- Corresponding Author:
- A. K. Pandey
NWFP Division, Tropical Forest Research Institute, P. O. RFRC, Mandla Road, Jabalpur-482020, India
E-mail: akpandey10@rediffmail.com
| Date of Submission | 26 July 2010 |
| Date of Revision | 4 January 2011 |
| Date of Acceptance | 2 March 2011 |
| Indian J Pharm Sci, 2011, 73 (1): 216-219 |
Abstract
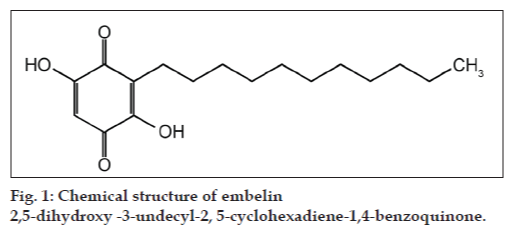
Embelin (2,5-dihydroxy-3-undecyl-2,5-cyclohexadiene-1,4-benzoquinone) is a phenolic compound found in the fruits of Embelia tsjeriam-cottam and is responsible for the medicinal properties of the plant Thus the fruits are harvested at a large scale before maturity leading to depletion of population. Since the chemical constituents of medicinal plants are directly associated with the harvesting time, a study was conducted in different forest areas of Chhattisgarh, India, to standardize the harvesting time of Baividang fruits on the basis of their embelin content during 2005-08. The embelin content was determined by RP-HPLC and varied from 1.09 to 5.21% (w/w). The immature fruits collected in October contain an average of 1.67% embelin whereas mature fruits collected in December on an average contain 4.64% embelin. On the basis of our findings it can be concluded that fruits should be harvested after attaining maturity to get better quality produce and also to maintain the sustainability of plant.
Keywords
Baividang, embelin, Embelia tsjeriam-cottam, harvest, sustainability
The family Myrsinaceae consists of nearly 1000 species of trees and shrubs spread over 33 genera including four genera namely Myrsine, Maesa, Rapanea and Embelia, which are widely used in herbal medicines [1]. Embelia ribes is the most correlated species of Vidanga (Sanskrit), a drug used in Ayurveda, Siddha as well as in Unani medicine system as anthelmintic and to cure skin diseases [2]. The fruits of E. tsjeriam-cottam are being used in place of E. ribes as there is no significant difference in the properties of two species [3]. It is a threatened woody shrub, which is sparsely distributed in the moist deciduous forests of the Western Ghats, India, SriLanka, Malaysia and South China [4]. The fruit is bitter in taste, good appetizer, cures tumors, ascites, bronchitis, jaundice and mental disorders [5]. Seeds are used as antibiotic, anthelmintic, antituberculosis, alterative and stimulative [4]. Leaves are astringent, demulcent, depurative and useful in pruritus, sore throat, mouth ulcers, indolecent, skin diseases and leprosy [6]. A gum obtained from the plant is being used to treat dysmenorrhoea. A decoction of the leaves of this plant has been reported to be a blood purifier [7]. Bhandari et al. [8,9] have reported antidiabetic, antidyslipidemic and antioxidant properties of E. ribes in streptozotocininduced diabetic rats, using gliclazide as a positive control. Recently, Bhandari et al [10]. have also reported cardioprotective activity of the aqueous extract of E. ribes in isoproterenol-induced myocardial infarction in the rat. Wound healing activity of the embelin isolated from the leaves of E. ribes was reported by Swamy et al [11]. Chemical structure of embelin resembles the structure of natural coenzyme Q10 (ubiquinones) and the role of this is well defined in various biochemical protective mechanisms.
Embelin (2,5-dihydroxy-3-undecyl-2,5-cyclohexadiene- 1,4-benzoquinone, fig. 1) is a phenolic compound found in the fruits of E. ribes, E. tsjeriam-cottam and other species of Myrsinaceae family [12]. This compound has been reported to possess antihelmentic, antioestrogenic, antiinflammatory, antioxidant, antitumour and antifertility activity [13-15]. Embelin is also reported to be a potent oral contraceptive [16] and is thus used by tribal people. There is no significant difference in embelin content between these two species [3].
The biosynthesis of secondary metabolites is not only controlled by genetic factors but also affected by environmental influences, developmental stages and functionally different plant parts. However, the research work pertaining to influence of harvesting time or maturity on chemical content has not been carried out in E. tsjeriam-cottam. Keeping above facts into consideration a study was conducted to standardize the harvesting time with special reference to embelin content (major active ingredient).
The fruits were collected from the forests of Jabarra, Keonchi, Marwahi, Bherosong and Ataria Lamni of Chhattisgarh using a non-destructive harvesting technique of by hand plucking. On maturity fruits turn red to black in color. The fruits were then packed in polythene bags and brought to the laboratory for further chemical analysis. The fruits were collected every year from 2005-08.
The collected fruits were cleaned properly by removing leaves and other foreign material. The fruits were weighed and kept for drying in shade at room temperature on elevated surface to maintain their quality. In good sunny days fruits dry within a week. Fruits were then crushed to coarse powder and stored in polythene bags for extraction. The embelin content was determined by using HPLC method given by Choudhary et al. [17] with some modifications.
Two grams of coarse powder of the dried sample was taken in 100 ml round bottom flask and refluxed in 50 ml HPLC grade methanol (Merck) for 3 h. The extract was then decanted to 250 ml conical flask and the residue was further extracted in 50 ml methanol for 3 h. This procedure was repeated until no color comes to the solvent. Typically, four extractions are sufficient for complete exhaustion of the samples. The volume of extract was then made up to 100 ml and filtered through 0.22 μm disc filter.
A standard solution of embelin was prepared by dissolving 1 mg standard embelin (Sigma) in 5 ml HPLC grade methanol and heating the flask over hot water bath at 60 for 5 min. The volume was then made up to 10 ml with HPLC grade methanol. The concentration of resulting solution was 100 μg/ml. The standard solution was then filtered through 0.22 μm pore size disc filter and stored in refrigerator till further use. Waters 515 HPLC instrument having C-18 Xbridge column with a photo diode array (PDA) detector was used during the experiment. Mobile phase consisted of the mixture of methanol, water, acetic acid and tetrahydrofuran in the ratio 80:15:4:1. Twenty microloitre of sample was injected at a flow rate of 1.6 ml/min.
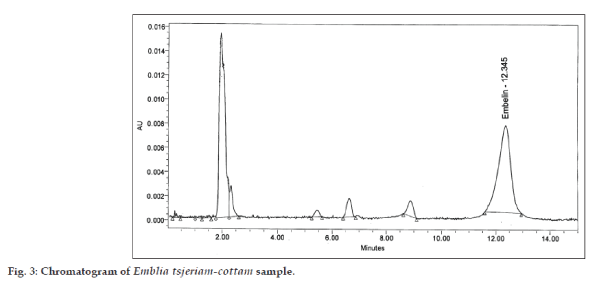
The embelin content estimated in fruits collected from different locations is shown in Table 1. The maximum embelin content (5.06%) was found in Ataria and minimum (3.06%) was found in Bherosong. The data shows that the embelin content varied in different locations. Pandey and Kori [18] also reported the variations in tannin and oxalic acid content in Terminalia Arjuna (Arjuna) bark with respect to different places in Madhya Pradesh. The embelin content in the fruits collected at different harvesting time was also estimated and is represented in Table 2. The chromatograms of embelin standard and baividang sample are shown in figs. 2 and 3, respectively. Maximum embelin content 5.21% was found in December 2007 followed by 5.09% in December 2008 and the minimum embelin content 1.09% was found in October 2005 followed by 1.62% in October 2007, depicting that the fruits harvested at right time i.e after attaining maturity (in December) contains more percentage of embelin than the immature fruits collected in October or November. Cirak et al. [19] studied variation of bioactive secondary metabolites in Hypericum origanifolium during its phenological cycle and reported increase in the concentration of secondary metabolite on fruit maturation. Similarly, variation in embelin content was observed in our study. Similar studies were also done by Mhamdi et al. [20] showing the increase in the concentration of total phenolic acids with the ripening of Borage seeds (Borago officinalis L.). Desouky et al. [21] also studied that free acidity, peroxide number and delta K increased during olive ripening. Seasonal variations in tannin and oxalic acid content in Terminalia Arjuna (Arjuna) bark [18] and variations in protein, phenol, tannin, nitrate, oxalate, vitamin C, anthocyanin and chlorophyll in the leaves [22] were also reported. Thus it can be interpreted that harvesting time influences the quality of produce.
| Location | Embelin content % (w/w) |
|---|---|
| Jabarra, Dhamtari | 3.97±0.010 |
| Atarialamni, Bilaspur | 5.06±0.02 |
| Bherosong, Bilaspur | 3.06±0.04 |
| Keonchi, Pedra Road | 3.65±0.03 |
Table 1: Embelin content of emblia tsjeriamcottam fruits
| Harvesting time | Embelin content (%) | |
|---|---|---|
| Year | Month of collection | (w/w) |
| 2005 | October | 1.09±0.02 |
| November | 2.82±0.03 | |
| December | 3.61±0.04 | |
| 2007 | October | 1.62±0.03 |
| November | 3.82±0.03 | |
| December | 5.21±0.03 | |
| 2008 | October | 2.29±0.04 |
| November | 3.51±0.01 | |
| December | 5.09±0.03 |
Table 2: Embelin content in emblia tsjeriamcottam fruits collected in different months
Some fruits were washed after collection and their fruit coat was removed before drying. Such fruits contain only 2.52% embelin content, which is less than that present in the whole fruit 4.77%. It shows that fruit coat of Baividang fruits contain good amount of embelin. Therefore fruit coat should not be removed before drying.
On dry weight basis the amount of embelin content varied from 1-5%. Fruits collected in December contain more embelin than the fruits collected in October. Thus it can be concluded that harvesting time plays a major role in the concentration of active ingredients present in the plant, mature fruits contains more amount of active ingredients as compared to immature fruits or half mature fruits. The best harvesting time for this species is December to get better quality produce and also to maintain the sustainability of plant.
References
- Januaro AH, Fatima DM, De Silva F, Viera PC, Fernandes JB. Dammarane and cycloartanetriterpenoids from three Rapanea species. Phytochemistry 1992;4:1251-3.
- Githiori, JB, Höglund J, Waller PJ, Baker RL. Evaluation of anthelmintic properties of some plants used as livestock dewormers against Haemonchuscontortus infections in sheep. Parasitology 2004;129:245-53.
- Pullaiah T. Medicinal Plants in India, Vol. 1. New Delhi: Regency Publication; 2002.
- Guhabakshi DN, Sensarma P, Pal DC. Lexicon medicinal plants of India. Kolkata: NayaPrakashan; 2001. p. 135-6.
- Kirthikar KR, Basu BD. Indian Medicinal Plants. Vol. 2. Allahabad: Lalit Mohan Basu; 1987. p. 1479.
- Sharma PC, Yelne MB, Dennis TJ. Database on medicinal plants used in Ayurveda. New Delhi: Central Council for Research in Ayurveda and Siddha; 2002. p. 479.
- Anonymous. Wealth of India: Raw materials, Vol 3. New Delhi: CSIR; 1952. p. 167-8.
- Bhandari U, Kanojia R, Pillai KK. Effect of ethanolic extract of Embeliaribeson dyslipidaemia in diabetic rats. Int J ExpDiab Res2002;3:159-62.
- Bhandari U, Jain N, Pillai KK. Further studies on antioxidant potential and protection of pancreatic ß-cells by Embeliaribesin Experimentaldiabetes. ExpDiab Res 2007;7:1-6.
- Bhandari U, Ansari MN, Islam F. Cardioprotective effect of aqueous extract of EmbeliaribesBurm fruits against isoproterenol-induced myocardial infarction in albino rats. Indian J ExpBiol 2008;46:35-40.
- Kumara SH, Krishna V, Shankarmurthy K, Abdul Rahiman B, MankaniKL, Mahadevan KM, et al. A wound healing activity of embelin isolated from the ethanol extract of leaves of EmbeliaribesBurm. J Ethnopharmacol 2007;109:529-34.
- Rao CB, Venkateswaralu V. Vilangin a new constituent of Embeliaribes. CurrSci 1961;30:250-60.
- Krishnaswamy M, Purushothaman KK. Antifertility properties of Embeliaribes: (Embelin). Indian J ExpBiol 1980;18:1359.
- Chitra M, Sukumar E, Suja V, Shyamala Devi CS. Antitumor, anti-inflammatory and analgesic property of Embelin, a Plant Product. Chemotherapy 1994;40:109.
- Li XH, McLaughlin JL. Bioactive compounds from the root of Myrsineafricana. J Nat Prod 1989;52:660-2.
- Prakash AO. Antifertility investigations on embelin an oral contraceptive of plant origin. Part I Biological properties. Planta Med 1981;41:259-66.
- Choudhury RP, Md. Ibrahim A, Bharathi, Padma V. Quantitative analysis of embelin in Myrsineafricana L. (Myrsinaceae) using HPLC and HPTLC. E-J Food Plants Chem 2007;2:20-4.
- Pandey AK, Kori DC. Variations in tannin and oxalic acid content in Terminalia Arjuna (Arjuna) Bark. Phcog Mag 2009;5:159-64.
- Çirak C, Jolita R, Liudas I, Valdimaras J. Variation of bioactive secondary metabolites in Hypericumoriganifolium during its phenological cycle. ActaPhysiologiaePlantarum 2007;29:197-203.
- Mhamdi B, Wannes WA, Sriti J, Jellali I, Ksouri R, Marzouk B. Effect of harvesting time on phenolic compounds and antiradical scavenging activity of Boragoofficinalis seed extracts. Indust Crops Prod 2010;31:e1-4.
- Desouky IM, Laila F, Haggag MM, Abd El M, El-Hady ES. Changes in some physical and chemical properties of fruit and oil in some olive oil cultivars during harvesting stage. World J AgriSci 2009;5:760-5.
- Srivastava N, Prakash D, Behl HM. Biochemical contents, their variation and changes in free amino acids during seed germination in Terminalia Arjuna . Int J Food SciNutr 1997;48:215-9.