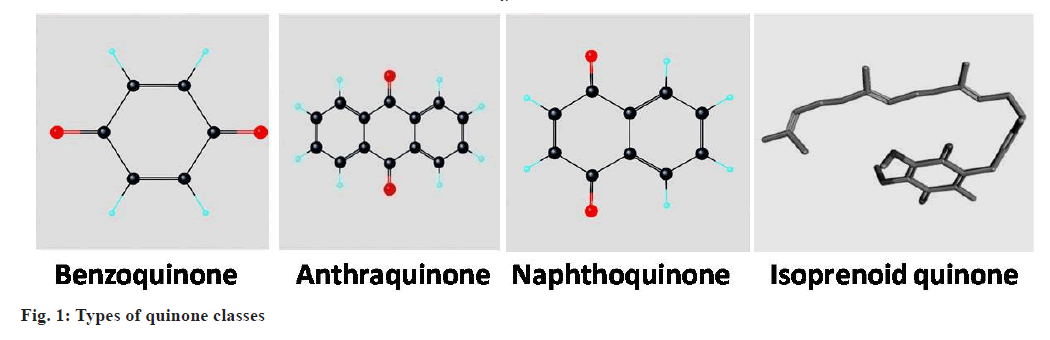
Types of Quinones
Considering the structure of quinones, they comprised of an unsaturated benzene ring with two oxygen atoms attached as carbonyl groups. Depending upon positioning of atoms, these can be subclassified as 1,4-benzoquinone, 1,2-benzoquinone, 1,4-naphthoquinone and 9,10-anthraquinone[17]. Quinones are basically consists of four classes of compounds viz. benzoquinones, anthraquinones, naphthoquinones and isoprenoid quinones (fig. 1) having more diverse biological activities[18].
Benzoquinones:
Benzoquinone (C6H4O2) is a cyclic conjugated diketone group, commonly known as 1,4-benzoquinone or p-benzoquinone.
Anthraquinones:
Anthraquinones (C14H8O2) are type of polycyclic aromatic hydrocarbons, commonly known as 9,10-anthraquinone.
Naphthoquinones:
These contain naphthalene nucleus with carbonyl groups. Structurally these are bicyclic in nature.
Isoprenoid quinones:
These consist of a polar head and a hydrophobic side chain.
Biochemistry of Quinones
The natural quinones are generally produced by the intermediate pathways of Acetate-Malonate and Shikimate Pathways[19]. Quinones have basic aromatic skeleton with several functional groups attached at different positions[20]. Parental quinone groups have the ability to add on several groups at 1,4-positions[21]. Quinones play vital role in the biochemical economy of living cells, particularly as redox-active cofactors such as plastoquinones, ubiquinones and vitamin C[12]. They can occur in a variety of forms, including monocyclic, extended or condensed form. These are oxidants, electrophiles and coloured[12] and play a pivotal role in biological functions, such as oxidative phosphorylation, electron transfer system. Quinones have several phyto-pharmacological properties like anti-parasitic, antitumor, antioxidant, antimicrobial activities[22]. These compounds show effective neurological, antithelmic, antiplasmodial activity. These are ubiquitously present with a myriad array of biological functions[23].
Anthraquinones
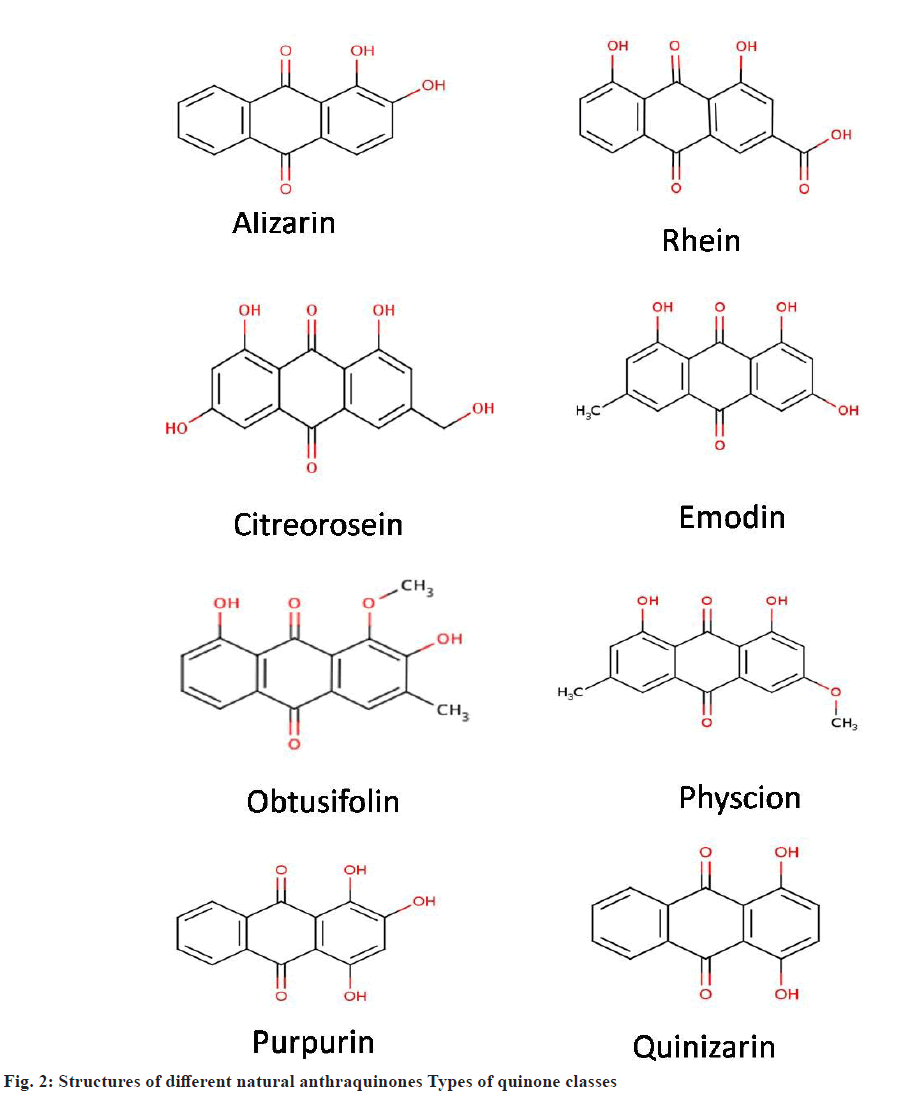
Anthraquinones are based on anthracene with a total of 3 benzene rings attached with each other. Each apex of the central ring is connected to a carbonyl group[24]. These are also known as anthracenedione. There are more than 700 types of anthraquinones found in plants along with some groups of fungi and lichens[25]. Till now 79 naturally occurring anthraquinones have been identified, isolated and characterized[26]. Anthraquinones like emodin, physcion, alizarin, catenarin, quinizarin, purpurin, obtusifolin (fig. 2) are synthesized via polyketide pathway or the shikimate pathway.
Natural source of anthraquinones:
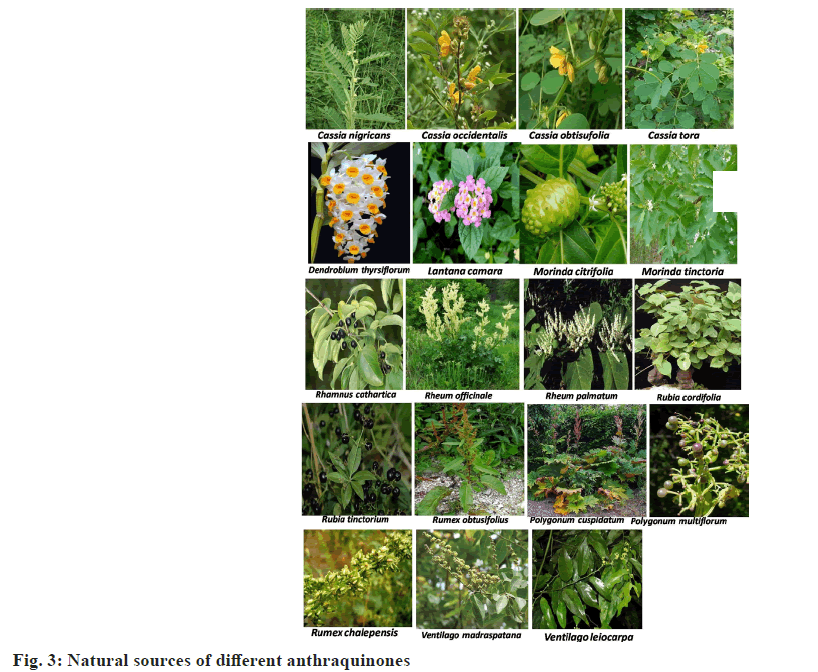
Quinones are widely distributed in the plant kingdom and mainly exist in higher plants, such as those from Polygonaceae, Rubiaceae[27,28], Myrsinaceae[15,16,29], Leguminosae[30], Rhamnaceae, Labiatae and Boraginaceae, Plumbaginaceae, Avicenniaceae, Euphorbiaceae families, among others[31]. Some natural sources of selective anthraquinones are given in (Table 1, fig. 3).
| S No | Name of Plant species | Family | Availability | Compounds |
|---|---|---|---|---|
| 1 | Cassia nigricans Vahl. | Fabaceae | Indian | Emodin |
| 2 | Cassia obtisufolia | Fabaceae | Indian | Obtusifolin |
| 3 | Cassia occidentalis L. var. aristata Collad. | Fabaceae | Indian | Emodin |
| 4 | Cassia tora Linn. | Fabaceae | Indian | Obtusifolin, Emodin |
| 5 | Dendrobium thyrsiflorum Rchb. f. ex Andre | Orchidaceae | Indian | Emodin |
| 6 | Lantana camara Linn | Verbenaceae | Indian | Emodin |
| 7 | Morinda citrifolia L. | Rubiaceae | Indian | Alizarin |
| 8 | Morinda tinctoria Roxb. | Rubiaceae | Indian | Alizarin |
| 9 | Polygonum cuspidatum Sieb. et Zucc. | Polygonaceae | Exotic | Emodin |
| 10 | Polygonum multiflorum Thunb. | Polygonaceae | Exotic | Emodin |
| 11 | Rhamnus cathartica L. | Rhamnaceae | Exotic | Emodin |
| 12 | Rheum officinale L. | Polygonaceae | Exotic | Emodin |
| 13 | Rheum palmatum L. | Polygonaceae | Indian | Emodin |
| 14 | Rubia cordifolia Linn. | Rubiaceae | Indian | Alizarin, purpurin, quinizarin |
| 15 | Rubia tinctorium L. | Rubiaceae | Indian | Alizarin, purpurin, quinizarin |
| 16 | Rumex chalepensis Mill. | Polygonaceae | Exotic | Emodin |
| 17 | Rumex obtusifolius L. | Polygonaceae | Exotic | Emodin |
| 18 | Ventilago leiocarpa Benth | Rhamnaceae | Exotic | Emodin |
| 19 | Ventilago madraspatana Gaertner | Rhamnaceae | Exotic | Emodin |
Table 1: Natural Source of Anthraquinones (Family & Availability)
Some natural anthraquinone compounds:
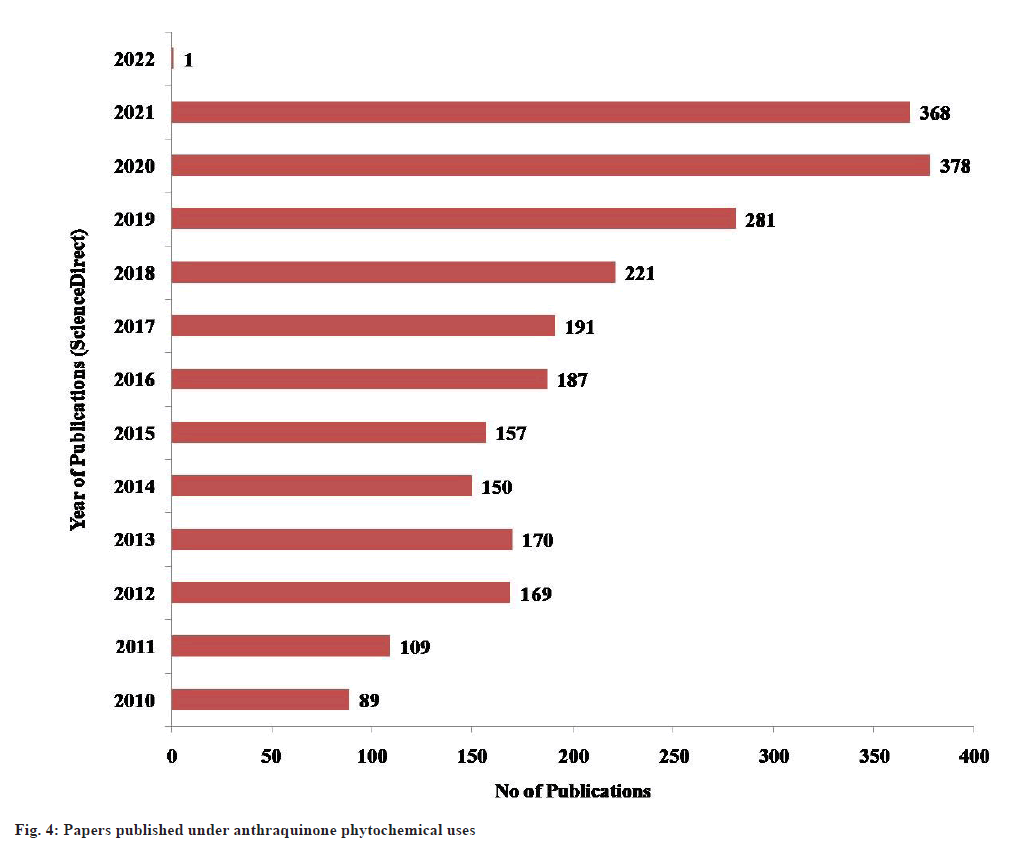
Quinones are mostly found in plants, fungi and bacteria. In angiosperms different types of quinines (biogenetically related) may occur even in the same plant[32]. Some of common natural anthraquinones are emodin, physcion, alizarin, catenarin, quinizarin, purpurin, obtusifolin, citreorosein, rhein and many more. Many studies have been done till now on anthraquinones structure, isolation, characterization and biological functions. The bibliometric analysis depicts a total publication of papers under “anthraquinone phytochemical uses” in the ScienceDirect database. The trend of research in this domain has extensively increased in the last 10 y (fig. 4).
Alizarin
Structural facts:
IUPAC Name: 1,2-dihydroxyanthracene-9,10-Dione;Group: Anthraquinone; Common name: Mordant Red 11, turkey red, rose madder; Molecular formula: C14H8O4; Molecular weight: 240.214 g/mol; Texture: Orange red crystal; Boiling point: 430°; Melting point: 279-283°; Density: 1.540 g/cm3; Solubility: Soluble in organic solvents like DMSO.
Natural source:
It is one of the main constituents found in plants of the Rubiaceae family, especially in Rubia tinctorium, Rubia cordifolia, Morinda tinctoria, Morinda citrifolia[27,28].
Description:
Alizarin, due to its red colouration, is broadly utilised in textile industries[33]. It has three equilibrium structures depending on the pH of the solution[34]. Alizarin forms stable complexes with Al3+, known as “lake pigments”.
Ethno-pharmacological uses:
Antioxidant activity: Free radicals mostly generated during normal metabolism of living cell. These are quite unstable and hamper normal cell activities to balance themselves leading to severe health crisis. However, neutralization of their activities is the vital process involved in diseases prevention. The antioxidant activity of alizarin has been well established by several researchers[35-38].
Osteotropic activity: Alizarin is being used as a neo technique for bone cancer treatment due to its antitumor properties[39]. In low dosages, it strongly inhibited the osteosarcoma and breast carcinoma cell proliferation. Furthermore its selective inhibitory activity on normal and cancerous cells makes it more efficient[40].
Dye: It is used as a chromophore and a dye. It is principally used for dyeing fabrics in textiles due to presence of red pigmentation[33]. It also enables red?mineralization for expression of osteogenesis markers (Runx2 and osteocalcin), which elevated osteoblast differentiation.
Anti-inflammatory activity: The anthraquinone has an effective anti-inflammatory efficacy in suppressing macrophages and neutrophils relevant to inflammation[41,42].
Chromogenic agent: Alizarin red S has been widely used for validation of antidepressant drug Dothiepin Hydrochloride (DOTH) in drug formulations by development of ion-pair complex between DOTH and alizarin red S[43]. It is also used for validation of anti-allergic drug, fexofenadine hydrochloride by development of ion-pair complex between fexofenadine hydrochloride and alizarin red S[44].
Emodin
Structural facts:
IUPAC name: 1,3,8-trihydroxy-6-methylanthracene- 9,10-Dione; Group: Anthraquinone; Common name: Emodol, frangula emodin; Molecular formula: C15H10O5; Molecular weight: 270.24 g/mol; Texture: Orange powder; Boiling point: sublimes; Melting point: 257°; Density: 1.231 g/cm3; Solubility: Insoluble in water; soluble in alcohol, alkali hydroxide, sodium carbonate and ammonia solution
Natural source:
Emodin is abundantly found in three families:
Fabaceae (Cassia tora, Cassia occidentalis and Cassia nigricans), Polygonaceae (Rheum palmatum, Rheum officinale, Rumex obtusifolius, Rumex chalepensis and Polygonum cuspidatum, Polygonum multiflorum)[45] and Rhamnaceae (Rhamnus cathartica and Ventilago leiocarpa, Ventilago madraspatana)[46]. It is also available in Asteraceae, Poaceae and Simaroubaceae[47]. It has a worldwide distribution, occurring in subtropical and tropical families Lantana camara, Dendrobium thyrsiflorum; in families that mainly inhabit the temperate region[48] and in families present in both tropics and temperate regions (Rhamnaceae and Clusiaceae)[49].
Description:
Emodin structurally is substituted by hydroxyl and methyl groups at 1st, 3rd, 8th and 6th positions. It is a tyrosine kinase inhibitor, an anti-neoplastic agent and a laxative[50,51].
Ethno-pharmacological uses:
Anti-bacterial activity: Emodin has been reported to have antibacterial activities against Arthrobacter globiformis, Chlorella pyrenoidosa, Bacillus megaterium, Rhizobium spp., and Azotobacter chroococcum with minimal concentrations of 10- 200 μg/ml[52-54]. It is also found to be potent against Helicobacter pylori[55].
Anti-Multidrug Resistance (MDR) activity: Due to wide spectrum utilization of antibiotics in each and every disease, their efficacy has been sacrificed in terms of both quantity and quality. This creates a serious concern as the targeted pathogens are kind of getting immunity or adapting to the biochemistry of the concerned antibiotics thereby producing MDR strains. The MDR activity of tumor cells is a crucial problem in treating cancer. In such situation, some bio-active compounds like emodin are potent enough to break this MDR action of cancer cells making them sensitive towards treatment by blocking certain pathways[56]. It also reduces glutathione level and down regulates MDR protein expression in cancer cells[46].
Anticancer activity: In particular, emodin exhibits cytotoxic effects through the arrest of cell cycle and induction of apoptosis in cancer cells[46]. Emodininduced apoptosis in human cervical cancer Bu25TK cell occurs through poly (adenosine diphosphate ribose) polymerase cleavage and activation of caspase-9[57]. Moreover, it triggers apoptosis in HepG2/C3A, PLC/PRF/5 and SK-HEP-1 cells through a p53-dependent pathway[58]. In addition, emodin accelerates arsenic trioxide-induced apoptosis[59] and even gene expression alteration occurs in HeLa cells through the redox-dependent enhancement of arsenic cytotoxicity[60]. It has cytotoxic activities against multiple myeloma[61] and also induces apoptosis in human tongue squamous cancer SCC-4 cells through mitochondria-dependent pathways in vitro[62].
Anti-diabetic activity: Emodin isolated from rhizome of Rheum palmatum exhibit anti-diabetic properties through insulin-stimulated glucose transport mechanism[63].
Obtusifolin
Structural facts:
IUPAC Name: 2,8-dihydroxy-1-methoxy- 3-methylanthracene-9,10-Dione; Group: Anthraquinone; Common name: Obtusifolin; Molecular formula: C16H12O5; Molecular weight: 284.267 g/mol; Texture: Yellowish powder; Boiling point: 528° at 760 mm Hg; Melting point: 242-243°; Density: 1.448 g/cm3; Solubility: It is soluble in chloroform, dichloromethane, ethyl acetate, DMSO.
Natural source:
It is ubiquitously found in plants from Caesalpinaceae family viz. Cassia obtisufolia, Cassia tora[64,65].
Description:
Obtusifolin is group of dihydroxy anthraquinone with several pharmaceutical medicament applications including antioxidant and antidiabetic activity[66].
Ethno-pharmacological uses:
Anti-obesity activity: Obtusifolin from Cassia tora was found to possess anti-obesity activity that regulates the lipid metabolism and used to treat obesity[66,67].
Pharmacokinetics study: The validation study of obtusifolin for pharmacokinetic activity through liquid chromatography with tandem mass spectrometry analysis in rats were detected through negative ion electrospray ionization with AUC0–t and AUC0–∞ values were 491.8±256.7 and 501.7±256.7 ng×h/ml and elimination half-life of 3.1±0.7 h. This provided pharmacological insight to obtusifolin[68].
Antibacterial activity: Ethanolic and aqueous extracts of leaves of Cassia tora were evaluated for antibacterial activity against selected pathogenic microbes. Both the extracts showed higher antimicrobial efficacy with minimum inhibitory concentration[69].
Antifungal activity: Crude extracts of leaves of Cassia tora were investigated for antifungal activities against dermatophytes basically trichophyton and epidermophyton, through well diffusion method[70].
Treatment of Alzheimer’s disease: A variety of traditionally used plants extracts were evaluated for modulation of Aβ-producing secretase activities and Aβ-degradation mechanism. In this study obtusifolin, was found to inhibit acetylcholinesterase activity in vitro and ex vivo along with possessing remarkable antioxidant and heavy metal chelating activities[71,72]. Obtusifolin, obtained from Cassia obtusifolia, lowers cellular reactive oxygen species and malondialdehyde production by enhancing antioxidant activities with mitochondrial complex I/ III. It also enables X chromosome linked inhibitor of apoptosis up regulation along with cysteinyl aspartate specific proteinase 3/9 and poly adenosine diphosphate ribose polymerase down regulation, thereby protecting mitochondrial against apoptosis by inhibiting Omi/HtrA2 release[73].
Anti-inflammatory activity: The anti-inflammatory activities of obtusifolin from Senna obtusifolia (L.) H.S.Irwin and Barneby was studied. The intake of Obtusifolin was found to inhibit metalloproteinase 3, metalloproteinase 13 and cyclooxygenase 2 along with lowering collagenase activity and the PGE2 level. It reduces the cartilage damage by regulation of metalloproteinases and cyclooxygenase 2 expressions[72].
Purpurin
Structural facts:
IUPAC Name: 1,2,4-trihydroxyanthracene-9,10- Dione; Group: Anthraquinone; Common name: Verantin, smoke brown G, hydroxylizaric acid; Molecular formula: C14H8O5; Molecular weight: 256.213 g/mol; Texture: Brown to brown-red powder; Boiling point: 359.45°; Melting point: 253- 256°; Density: 1.659 g/cm3; Solubility: Soluble in water (partly), alcohol, ether DMSO (~0.5 mg/ml)
Natural Source:
It is found in the plant Rubia cordifolia, Rubia tinctorium (Rubiaceae)[74].
Description:
It is a trihydroxy anthraquinonederrived from anthracene by substitution with oxo groups at C-9 and C-10 and with hydroxyl groups at C-1 and C-4.
Ethno-pharmacological uses:
Antioxidant activity: Antioxidant effect of purpurin was studied by several researchers using various in vitro and in vivo assays. Its antioxidant activity was studied against butrylcholinesterase, tyrosinase, acetylcholinesterase a-amylase and a-glucosidase. Purpurin found to have antioxidant and enzyme inhibition activity[37]. In another study antioxidant activity and phenolic compounds of traditional Chinese medicinal plants associated with anticancer, comprising 112 species from 50 plant families including Rubia cordifolia (purpurin) were measured showing higher values[75]. Purpurin treatment directly lowers antioxidative stress, thereby controlling down regulation system of NLRP3 leading to inhibition of inflammasome assembly scaffold[76].
Dye: Many of the plants used for dye extraction are classified as medicinal due to possession of vast array of secondary metabolites. Purpurin is actively used as a natural dye in fibre and textile industries, extracted from Rubia cordifolia and Rubia tinctorum[77,78].
Anti-fungal activity: The susceptibility of biofilm production by C. albicans against purpurin was studied in which results suggested the sub-lethal amount of Purpurin (3 μg/ml) can stop yeast-hypha transition. It also inhibited biofilm formation and reduced the metabolic activity of mature biofilms [79].
Pharmacokinetics study: The pharmacokinetic study on purpurin through Ultra Performance Liquid Chromatography Tandem Mass Spectrometery (UHPLC-MS/MS) method on rodent model found to has highest plasma concentration with 70.10±11.78 ng/ml Cmax values with 0.82 g/kg of oral administration and maximal concentration at 1.61±0.24 h thereby giving slower absorption and metabolism[80].
Anti-inflammatory activity: Purpurin along with other natural had showed promising anti-inflammatory activity[41]. It also showed the anti-inflammatory activities by regulating the pro-inflammatory Interleukins (IL)-1β and IL-18 systems[76].
Neuro-protective activity: Long duration treatment with purpurin validated serotonin linked activity on p-chloro phenylalanine-induced depression by suppressing Monoamine Oxidase (MAO) associated brain metabolism[81]. Even molecular docking simulation validated major purpurin and MAO-A binding affinity than that of purpurin and MAO-B[82]. Purpurin also crosses blood–brain barrier thereby regulating neurological disorders[83].
Quinizarin
Structural facts:
IUPAC name: 1,4-dihydroxyanthracene-9,10- Dione; Group: Anthraquinone; Common name: 1,4-dihydroxyanthraquinone; Molecular formula: C14H8O4; Molecular weight: 240.214 g/mol; Texture: Orange reddish crystalline powder; Boiling point: 450°; Melting point: 200°; Density: 1.3 g/cm3; Solubility: Moderately soluble in alcohol, soluble in ether and benzene.
Natural source:
It is found in plants of Rubiaceae family (Rubia tinctorium, Rubia cordifolia)[79].
Description:
Quinizarin has hydroxyl substituent at 1st and 4th positions through alternation of H atom from hydroxyl group hence called as dihydroxyanthraquinone.
Ethno-pharmacological uses:
Anti-inflammatory activity: The anti-inflammatory activity of quinizarin along with other natural quinones was evaluated[41].
Antioxidant activity: The antioxidative activity of quinizarin was measured through various enzymatic assays. The enzyme inhibitory activities were also analyzed against acetylcholinesterase, butrylcholinesterase, tyrosinase, a-amylase and a-glucosidase. It was found to have antioxidant and enzyme inhibition activity[37].
Anti-proliferative and anti-metastatic activity: Quinizarin anticancer activity was done against B16-F10 melanoma murine cells[84], in which the tumor cell growth was inhibited after treatment with danthron and quinizarin. The overall result suggested that both the compounds possess significant antineoplastic activity[85].
Anti-cancer activities: Photosensitive compounds like emodin and quinizarin have shown antineoplastic activity and antitumor activities and are utilized for photodynamic therapy for cancer[86]. The molecular docking and dynamics simulation research had verified its binding affinity and structural stability towards anti-apoptotic Bcl-2 protein[87].
Conclusion
Aanthraquinones are one of unique quinones with broad spectrum pharmaco-therapeutic utilization in several drug formulations. The versatility of these compounds is mostly due to their structural pattern along with the plants in which they are being synthesized. The 6 unique anthraquinone compounds depicted in the review article helps in depicting their natural resources, structural pattern and pharmacological potency. The gathered information would be helpful in precise identification of these compounds from a wide range of natural resources for further pharmao-clinical studies for drug formulation applications thereby lessening the threat status of the frequently used rare, endangered and threatened plants.
Conflict of Interest:
The authors report no conflicts of interest.
References
- Tonthubthimthong P, Chuaprasert S, Douglas P, Luewisutthichat W. Supercritical CO2 extraction of nimbin from neem seeds-an experimental study. J Food Eng 2001;47(4):289-93.
- Soetan KO, Aiyelaagbe OO. The need for bioactivity-safety evaluation and conservation of medicinal plants-A review. J Med Plants Res 2009;3(5):324-8.
- Doughari JH. Phytochemicals: Extraction methods, basic structures and mode of action as potential chemotherapeutic agents. Rijeka, Croatia: INTECH Open Access Publisher; 2012.
- Hadacek F. Secondary metabolites as plant traits: current assessment and future perspectives. Critic Rev Plant Sci 2002;21(4):273-322.
- Nweze EI, Okafor JI, Njoku O. Antimicrobial activities of methanolic extracts of Trema guineensis (Schumm and Thorn) and Morinda lucida benth used in Nigerian. Bio Res 2004;2(1):39-46.
- Doughari JH, Human IS, Benadé AJ, Ndakidemi PA. Phytochemicals as chemotherapeutic agents and antioxidants: Possible solution to the control of antibiotic resistant verocytotoxin producing bacteria. J Med Plants Res 2009;3:839-848.
- Mukherjee PK, Bahadur S, Harwansh RK, Chaudhary SK. Shifting paradigm for validation of medicinal plants in Indian traditional medicine. Indian Drugs 2014;51:5-14.
- Sarwat M, Nabi G, Das S, Srivastava PS. Molecular markers in medicinal plant biotechnology: Past and present. Critic Rev Biotechnol 2012;32(1):74-92.
[Crossref] [Google Scholar] [PubMed]
- Alvin A, Miller KI, Neilan BA. Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol Res 2014;169(7-8):483-95.
[Crossref] [Google Scholar] [PubMed]
- Engelmeier D, Hadacek F. Antifungal natural products: Assays and applications. Adv Phytomed 2006;3:423-67.
- Zainal A, I B. A review on bioactive compounds isolated from plants against plant pathogenic fungi. J Med Plants Res 2011;5(30):6584-9.
- Monks TJ, Hanzlik RP, Cohen GM, Ross D, Graham DG. Quinone chemistry and toxicity. Toxicol Appl Pharmacol 1992;112(1):2-16.
- Lu JJ, Bao JL, Wu GS, Xu WS, Huang MQ, Chen XP, et al. Quinones derived from plant secondary metabolites as anti-cancer agents. Anticancer Agents Med 2013;13(3):456-63.
[Crossref] [Google Scholar] [PubMed]
- Mohaptra M, Basak UC. Assessment of Embelin in Fruits of Embelia tsjeriam-cottam A. DC., A threatened medicinal Plant of Odisha, India. Am J Pharm Tech Res 2014;4:212-21.
- Mohapatra M, Basak UC. Quantitative reckoning of embelin from fruits of Embelia tsjeriam-cottam using water bath process as an alternate method of extraction. Indian J Pharm Biol Res 2015;3(3):15-23.
- Mohapatra M, Basak UC. Quantitative assessment of embelin content from leaf, stem bark, roots of Embelia tsjeriam-cottam. J Pharm Sci Innov 2017;6:44-49.
- Saibu M, Sagar S, Green I, Ameer F, Meyer M. Evaluating the cytotoxic effects of novel quinone compounds. Anticancer Res 2014;34(8):4077-86.
[Google Scholar] [PubMed]
- Chansukh K. Antimicrobial activities of selected thai medicinal plants bearing quinonoids. Res J Pharm Biol Chem Sci 2014;5:425-432.
- Thomson RH. Naturally occurring quinones. Elsevier; 2012.
- Talalay P, Dinkova-Kostova AT. Role of nicotinamide quinone oxidoreductase 1 (NQO1) in protection against toxicity of electrophiles and reactive oxygen intermediates. Methods Enzymol 2004;382:355-64.
[Crossref] [Google Scholar] [PubMed]
- Lindsey AS. Polymeric quinones. In: The Chemistry of Quinoid Compounds, Part II 1974;793-855.
- Liu H. Extraction and isolation of compounds from herbal medicines. Traditional herbal medicine research methods: Identification, analysis, bioassay, and pharmaceutical and clinical studies. 2011:81-138.
- Eyong KO, Kuete V, Efferth T. Quinones and benzophenones from the medicinal plants of Africa. Med Plant Res Africa 2013;351-91.
- Bone K, Mills S. Principles of herbal pharmacology, Modern Herbal Medicine. In: Principles and Practice of Phytotherapy, 2nd ed, Churchill Livingstone; 2013. p. 17-82.
- Yadav AN, Kour D, Rana KL, Yadav N, Singh B, Chauhan VS, et al. Metabolic engineering to synthetic biology of secondary metabolites production. New Future Dev Microbial Biotechnol Bioeng 2019;279-320.
- Chien SC, Wu YC, Chen ZW, Yang WC. Naturally occurring anthraquinones: Chemistry and therapeutic potential in autoimmune diabetes. Evid Based Complement Alternat Med 2015;2015:357357.
[Crossref] [Google Scholar] [PubMed]
- Anjum L, Ahmad J, Mughees M, Ahmad A. A validated quantitative determination of alizarin in Rubia cordifolia Linn. by isocratic RP-HPLC. Int J Pharm Bio Sci 2014;5(3):P1-6.
- Ramesh S, Muthubalaji R, Elangomathavan R. Phytochemical and in vitro antimicrobial assay of fruit extracts of Morinda tinctoria Roxb. Int J PharmTech Res 2014;6(2):834-41.
- Madhavan SN, Arimboor R, Arumughan C. RP?HPLC?DAD method for the estimation of embelin as marker in Embelia ribes and its polyherbal formulations. Biomed Chromatogr 2011;25(5):600-5.
[Crossref] [Google Scholar] [PubMed]
- Hussain H, Krohn K, Ahmad VU, Miana GA, Green IR. Lapachol: An overview. Arkivoc 2007;2(1):145-71.
- Nowicka B, Kruk J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim Biophys Acta 2010;1797(9):1587-605.
[Crossref] [Google Scholar] [PubMed]
- Thomson RH. Distribution of naturally occurring quinones. Pharm Weekbl Sci 1991;13:70-3.
[Crossref] [Google Scholar] [PubMed]
- Kaur P, Chandel M, Kumar S, Kumar N, Singh B, Kaur S. Modulatory role of alizarin from Rubia cordifolia L. against genotoxicity of mutagens. Food Chem Toxicol 2010;48(1):320-5.
[Crossref] [Google Scholar] [PubMed]
- Fujikawa H, Yamaguchi S, Matsui K. Spectroscopic study of alizarin and alizarin red adsorbed on anodic aluminum oxide films. Trans Mat Res Soc Japan 2018;43(3):197-200.
- Jin R, Bao H, Bai Y, Li X. Theoretical study on the antioxidant activity of alizarin, purpurin, and pseudopurpurin. Interdiscip Res Appl Bioinformat Comput Biol Environ Sci 2011;130-40.
- Kumar M, Chandel M, Kumar S, Kaur S. Amelioration of oxidative stress by anthraquinones in various in vitro assays. Asian Pac J Tropic Dis 2012;2:S692-8.
- Zengin G, Degirmenci NS, Alpsoy L, Aktumsek A. Evaluation of antioxidant, enzyme inhibition, and cytotoxic activity of three anthraquinones (alizarin, purpurin, and quinizarin). Hum Exp Toxicol 2016;35(5):544-53.
[Crossref] [Google Scholar] [PubMed]
- Sadatsharifi M, Purgel M. Radical scavenger competition of alizarin and curcumin: A mechanistic DFT study on antioxidant activity. J Mol Model 2021;27(6):166.
[Crossref] [Google Scholar] [PubMed]
- Kumar M, Rawat P, Dixit P, Mishra D, Gautam AK, Pandey R, et al. Anti-osteoporotic constituents from Indian medicinal plants. Phytomedicine 2010;17(13):993-9.
[Crossref] [Google Scholar] [PubMed]
- Fotia C, Avnet S, Granchi D, Baldini N. The natural compound Alizarin as an osteotropic drug for the treatment of bone tumors. J Orthop Res 2012;30(9):1486-92.
[Crossref] [Google Scholar] [PubMed]
- Elsharkawy E, Elshathely M, Jaleel GA, Al-Johar HI. Anti-inflammatory effects of medicinal plants mixture used by Bedouin people in Saudi Arabia. Herba polonica 2013;59(3):76-88.
- Shen CH, Liu CT, Song XJ, Zeng WY, Lu XY, Zheng ZL, et al. Evaluation of analgesic and anti-inflammatory activities of Rubia cordifolia L. by spectrum-effect relationships. J Chromatogr B 2018;1090:73-80.
[Crossref] [Google Scholar] [PubMed]
- Abdulrahman SA, Basavaiah K. Use of alizarin red S as a chromogenic agent for the colorimetric determination of dothiepin hydrochloride in pharmaceutical formulations. J Saudi Chem Soc 2014;18(2):107-14.
- Raghu MS, Basavaiah K, Prashanth KN, Vinay KB. Use of Alizarin Red S as an Ion-Pair reagent for the Spectrophotometric assay of Fexofenadine in pharmaceuticals and in spiked human urine. Int Scholar Res Notice 2012;2012:648510.
- Wang M, Zhao R, Wang W, Mao X, Yu J. Lipid regulation effects of Polygoni multiflori Radix, its processed products and its major substances on steatosis human liver cell line L02. J Ethnopharmacol 2012;139(1):287-93.
[Crossref] [Google Scholar] [PubMed]
- Hsu SC, Chung JG. Anticancer potential of emodin. Biomedicine 2012;2(3):108-16.
[Crossref] [Google Scholar] [PubMed]
- Liang HX, Dai HQ, Fu HA, Dong XP, Adebayo AH, Zhang LX, et al. Bioactive compounds from Rumex plants. Phytochem Lett 2010;3(4):181-4.
- Xing YM, Chen J, Cui JL, Chen XM, Guo SX. Antimicrobial activity and biodiversity of endophytic fungi in Dendrobium devonianum and Dendrobium thyrsiflorum from Vietnam. Curr Microbiol 2011;62:1218-24.
[Crossref] [Google Scholar] [PubMed]
- Locatelli M, Epifano F, Genovese S, Carlucci G, Kon?i? MZ, Kosalec I, et al. Anthraquinone profile, antioxidant and antimicrobial properties of bark extracts of Rhamnus catharticus and R. orbiculatus. Nat Prod Commun 2011;6(9):1934578X1100600917.
[Google Scholar] [PubMed]
- Thomson RH. Naturally occurring quinones IV. Springer Science & Business Media; 1997.
- Harborne JB, Baxter H, Moss GP. Phytochemical dictionary: a handbook of bioactive compounds from plants. 2nd ed, London, UK: Taylor & Francis, 1999.
- Kitanaka S, Takido M. Studies on the constituents in the roots of Cassia obtusifolia L. and the antimicrobial activities of constituents of the roots and the seeds. Yakugaku zasshi 1986;106(4):302-6.
[Crossref] [Google Scholar] [PubMed]
- Coopoosamy RM, Magwa ML. Antibacterial activity of aloe emodin and aloin A isolated from Aloe excelsa. African J Biotechnol 2006;5(11):1092-4. [Crossref] [Google Scholar] [PubMed]
- Ayo RG, Amupitan JO, Zhao Y. Cytotoxicity and antimicrobial studies of 1, 6, 8-trihydroxy-3-methyl-anthraquinone (emodin) isolated from the leaves of Cassia nigricansVahl. Afr J Biotechnol 2007;6:1276-1279.
- Ibrahim M, Khan AA, Tiwari SK, Habeeb MA, Khaja MN, Habibullah CM. Antimicrobial activity of Sapindus mukorossi and Rheum emodi extracts against H. pylori: In vitroand in vivo studies. World J Gastroenterol 2006;12(44):7136.
[Crossref] [Google Scholar] [PubMed]
- Huang XZ, Wang J, Huang C, Chen YY, Shi GY, Hu QS, et al. Emodin enhances cytotoxicity of chemotherapeutic drugs in prostate cancer cells: the mechanisms involve ROS-mediated suppression of multidrug resistance and hypoxia inducible factor-1. Cancer Biol Ther 2008;7(3):468-75.
[Crossref] [Google Scholar] [PubMed]
- Tavazoie M, van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008;3(3):279-88.
[Crossref] [Google Scholar] [PubMed]
- Hovinga KE, Shimizu F, Wang R, Panagiotakos G, van Der Heijden M, Moayedpardazi H, et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells 2010;28(6):1019-29.
[Crossref] [Google Scholar] [PubMed]
- Lathia JD, Rao MS, Mattson MP, ffrench?Constant C. The microenvironment of the embryonic neural stem cell: Lessons from adult niches? Dev Dyn 2007;236(12):3267-82.
[Crossref] [Google Scholar] [PubMed]
- Ljubimova JY, Fugita M, Khazenzon NM, Das A, Pikul BB, Newman D, et al. Association between laminin?8 and glial tumor grade, recurrence, and patient survival. Cancer 2004;101(3):604-12.
[Crossref] [Google Scholar] [PubMed]
- Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 2010;6(2):141-52.
[Crossref] [Google Scholar] [PubMed]
- Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res 2006;66(15):7445-52.
[Crossref] [Google Scholar] [PubMed]
- Lee MS, Sohn CB. Anti-diabetic properties of chrysophanol and its glucoside from rhubarb rhizome. Biol Pharm Bull 2008;31(11):2154-7.
[Crossref] [Google Scholar] [PubMed]
- Singh S, Singh SK, Yadav A. A review on Cassia species: Pharmacological, traditional and medicinal aspects in various countries. Am J Phytomed Clin Ther 2013;1(3):291-312.
- Kim DH, Hyun SK, Yoon BH, Seo JH, Lee KT, Cheong JH, et al. Gluco-obtusifolin and its aglycon, obtusifolin, attenuate scopolamine-induced memory impairment. J Pharmacol Sci 2009;111(2):110-6.
[Crossref] [Google Scholar] [PubMed]
- Cho IJ, Lee C, Ha TY. Hypolipidemic effect of soluble fiber isolated from seeds of Cassia tora Linn. in rats fed a high-cholesterol diet. J Agri Food Chem 2007;55(4):1592-6.
[Crossref] [Google Scholar] [PubMed]
- Akram M. Anti-obesity activity of medicinal plants: A review. Diabetic Complicat. 2017;1-13.
- Huang Z, Sun Q, Hao W, Zhao J. Pharmacokinetics and tissue distribution study of obtusifolin in rats by liquid chromatography–tandem mass spectrometry. Biomed Chromatogr 2021;35(3):e5009.
[Crossref] [Google Scholar] [PubMed]
- Bharathirajan R, Prakash M. In vitro antibacterial activity and phytochemical screening of Cassia tora leaves. Int J Curr Microbiol App Sci 2013;2:463-5.
- Jain S, Dubey P, Singh R. Antifungal properties of weed plant (C. tora) on dermatophytosis. Int J Chem Sci 2012;10:1451-8.
- Eckert GP. Traditional used plants against cognitive decline and Alzheimer disease. Front Pharmacol 2010;1:138.
[Crossref] [Google Scholar] [PubMed]
- Nam J, Seol DW, Lee CG, Wee G, Yang S, Pan CH. Obtusifolin, an anthraquinone extracted from Senna obtusifolia (L.) HS Irwin & Barneby, reduces inflammation in a mouse osteoarthritis model. Pharmaceuticals 2021;14(3):249.
[Crossref] [Google Scholar] [PubMed]
- Tang Y, Zhong ZY, Liu YF, Sheng GT. Obtusifolin inhibits high glucose?induced mitochondrial apoptosis in human umbilical vein endothelial cells. Mol Med Rep 2018;18(3):3011-9.
[Crossref] [Google Scholar] [PubMed]
- Sharma L, Agarwal G, Kumar A. Medicinal plants for skin and hair care. Indian J Tradit Knowl 2003;2:62-8.
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 2004;74(17):2157-84.
[Crossref] [Google Scholar] [PubMed]
- Nam W, Kim SP, Nam SH, Friedman M. Structure-antioxidative and anti-inflammatory activity relationships of purpurin and related anthraquinones in chemical and cell assays. Molecules 2017;22(2):265.
[Crossref] [Google Scholar] [PubMed]
- Gokhale SB, Tatiya AU, Bakliwal SR, Fursule RA. Natural dye yielding plants. Nat Prod Radiance 2004;3:228-34.
- Chengaiah B, Rao KM, Kumar KM, Alagusundaram M, Chetty CM. Medicinal importance of natural dyes-a review. Int J PharmTech Res 2010;2(1):144-54.
- Tsang PW, Bandara HM, Fong WP. Purpurin suppresses Candida albicans biofilm formation and hyphal development. PloS one. 2012;7(11):e50866.
[Crossref] [Google Scholar] [PubMed]
- Gao M, Yang J, Wang Z, Yang B, Kuang H, Liu L, et al. Simultaneous determination of purpurin, munjistin and mollugin in rat plasma by ultra-high performance liquid chromatography-tandem mass spectrometry: Application to a pharmacokinetic study after oral administration of Rubia cordifolia L. extract. Molecules 2016;21(6):717.
[Crossref] [Google Scholar] [PubMed]
- Ksouri R, Ksouri WM, Jallali I, Debez A, Magné C, Hiroko I, et al. Medicinal halophytes: potent source of health promoting biomolecules with medical, nutraceutical and food applications. Critic Rev Biotechnol 2012;32(4):289-326.
[Crossref] [Google Scholar] [PubMed]
- Rossi S, Tabolacci C, Lentini A, Provenzano B, Carlomosti F, Frezzotti S, et al. Anthraquinones danthron and quinizarin exert antiproliferative and antimetastatic activity on murine B16-F10 melanoma cells. Anticancer Res 2010;30(2):445-9.
[Google Scholar] [PubMed]
- Ma L, Hu P, Zhang J, Cui W, Zhao X. Purpurin exerted antidepressant-like effects on behavior and stress axis reactivity: Evidence of serotonergic engagement. Psychopharmacol 2020;237:887-99.
[Crossref] [Google Scholar] [PubMed]
- Lee HW, Ryu HW, Kang MG, Park D, Oh SR, Kim H. Selective inhibition of monoamine oxidase A by purpurin, an anthraquinone. Bioorg Med Chem Lett 2017;27(5):1136-40.
[Crossref] [Google Scholar] [PubMed]
- Viswanathan GK, Shwartz D, Losev Y, Arad E, Shemesh C, Pichinuk E, et al. Purpurin modulates Tau-derived VQIVYK fibrillization and ameliorates Alzheimer’s disease-like symptoms in animal model. Cell Mol Life Sci 2020;77:2795-813.
[Crossref] [Google Scholar] [PubMed]
- Verebová V, Beneš J, Stani?ová J. Biophysical characterization and anticancer activities of photosensitive phytoanthraquinones represented by hypericin and its model compounds. Molecules 2020;25(23):5666.
[Crossref] [Google Scholar] [PubMed]
- Sachithanandam V, Lalitha P, Parthiban A, Muthukumaran J, Jain M, Misra R, et al. A comprehensive in silico and in vitro studies on quinizarin: A promising phytochemical derived from Rhizophora mucronata Lam. J Biomol Struct Dyn 2022;40(16):7218-29.
[Crossref] [Google Scholar] [PubMed]