Varsha R. Galande, K. G. Baheti*, S. Indraksha1 and M. H. Dehghan
Department of Pharmaceutical Chemistry, Y. B. Chavan College of Pharmacy, Dr. Rafiq Zakaria Campus, Rauza Bagh,
P. B. No. 33, Aurangabad–431 001,
1APL Research Centre, Survey No. 313, Bachupally Village, Quthubullapur Mandal,
Hyderabad-500 072, India.
- *Corresponding Author:
- K. G. Baheti
Department of Pharmaceutical Chemistry, Y. B. Chavan College of Pharmacy, Dr. Rafiq Zakaria Campus, Rauza Bagh, P. B. No. 33, Aurangabad–431 001,India.
E-mail: nk_baheti@yahoo.com
| Date of Submission | 20 March 2011 |
| Date of Revision | 23 December 2011 |
| Date of Acceptance | 28 December 2011 |
| Indian J Pharm Sci, 2012, 74 (1): 18-23 |
DOI: 10.4103/0250-474X.102538
Abstract
A simple, precise, accurate and economic simultaneous UV spectrophotometric method has been developed for the estimation of amlodipine besylate, valsartan and hydrochlorothiazide in combination in bulk mixture and tablet. The estimation was based upon measurement of absorbance at absorbance maxima of 359 nm, 317 nm and 250 nm for amlodipine besylate, hydrochlorothiazide and valsartan in methanol, respectively in bulk mixture and tablet. The Beer Lambert’s law obeyed in the concentration range 5-25 μg/ml, 10-50 μg/ml and 5-25 μg/ml for amlodipine besylate, hydrochlorothiazide and valsartan, respectively. The estimation of bulk mixture and tablet was carried out by simultaneous equation, Q-analysis and area under curve method for estimation of amlodipine besylate and hydrochlorothiazide and standard curve method for estimation of valsartan. The results were found to be in the range of 99.6±1.52% to 102±0.51%. Method was validated with respect to specificity, linearity, range, accuracy, precision, LOD, LOQ, robustness, ruggedness and can be applied for routine analysis of tablet dosage forms.
Keywords
Amlodipine besylate, hydrochlorothiazide, UV spectrophotometry, valsartan
Introduction
Recent rapid socioeconomic changes have led to a concomitant change in people’s lifestyle, leading to work-related stress and altered food habits, raising the risk of hypertension. About 85% of patients may need multiple medications of three or more components to control their blood pressure. Hence there was a need of single pill containing three or more components. The Exforge HCT tablet of Novartis International AG was recently approved by USFDA for the treatment of blood pressure contains three components, amlodipine besylate 10 mg, valsartan 160 mg and hydrochlorothiazide 25 mg. It is an important new option for the patients who have tried taking dual combinations of blood pressure medications without success. Patients may find treatment more convenient with single pill rather than separate pills. Exforge HCT provides additional reductions of 18-29% in systolic blood pressure and 19-32% in diastolic blood pressure. Amlodipine besylate (AMLB) is 3-ethyl-5-methyl (±)-2-[(2-aminoethoxy) methyl]-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzenesulfonate, a potent dihydro calcium channel blocker depresses myocardial contractility as well as reduces peripheral vascular resistance to prevent a coronary vascular spasm. Valsartan (VAT), N-(1-oxopentyl)-N-[[2′-(1Htetrazol- 5-yl) [1,1′-biphenyl]-4-yl] methyl]-L-valine, is a potent angiotensin-II receptor blocker which prevent vasoconstriction and aldosterone secretion thus decreases blood pressure. Hydrochlorothiazide (HCT), 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7- sulfonamide-1,1-dioxide, is a thiazide type diuretic, which reduces reabsorption of electrolytes from the renal tubules there by increasing the excretion of sodium, potassium, chloride (fig. 1)[1].
Materials and Methods
UV spectrophotometric methods[2,3], HPLC[4-8] and HPTLC[9] were reported for the estimation of AMLB alone or in combination with other antihypertensive agents. Methods such as LC[10-12], HPLC[13,14], simultaneous UV spectrophotometry[11,12,15] and LCESI- MS/MS[16] were reported for estimation of VAT alone or in combination with other agents. There are several reports of the determination of HCT individually or in combination with other drugs, including spectrophotometry[17,18], LC-MS[19], LC[20], HPLC[21] and HPTLC[18]. Recently, first derivative spectrophotometry and absorption correlation method was published for determination of this combination[22,23]. But in both the method the linearity range of determination of components does not fall in final concentration of tablet solution. Hence there is need to develop the method to overcome the limitation of present method. Also in the literature the AUC and Q-analysis methods were not reported. Hence, the present study aims at developing a simple, accurate and precise method for analysis of AMLB, VAT and HCT in combination.
The instrument used for present study was UV/Vis spectrophotometer (Jasco- UV V-630) with 1.0 cm matched quartz cells. The SPSS 9 application software for statistical treatment and analytical grade chemicals and solvents of Qualigen were used.
Standard stock solution (1000 μg/ml) of AMLB was prepared by dissolving 25 mg in 25 ml of methanol. Appropriate volume of stock solution of AMLB was diluted to get a series of solutions containing 5-25 μg/ml of AMLB. Similarly the 1000 μg/ml solution of VAT and HCT were prepared and the stock solution was diluted to get series of solutions containing 10-50 μg/ml of HCT and 5-25 μg/ ml of VAT. All the solutions were protected from light throughout the experiments. Bulk mixture was prepared by dissolving AMLB (10 mg), VAT (160 mg) and HCT (25 mg) in 100 ml volumetric flask[1]. The 1 ml bulk mixture was diluted to 10 ml to obtain 10, 160 and 25 μg/ml of AMLB, VAT and HCT, respectively, referred as bulk mixture-I, which was used for determination of AMLB and HCT. The 1 ml of bulk mixture-I was further diluted to 10 ml to obtain 1, 16 and 2.5 μg/ml of AMLB, VAT and HCT, respectively referred as bulk mixture-II which was used for determination of VAT. Tablets of composition AMLB (10 mg), VAT (160 mg) and HCT (25 mg) were formulated in our laboratory [1]. Sample solution was prepared by taking twenty tablets accurately weighed and powdered. The powder weight equivalent to the label claims was transferred to volumetric flask and to this 50 ml methanol was added and the solution was ultrasonicated for 15 min, the volume was made up to 100 ml with methanol. This tablet stock solution was filtered through Whatmann filter paper no. 40 prior to use. The tablet stock solution (1 ml) was further diluted to 10 ml to obtain 10, 160 and 25 μg/ml of AMLB, VAT and HCT, respectively referred as tablet solution-I, which was used for determination of AMLB and HCT. The l ml of tablet solution-I was further diluted to 10 ml to obtain 1, 16 and 2.5 μg/ ml of AMLB, VAT and HCT, respectively referred as tablet solution-II, which was used for determination of VAT.
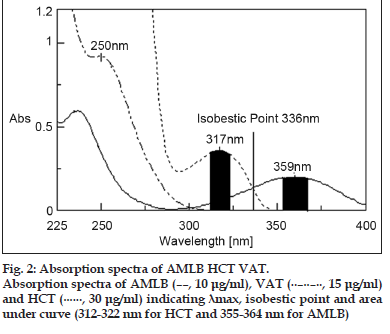
The standard solutions were scanned over the UV range of 200-400 nm and absorbance maxima of 359 and 317 nm were selected for the determination of AMLB and HCT, respectively by simultaneous equation method. For Q-analysis the isobestic point at 336 nm and for area under curve method, the absorbance range of 312-322 nm and 355-364 nm for HCT and AMLB, respectively was selected. The absorbance maximum of 250 nm was considered for detection of VAT by standard curve method (fig. 2).
Estimation of AMLB and HCT in presence of VAT by simultaneous equation method
Absorbance and absorptivity of standard AMLB and HCT solutions and bulk mixture-I was determined at 359 and 317 nm, respectively (fig. 2) and the concentration of AMLB and HCT was estimated in bulk mixture-I by simultaneous equations, CAMLB = (A2ay1–A1ay2)/(ax2ay1–ax1ay2) and CHCT = (A1ax2–A2ax1)/(ax2ay1–ax1ay2). Where, CAMLB and CHCT- Concentration of AMLB and HCT, A1 and A2- Absorbance of bulk mixture-I or tablet solution-I at 359 and 317 nm, ax1 and ax2- Absorptivity of AMLB at 359 and 317 nm, ay1 and ay2- Absorptivity of HCT at 359 and 317 nm. Similarly the absorbance and absorptivity of tablet solution-I was determined and the concentration of AMLB and HCT in tablet solution-I was estimated by using above equation[24].
Estimation of AMLB and HCT in presence of VAT by Q-analysis method
Absorbance of standard AMLB and HCT solutions and bulk mixture-I were determined at 359 nm and at isobestic point 336 nm (fig. 2) and the concentration of AMLB and HCT in bulk mixture-I was estimated by simultaneous equations CAMLB = (QSample–QHCT/QAMLB–QHCT)×(ASample/aAMLB) and CHCT = (QSample–QAMLB/QHCT–QAMLB)×(ASample/aHCT). Where, CAMLB and CHCT- Concentration of AMLB and HCT, QAMLB- Absorption ratio of AMLB, QHCT- Absorption ratio of HCT, QSample- Absorption ratio of Sample, ASample- Absorbance of sample at 336 nm (Sample may be bulk mixture-I or tablet solution-I), aAMLB and aHCT- Absorptivity of AMLB and HCT at 336 nm. Similarly the concentration of AMLB and HCT in tablet solution-I was estimated by above equations[22].
Estimation of AMLB and HCT in presence of VAT by area under curve method
The area under curve of standard AMLB and HCT solutions and bulk mixture-I were determined at wavelength range of 355-364 nm for AMLB and 312-322 nm for HCT (fig. 2). X values (i.e. Area under curve divided by concentration of analyte) for AMLB and HCT was calculated independently and the concentration of AMLB and HCT was estimated by simultaneous equations. CAMLB = (XHCT at 355-364 × AUCHCT at 312-322 ×- XHCT at 312-322 × AUCAMLB at 355-364)/ (XHCT at 355-364 × XAMLB at 312-322 - XHCT at 312-322 × XAMLB at 355-364), and CHCT = (XAMLB at 312-322 × AUCAMLB at 355-364 – XAMLB at 355-364 × AUCHCT at 312-322)/ (XHCT at 355-364 × XAMLB at 312- 322 - XHCT at 312-322 × XAMLB at 355-364)
Where, CAMLB and CHCT- Concentration of AMLB and HCT, respectively, AUCAMLB and AUCHCT- Area under curve of AMLB and HCT in bulk mixture-I or tablet solution-I. Similar procedure was applied for determination AMLB and HCT in tablet solution-I[25].
Estimation of VAT in presence of AMLB and HCT by standard curve method
The absorbance of standard VAT solutions at different concentration ranging from 5-25 μg/ml at 250 nm was measured. The regression equation was established by plotting the calibration curve of absorbance Vs concentration. The absorbance of bulk mixture-II and tablet solution-II was measured at 250 nm for VAT. The concentration of VAT was estimated by regression equation, y = 0.0602x + 0.0143[22]. Where, y– Absorbance of VAT in bulk mixture-II or tablet solution-II, x– Concentration of VAT in bulk mixture- II or tablet solution-II
Specificity
The specificity of the method was investigated by observing any interference of one drug with other two drugs in bulk mixture and tablet solution. Similarly the interference of excipients of tablet with drugs was investigated.
Linearity and range
The linearity of method is its ability within a given range to obtain test results which are directly or through a mathematical transformation, proportional to the concentration of analyte. Linearity of the method was determined at five concentration levels for AMLB, HCT and VAT independently.
Accuracy
The accuracy of an analytical method is the closeness of the test results to the true value. It was tested by spiking standard AMLB solution in different concentration 80, 100 and 120% to a tablet solution.The tablet solution was analyzed at 359 nm for estimation of AMLB. Similarly, the accuracy for HCT and VAT was determined at 317 nm and 250 nm, respectively./p>
Precision
The intra-day precision (repeatability) of method was determined by measuring the absorbance of tablet solution-I at 359 and 317 nm for AMLB and HCT, respectively. within a laboratory over a short period of time. The inter-day precision (intermediate precision) was determined by measuring the absorbance of tablet solution-I at 359 and 317 nm for AMLB and HCT, respectively. within a laboratory on three consecutive days, by different analysts. The %RSD was calculated for intra and inter-day precision. Similar procedure was applied for determination of precision in tablet solution-II for VAT at 250 nm.
LOD and LOQ
The LOD of an analytical method is the lowest amount of analyte in a sample which can be detected but not necessarily quantified. The detection limit (DL) of method was determined by equation, DL = (3.3 σ)/S, where, σ– standard deviation of blank response, S– slope of the calibration curve. The quantitation limit (QL) of analyte was determined by equation DL = (10 σ)/S, where, σ– standard deviation of blank response, S– slope of the calibration curve
Robustness and ruggedness
Robustness and ruggedness of the method has been evaluated at four different levels i.e. analyst, days, wavelength (±2 nm) and pH (±0.5). pH of solution was changed using 0.1 N hydrochloric acid or 0.1 N sodium hydroxide.
Results and Discussion
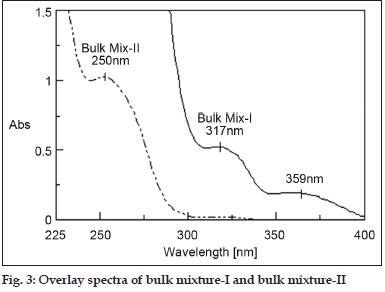
The absorbance wavelength for AMLB, HCT and VAT was found to be 359, 317 and 250 nm, respectively, which are different and hence non-overlapping. Thus simultaneous determination of AMLB and HCT in presence of VAT in bulk mixture-I and tablet solution-I was found to be successful by simultaneous equation, Q-analysis and area under curve method. The concentration of VAT in bulk mixture-I and tablet solution-I was 160 μg/ml which is not in the linearity range and hence it was diluted 10 times so that the concentration of VAT should be in the range of 5-25 μg/ml. At this concentration of VAT, the concentration of AMLB and HCT was 1 and 2.5μg/ ml which is negligible and thus estimation of VAT in bulk mixture-II and tablet solution-II was found to be successful. Zero order spectra of the bulk mixture were shown in fig. 3. The negligible absorption of HCT and VAT at a wavelength of 359 nm made it easier for the estimation of AMLB. Similarly the negligible absorption of AMLB and VAT at wavelength of 317 nm made easier for determination of HCT.
The analysis of AMLB and HCT in presence of VAT at 359 and 317 nm in bulk mixture-I and tablet solution-I was performed by simultaneous equation, Q-analysis and area under curve method. The analysis of VAT at 250 nm in bulk mixture-II and tablet solution-II was performed by standard curve method. The results were found to be in between 99.6–102.5% with ≤1.58% RSD given in the Table 1.
| Method | Sample | Contents | Amount present (µg/ml) | Amount found (µg/ml) | % Mean found*±SD | % RSD |
|---|---|---|---|---|---|---|
| Simultaneous equation | Bulk mix.-I | AMLB | 10 | 10.02 | 100.2±0.84 | 0.84 |
| HCT | 25 | 25.3 | 101.2±0.89 | 0.88 | ||
| Tab. sol.-I | AMLB | 10 | 10.23 | 102.3±0.38 | 0.35 | |
| HCT | 25 | 25.37 | 101.5±0.08 | 0.08 | ||
| Q- analysis | Bulk mix.-I | AMLB | 10 | 10 | 100.0±1.58 | 1.58 |
| HCT | 25 | 25 | 100.0±0.40 | 0.40 | ||
| Tab. sol.-I | AMLB | 10 | 10.12 | 101.2±0.39 | 0.36 | |
| HCT | 25 | 25.43 | 101.7±0.04 | 0.04 | ||
| Area under curve | Bulk mix.-I | AMLB | 10 | 9.96 | 99.6±1.52 | 1.52 |
| HCT | 25 | 25.1 | 100.4±0.48 | 0.48 | ||
| Tab. sol.-I | AMLB | 10 | 10.25 | 102.5±0.51 | 0.46 | |
| HCT | 25 | 25.18 | 100.7±0.04 | 0.04 | ||
| Standard curve | Bulk mix.-II | VAT | 16 | 16.19 | 101.2±1.50 | 1.44 |
| Tab. sol.-II | VAT | 16 | 16.29 | 101.8±0.09 | 0.09 |
Table 1: Estimation of amlb, hct and vat in bulk mixture and tablet by different uv Spectroscopic methods
Validation of method was carried out as per the ICH guidelines[25]. Specificity of method was determined as there is no interference of AMLB, HCT and VAT with one another during the determination at 359, 317 and 250 nm, respectively. No interference of excipients in tablet was observed during analysis. Hence the method was found to be specific. The analytical range was found to be 5-25, 10-50 and 5-25 μg/ml for AMLB, HCT and VAT, respectively and it is linear. An excellent correlation coefficients were obtained for each drug within the analytical range with r2 = 0.999, 0.999 and 0.999 for AMLB, HCT and VAT, respectively. The regression equations for AMLB, HCT and VAT were y = 0.021x – 0.000, y = 0.019x – 0.004 and y = 0.060x + 0.014, respectively. This demonstrates a significant correlation between the concentration of analytes and detector response and hence the method is suitable for analysis. Accuracy of the method was tested by adding drugs of three different concentrations ranging from 80−120%. The recovery range was from 99.32–102.2% with ≤0.97 % RSD indicates that the method gives sufficient accuracy given in Table 2. The intra-day and inter-day precision of the method was evaluated using % RSD The intra-day % RSD for simultaneous equation, Q-analysis and area under curve method was found to be ≤2.03, ≤0.50 and ≤1.23, respectively for both AMLB and HCT. The inter-day % RSD for simultaneous equation, Q-analysis and area under curve method was found to be ≤0.97, ≤0.15 and ≤0.45, respectively for both AMLB and HCT. The intra and inter-day % RSD for standard curve method determining VAT was found to be ≤0.82 and ≤0.79, respectively. The method was found to be precise. The LOD of AMLB, HCT and VAT was found to be 0.51, 0.91 and 1.57 μg/ml, respectively. The LOQ of AMLB, HCT and VAT was found to be 1.68, 3.02 and 4.77 μg/ml, respectively. The robustness and ruggedness of method was evaluated with % RSD For simultaneous equation method, the % RSD at different days, analyst, wavelength and pH was found to be ≤0.91, ≤0.97, ≤0.67 and ≤1.85, respectively for both AMLB and HCT. The % RSD for Q-analysis method at different days, analyst, wavelength and pH was found to be ≤0.91, ≤0.97, ≤0.67 and ≤1.85, respectively for both AMLB and HCT. The % RSD for area under curve method at different days, analyst, wavelength and pH was found to be ≤0.56, ≤0.46, ≤0.40 and ≤0.80, respectively for both AMLB and HCT. The % RSD for standard curve method at different days, analyst, wavelength and pH was found to be ≤0.50, ≤0.83, ≤0.67 and ≤1.36, respectively for VAT. The developed method has sufficient robustness and ruggedness.
| Method | Simultaneous equation | Q- analysis | Area under curve | Standard curve | |||
|---|---|---|---|---|---|---|---|
| Level | Tab. sol.-I | Tab. sol.-I | Tab. sol.-I | Tab. sol.-II | |||
| AMLB | HCT | AMLB | HCT | AMLB | HCT | VAT | |
| 80*% | 99.46±0.45 | 99.32±0.41 | 99.46±0.43 | 99.33±0.32 | 100.5±0.56 | 100±0.07 | 101.6±0.65 |
| 100*% | 100.5±0.46 | 99.37±0.35 | 100.1±0.12 | 100.2±0.18 | 100.9±0.33 | 101.2±0.45 | 100.5±0.47 |
| 120*% | 101.9±0.97 | 102.2±0.43 | 100.9±0.55 | 100.3±0.69 | 100.7±0.44 | 100.4±0.51 | 100.7±0.77 |
Table 2: Accuracy Study With Tablets
The validation of method with reference to all above parameters showed % RSD less than 2% and found to be satisfactory. The solutions were stable throughout the study. A UV spectrophotometric method was developed for estimation of AMLB, VAT and HCT in combination. The method was found to be simple, accurate, precise, economic and specific for determination and quantitation of these drugs in bulk mixture and tablet formulation.
Acknowledgements
The authors express their gratitude to Padmashree Mrs. Fatma Rafiq Zakaria, Chairman of Maulana Azad Educational Trust, Aurangabad for encouragement and the laboratory facilities. The authors are also thankful to ATL Research center, Hyderabad, India for providing gift samples of pure drugs.
References
- Available from:http://www.pharma.us.novartis.com/product/pi/pdf/ exforge_hct.pdf [Last accessed 2009 Aug 01].
- Ragno G, Garofalo A, Vetuschi C. Photo degradation monitoring ofamlodipine by derivative spectrophotometry. J Pharm Biomed Anal2002;27:19-24.
- Rahman N, Azmi SN. Spectrophotometric method for the determinationof amlodipine besylate with ninhydrin in drug formulations. Farmaco2001;56:731-5.
- Bahrami GH, Mirzaeei SH. Simple and rapid HPLC method fordetermination of amlodipine in human serum with fluorescencedetection and its use in pharmacokinetic studies. J Pharm Biomed Anal2004;36:163-8.
- Chaudhari BG, Patel NM. Development and validation of HPLCmethod for simultaneous estimation of atorvastatin calcium andamlodipine besylate. J Pharm Res 2006;5:141-4.
- Chaudhari BG, Patel NM, Sham PB. Stability indicating RP-HPLCfor simultaneous determination of atorvastatin calcium and amlodipinebesylate from their combination drug products. Chem Pharm Bull2007;55:241-6.
- Chitlange SS, Mohammed I, Sakarkar DM. RP-HPLC method forsimultaneous estimation of amlodipine and metoprolol in tabletformulation. Asian J Pharm 2008;2:232-4.
- Agrekar AP, Powar SG. RP-HPLC determination of ramipril andamlodipine in tablets. J Pharm Biomed Anal 2000;21:1137-42.
- Ilango K, Kumar PB, Prasad VR. Simple and rapid high performancethin layer chromatographic estimation of amlodipine frompharmaceutical dosage forms. Indian J Pharm Sci 1997;59:336-7.
- Doshi AS, Bhagwan SS, Mehta TN. Determination of nebivolol andvalsartan in a fixed-dose combination by liquid chromatography.J AOAC Int 2008;91:292-8.
- Atana E, Altinay A. Simultaneous determination of valsartanand hydrochlorothiazide in tablets by first-derivative ultravioletspectrophotometry and LC. J Pharm Biomed Anal 2001;25:1009-13.
- Tatar S, Saglik S. Comparison of UV and second derivativespectrophotometric and LC methods for the determination of valsartanin pharmaceutical formulation. J Pharm Biomed Anal 2002;30:371-5.
- Piao ZZ, Lee ES. Improved analytical validation and pharmacokineticsof valsartan using HPLC with UV detection. Arch Pharm Res2008;31:1055-9.
- Ivanovic D, Malenovic A, Jancic B, Medenica M, Ma?kovic M.Monitoring of impurity level of valsartan and hydrochlorothiazideemploying an RP-HPLC gradient mode. LiqChromatogrRel Tech2007;30:2879-90.
- Erk N. Spectrophotometric analysis of valsartan andhydrochlorothiazide. Anal Lett 2002;35:283-302.
- Ramani AV, Sengupta P, Mullangi R. Development and validation of ahighly sensitive and robust LC-ESI-MS/MS method for simultaneousquantitation of simvastatin acid, amlodipine and valsartan in humanplasma: Application to a clinical pharmacokinetic study. BiomedicalChromatogr 2009;23:615-22.
- Dinc E, Ustundag O. Chemometric resolution of a mixture containinghydrochlorothiazide and amiloride by absorption and derivativespectrophotometry. J Pharm Biomed Anal 2002;29:371-9.
- Shah SA, Rathod IS, Suhagia BN. Simultaneous determinationof losartan and hydrochlorothiazide in combined dosage formsby first-derivative spectroscopy and high-performance thin-layerchromatography. J AOAC Int 2001;84:1715-23.
- Liu F, Xu Y, Gao S, Zhang J. Determination of hydrochlorothiazide inhuman plasma by liquid chromatography/tandem mass spectrometry.J Pharm Biomed Anal 2007;44:1187-91.
- Tagliari MP, Stulzer HK, Murakami FS. Development and validation ofa stability-indicating LC method to quantify hydrochlorothiazide in oralsuspension for pediatric use. Chromatographia 2008;67:647-52.
- Rane VP, Sangshetti JN, Shinde DB. Simultaneous highperformanceliquid chromatographic determination of telmisartan andhydrochlorothiazide in pharmaceutical preparation. ChromatogrSci2008;46:887-91.
- Anandakumar K, Jayamariappan M. Absorption correction methodfor the simultaneous estimation of Amlodipine besylate, valsartan andhydrochlorothiazide in bulk and in combined tablet dosage form. Int JPharm PharmSci 2011;3:23.
- Nikam MB, Dhamane H, Aligave A, Kondawar MS. Simultaneousestimation of valsartan, amlodipine besylate and hydrochlorothiazideby first order derivative UV Spectrophotometric method. Int J PharmTechnol 2010;2:642.
- Beckett AH, Stenlake JB. Principle Pharmaceutical Chemistry. NewDelhi: CBS Publishers; 2004. p. 275-314.
- Available from: www.ich.org/products/guidelines/quality/article/qualityguidelines .html [Last accessed on 2011 Jul 15]