- *Corresponding Author:
- Hongtao Lei
Guangdong Laboratory for Lingnan Modern Agriculture, Guangzhou 510642, China
E-mail: hongtao@scau.edu.cn
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “73-80” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Polyclonal antibodies that recognize sibutramine and an indirect competition enzyme-linked immunosorbent assay were prepared to detect the content of Western butramine to be tested. Immunogen and coating progen were obtained by conjugation with bovine serum albumin and ovalbumin by active ester method with 1-(4-phenol) cyclobutamine as hapten, and the New Zealand white rabbit was immunized after the conjugate was successfully conjugated by ultraviolet scanning, and the obtained antibody serum was screened by indirect competition enzyme-linked immunosorbent assay detection to screen out the best antibody, and then the coating progen was replaced with 5-hydroxytryptamine-ovalbumin to compare the titer and inhibition rate, and the heterologous coating effect was better, and the heterologous coating was used for follow-up experiments. By changing the concentration of coating and antibody, the inhibition rate was found when the coating was 31.25 ng/ml and the antibody was diluted 8000 times. The antibody had the highest sensitivity for the two standards at the half maximal inhibitory concentration of 4.89 ng/ml, a limit of detection of 0.21 ng/ml, and a detection range of 0.68-35.21 ng/ml for the 8000 fold-dilution of the original 31.25 ng/ml and an 8000-fold dilution of the antibody. The recovery under these conditions ranged between 88 % and 105 %, which was within a reasonable range, indicating that the rapid enzyme linked immunosorbent assays was successfully established.
Keywords
Sibutramine, polyclonal antibody, enzyme linked immunosorbent assay, ovalbumin, norepinephrine
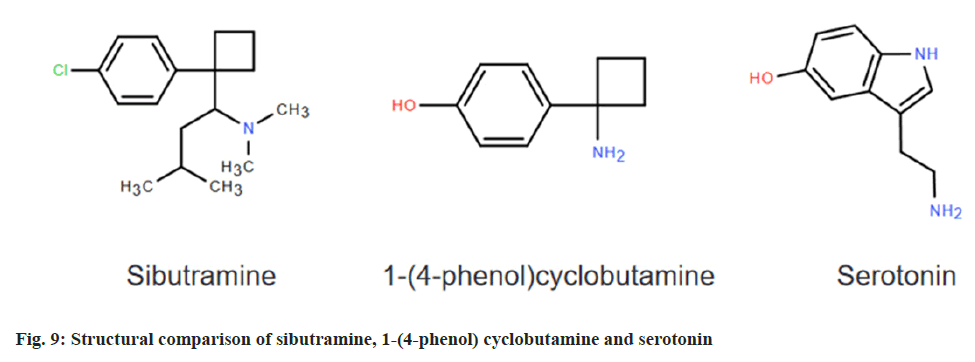
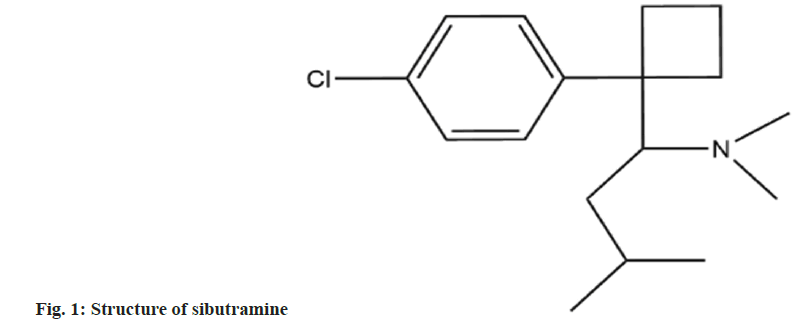
Sibutramine, chemically known as N-(1-(1-(4-chlorophenyl) cyclobutyl)-3-methylbutyl)-N,N-dimethylamine (fig. 1), is a drug approved by the United States of America (USA)[1]. Food and drug administration in 1997 for the long-term treatment of obesity[2]. The drug acts as a norepinephrine inhibitor and plays a role in weight loss by inhibiting nerve centers, reducing appetite, etc.,[3]. But with the passage of time, its safety and side effects have gradually attracted attention[4]. In 2010, the European Union’s committee on medicinal products for human use recommended a moratorium on the sale and use of all weight loss drugs containing sibutramine in the EU; In the same year, the US[5] Food and Drug Administration also announced a ban on the sale and use of sibutramine; China’s food and drug administration also required the domestic production, sale and use of sibutramine preparations and Active Pharmaceutical Ingredient (API) in 2010. However, due to its obvious weight loss effect and low cost, some unscrupulous traders will add sibutramine to apple cider vinegar, coffee and other drinks with weight loss effect to enhance their weight loss effect, so that it is favored by more consumers, so it is necessary to establish a method to detect sibutramine content[6]. At present, the detection methods for sibutramine mainly include instrumental analysis represented by high performance liquid chromatography and capillary electrophoresis and immunoassay represented by Enzyme-Linked Immunosorbent Assay (ELISA)[7]. Although instrumental analysis can achieve accurate detection of analytes, it is only suitable for laboratory quantitative analysis due to its complex sample preparation, expensive instruments and equipment, and long detection time[8]. The immunoassay method is simple to operate, low detection cost and fast detection speed, which is suitable for on-site large-scale rapid testing of samples, and can better meet the testing work of consumers, enterprises and regulatory departments[9]. At present, most of the ELISA methods used for sibutramine are used as hapten for antibody preparation, and the sibutramine analogue 1-(4-phenol) cyclobutamine as shown in fig. 2, was selected as the hapten for the establishment of enzyme-linked immunoassay method[10,11].
Materials and Methods
Materials:
Materials and reagents: Bovine Serum Albumins (BSA), chicken Ovalbumin (OVA), 1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide (EDC), N,N-Dimethylamide (DMF), N-Carbonylsuccinimide (NHS), Freund’s adjuvant, Freund’s incomplete adjuvant, Horseradish Peroxidase (HRP)-sheep anti-rabbit Immunoglobulin G (IgG) enzyme-labeled secondary antibody were purchased from Sigma in the USA; sibutramine standard was purchased from TM standard in the USA; 1-(4-Phenol) cyclobutamine was purchased from Shanghai Ennuo Biotechnology Co., Ltd., apple cider vinegar and canned coffee are purchased from local supermarkets.
Instruments: 6K15 refrigerated centrifuge (Sartorius-Sigma, United States of America (USA)); ND1000 Ultra Violet (UV) spectrophotometer (Thermo Company, USA); M2E multifunctional microplate reader (molecular device, USA); DK-8D electric heating constant temperature sink (Shanghai Medical Constant Temperature Equipment Factory); Single-channel and 12-channel pipettes (Eppendorf, USA); QL-861 oscillator (Haimen Qilin Bell Instrument Co., Ltd.).
Buffers: Phosphate Buffered Saline (PBS) (0.01 mol/l pH 7.4), coating buffer (0.1 mol/l carbonate buffer, pH 9.6), wash Phosphate-Buffered Saline with Tween 20 (PBST) (0.02 mol/l PBS), chromogenic buffer (phosphate-citric acid buffer), blocking solution (5 % skimmed milk powder), 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate solution, stop solution (10 % Sulfuric Acid (H2SO4)), and chromogenic solution were prepared according to the standard method. PBS (0.01 mol/l pH 7.4), coating buffer (0.1 mol/l carbonate buffer, pH 9.6), wash buffer PBST (0.02 mol/l PBS), chromogenic buffer (phosphate-citric acid buffer), blocking solution (5 % skimmed milk powder), TMB substrate solution, stop solution (10 % H2SO4), and chromogenic solution were prepared according to the standard method.
Methods:
Preparation of artificial antigens: Using the active ester method, 0.36 mg of 1-(4-phenol) cyclobutamine and 0.18 mg of 5-hydroxytryptamine were dissolved in 0.125 ml of Dimethylformamide (DMF), 5 mg of N-Hydroxysuccinimide (NHS) was dissolved in 0.25 ml of DMF, 5 mg of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide (EDC) was dissolved in 0.5 ml of DMF, mixed and stirred overnight at 16° in the dark, and the supernatant was obtained by centrifugation at 4° and 6000 rpm/ min for 8 min. 3 mg of BSA was dissolved in 2 ml of coating buffer, and 2 mg of OVA was dissolved in 1 ml of coating buffer. 0.15 ml and 0.1 ml supernatants were slowly added to the BSA/OVA mixture, respectively, and the reaction was stirred for 3 h at room temperature. Dialysis was carried out with 0.85 % sodium chloride solution for 3 d (dialysate was changed every 12 h), the immunogen 1-(4-phenol) cyclobutamine-BSA was obtained by measuring to 3 ml with the coating solution, and the original 1-(4-phenol) cyclobutylamine-OVA and 5-hydroxytryptamine were obtained by measuring the volume to 2 ml with the coating solution. Use an UV Visible (UV-Vis) spectrophotometer to scan the conjugate at full wavelength to confirm whether the conjugate was successfully conjugated.
Preparation of polyclonal antibody: Healthy New Zealand white rabbits at 12 w were selected for immunization. The first immunization was emulsified with immunogen and equal amount of Freund’s complete adjuvant, and subcutaneous multi-point injection was used for immunization, and each immunogen was immunized with 720 μl. Follow-up immunization was emulsified with Freund’s incomplete adjuvant every 3 w, and blood was taken from the orbit 10 d after the fourth immunization, and the antiserum was purified by caprylic-saturated ammonium sulfate salting out method for follow-up testing.
ELISA procedure: The 96-well plates were coated with the coating antigen (50 ng/ml, 100 μl/well) in carbonate buffer (0.05 mol/l, pH 9.6) and kept at 37° overnight. After washing 2 times with PBST, the plates were blocked with 5 % skim milk in PBS (120 μl/well) for 3 h. After blocking, the solution was poured out and the plates were dried at 37° overnight. Sibutramine standard solution (50 μl/well) and sibutramine antibody diluted with PBST (0.01mol/l, pH 7.4) were added to the wells. After incubation for 40 min at 37°, the plates were washed 5 times with PBST. HRP-IgG diluted 1:5000 in PBST (100 μl/well) was then added to the wells and incubated for 30 min at 37°. The plates were washed 5 times with the PBST solution, and the TMB solution (100 µl/well) was added to the wells and incubated for 10 min at 37°. The reaction was stopped by addition of 2 mol/l H2SO4 (50 μl/well), and the absorbance was recorded at 450 nm. The best antibody is selected based on the titer and inhibition rate of each antibody.
Determination of appropriate coating concentration and antibody dilution: The checkerboard method was used to determine the appropriate coating concentration and antibody dilution factor. The microplate was enriched with coating progens diluted from 1 μg/ml to 0.03125 μg/ ml in each column, antibody serum diluted from 1000 to 64 000 was added to each row of the microplate plates, 50 μl of PBST per well was added to the titer group, and 50 μl of sibutramine standard diluted to 1 μg/ml per well in the inhibition group was added to each well for indirect competition ELISA (ic-ELISA).
Establishment of standard curves: The three most suitable combinations were selected, the sibutramine standard was diluted from 1 mg/ml to 0.001 μg/ml with PBST, 50 μl of serum and 50 μl of standard were added to each well for ic-ELISA, and the logistic function of origin software was used to fit the standard curve of sibutramine for each combination, and the optimal combination was compared.
Recovery experiments: 2 ng, 1 ng, and 0.5 ng of sibutramine standard were added to 1 ml of apple cider vinegar and canned coffee for detection, respectively[12-17].
Results and Discussion
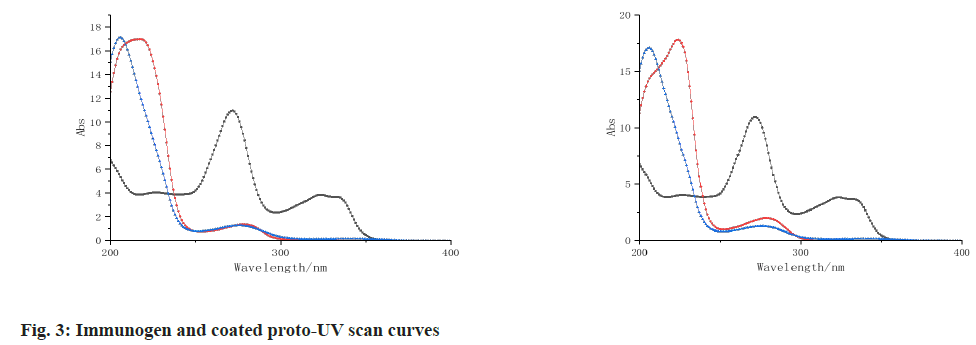
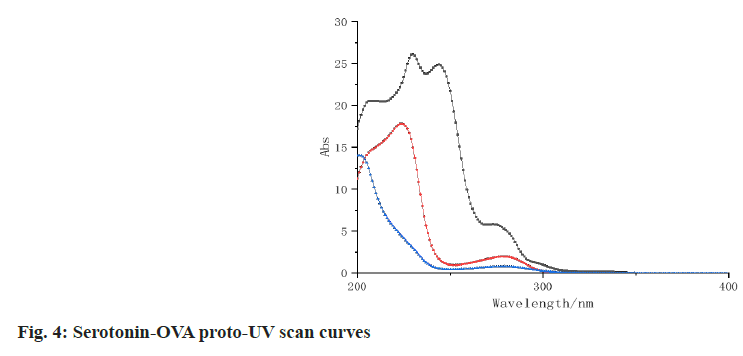
The UV scans of 1-(4-phenol) cyclobutylamine, 5-hydroxytryptamine, BSA, OVA, immunogen 1-(4-phenol) cyclobutylamine-BSA, coated proto-1-(4-phenol) cyclobutylamine-OVA and 5-hydroxytryptamine-OVA are shown in fig. 3 and fig. 4, 1-(4-phenol) cyclobutamine has an absorption peak at 287 nm, 5-hydroxytryptamine has an absorption peak at 289 nm, BSA has an absorption peak at 260 nm and OVA has an absorption peak at 279 nm. The absorption peaks of the three conjugates were significantly different from those of the reactants, indicating that the three conjugates were successfully coupled.
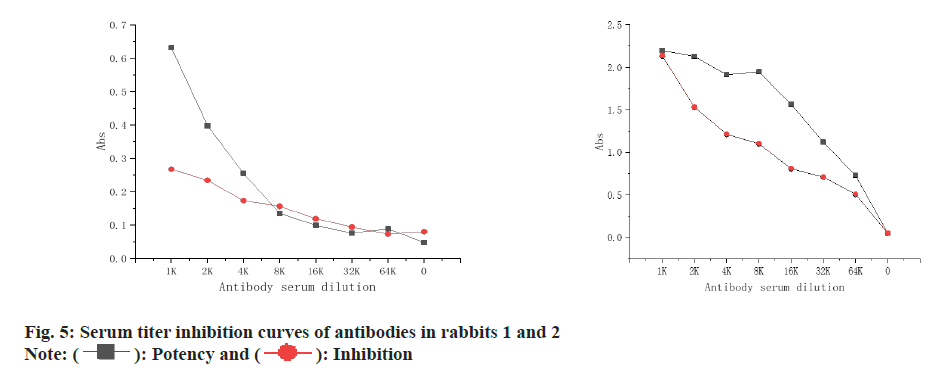
The titer inhibition curve of rabbit antibody serum 1 and 2 was shown in fig. 5, and it can be seen that the titer and inhibition rate of rabbit 2 are higher than those of rabbit 1, so the effect of rabbit antibody serum 2 is better, so rabbit antibody serum 2 is used as the best antibody as shown in fig. 6.
The homologous and heterologous coating results are shown in Table 1 and Table 2, and the combination of absorbance between 1.2-1.5 and inhibition rate of >80 % is usually selected, so three combinations of (0.125, 16000), (0.0625, 16000) and (0.03125, 8000) are selected to establish the standard curve.
| Serum dilution | Coated original concentration (µg/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 0.25 | 0.125 | 0.0625 | 0.03125 | |||||||
| Potency | Inhibition rate | Potency | Inhibition rate | Potency | Inhibition rate | Potency | Inhibition rate | Potency | Inhibition rate | Potency | Inhibition rate | |
| 1000 | 0.699 | 63.81 % | 0.695 | 69.64 % | 0.602 | 60.96 % | 0.832 | 63.32 % | 0.542 | 59.23 % | 0.599 | 54.92 % |
| 2000 | 0.349 | 58.45 % | 0.31 | 54.52 % | 0.34 | -7.06 % | 1.071 | 61.34 % | 0.972 | 68.11 % | 0.401 | 42.64 % |
| 4000 | 0.284 | 16.20 % | 0.251 | 28.69 % | 0.258 | -25.97 % | 0.991 | 67.27 % | 0.533 | 64.17 % | 0.284 | 26.06 % |
| 8000 | 0.169 | 40.24 % | 0.23 | 13.48 % | 0.179 | -6.15 % | 0.584 | 75.00 % | 0.238 | -180.67 % | 0.217 | 18.89 % |
| 16 000 | 0.2 | -27.00 % | 0.145 | -19.31 % | 0.207 | 21.74 % | 0.249 | 28.11 % | 0.168 | -5.36 % | 0.188 | 20.21 % |
| 32 000 | 0.189 | -59.79 % | 0.119 | -233.61 % | 0.183 | 26.23 % | 0.156 | 10.90 % | 0.166 | -113.86 % | 0.171 | -127.49 % |
| 64 000 | 0.102 | 8.82 % | 0.092 | -16.30 % | 0.121 | -12.40 % | 0.13 | -1.54 % | 0.166 | 16.87 % | 0.676 | 57.99 % |
| 0 | 0.062 | 4.84 % | 0.194 | 58.76 % | 0.078 | -11.54 % | 0.087 | -10.34 % | 0.094 | -11.70 % | 0.231 | 27.27 % |
Table 1: Inhibition Rate of Sibutramine by Homologous Coating with Different Coating Concentrations and Antibody Dilution Factors
| Serum dilution | Coated original concentration (µg/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 0.25 | 0.125 | 0.0625 | 0.3125 | |||||||
| Potency | Inhibition rate | Potency | Inhibition rate | Potency | Inhibition rate | Potency | Inhibition rate | Potency | Inhibition rate | Potency | Inhibition rate | |
| 1000 | 1.501 | -18.65 % | 1.89 | -8.89 % | 1.7 | -9.29 % | 2.127 | 11.00 % | 1.803 | 28.51 % | 1.539 | 28.01 % |
| 2000 | 1.769 | 11.98 % | 2.156 | 14.84 % | 1.902 | 10.52 % | 1.894 | 16.47 % | 1.832 | 38.21 % | 1.795 | 52.26 % |
| 4000 | 1.482 | 39.00 % | 1.714 | 32.56 % | 1.868 | 43.58 % | 1.539 | 40.16 % | 1.455 | 59.73 % | 1.263 | 61.20 % |
| 8000 | 1.047 | 50.62 % | 1.351 | 54.48 % | 1.399 | 52.75 % | 1.351 | 59.29 % | 1.128 | 63.65 % | 0.91 | 68.68 % |
| 16 000 | 0.509 | 47.54 % | 0.736 | 57.20 % | 0.71 | 55.21 % | 0.737 | 64.99 % | 0.576 | 67.53 % | 0.537 | 26.26 % |
| 32 000 | 0.281 | 38.43 % | 0.35 | 41.14 % | 0.349 | 49.57 % | 0.39 | 57.18 % | 0.334 | 56.29 % | 0.287 | 52.96 % |
| 64 000 | 0.176 | 28.41 % | 0.227 | 43.17 % | 0.302 | 51.66 % | 0.222 | 47.75 % | 0.2 | 50.50 % | 0.163 | -43.56 % |
| 0 | 0.08 | -166.25 % | 0.091 | 7.69 % | 0.1 | -9.00 % | 0.096 | 3.13 % | 0.086 | -6.98 % | 0.082 | -62.20 % |
Table 2: Inhibition Rate of Sibutramine by Heterologous Coating with Different Coating Concentrations and Antibody Dilution Factors
The results are shown in fig. 7 and Table 3, when the coating was 0.125 ng/ml and the antibody was diluted at 4000-fold, the sensitivity to the two standards was the highest, the semi-inhibitory concentration half maximal Inhibitory Concentration (IC50) was 61.83 ng/ml, the detection limit IC10 was 14.29 ng/ml, and the detection range IC20-IC80 was (1.78-222.42) ng/ ml, so the combination of (0.125, 1000) was selected to establish a rapid detection method.
| Parameter | (0.125, 1K) | (0.125, 2K) | (0.125,4K) |
|---|---|---|---|
| IC50 (ng/ml) | 61.83 | 61.76 | 74.79 |
| LOD (ng/ml) | 14.29 | 14.31 | 14.29 |
| (IC20-IC80) (ng/ml) | 1.78-222.42 | 1.79-222.98 | 8.45-256.21 |
Note: (LOD): Limit of Detection
Table 3: Comparison of the Parameters of the Three Standard of the Homologous Coating
As shown in fig. 8 and Table 4, when the heterologous coating was 0.03125 μg/ml and the antibody was diluted 8000 times, the sensitivity to sibutramine standard was the highest, with a semi-inhibitory concentration of IC50 of 4.89 ng/ml, a detection limit of IC10 of 0.21 ng/ml, and a detection range of IC20-IC80 of 0.68-35.21 ng/ml, so a combination of (0.03125, 8000) was selected to establish a rapid detection method.
| Parameter | (0.125, 16K) | (0.0625, 16K) | (0.03125, 8K) |
|---|---|---|---|
| IC50 (ng/ml) | 7.46 | 6.94 | 4.89 |
| LOD (ng/ml) | 0.35 | 0.27 | 0.21 |
| (IC20-IC80) (ng/ml) | 2.02-27.59 | 0.79-60.87 | 0.68-35.21 |
Note: (LOD): Limit of Detection
Table 4: Comparison of the Parameters of the Three Heterologous Coated Standards
The results are shown in Table 5, and the recovery rate of this experiment is between 88 % and 105 %, which is within a reasonable range, indicating that the ELISA rapid detection method has been successfully established.
| Amount added (ng/ml) | Recovery (ng/ml) | Recovery rate % | |
|---|---|---|---|
Apple cider vinegar |
2 | 2.07 | 103.5 |
| 1 | 0.94 | 94 | |
| 0.5 | 0.45 | 90 | |
Canned coffee |
2 | 1.87 | 93.5 |
| 1 | 1.05 | 105 | |
| 0.5 | 0.44 | 88 |
Table 5: Recover the Results of the Experiment
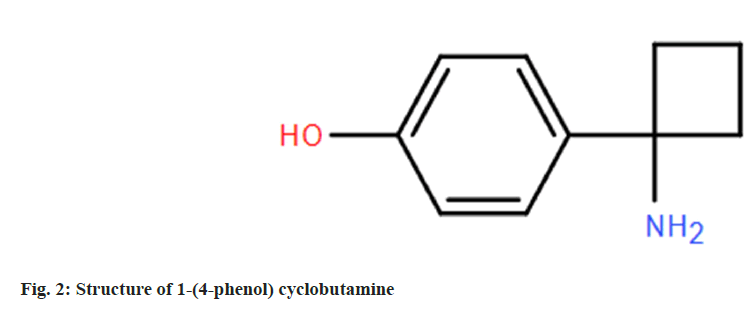
Because 1-(4-phenol) cyclobutamine (fig. 9) and serotonin themselves have amino groups, they can be directly used as small molecule haptens to link their amino groups with the carboxyl groups of carrier proteins BSA and OVA by reactive ester method to prepare immunogen 1-(4-phenol) cyclobutylamine-BSA and coated proto-1-(4-phenol) cyclobutamine-OVA and 5-hydroxytryptamine-OVA. The characteristic absorption peaks of the artificial antigen are shifted to a certain extent compared with the reactants by UV full wavelength scanning, so the successful conjugation of the artificial antigen can be determined[18]. The immunogen was used to immunize the New Zealand white rabbits, and the polyclonal antibodies were obtained by blood collection and purification, and then the titer and inhibition rate of the antibodies were detected by indirect competitive ELISA to screen for high-quality antibodies[19,20]. The titer and inhibition rate of rabbit antibody serum no. 2 were higher than those of rabbit no. 1, so rabbit antibody serum no. 2 was a high-quality antibody, and then serotonin-OVA was used as the coating source for heterologous coating, and the titer and inhibition rate of heterologous coating were higher than those of homologous coating. The second step is to establish an ELISA rapid detection method, in this paper, the chessboard method was used to obtain three ideal concentration combinations of homologous coating and heterologous coating respectively, and the corresponding standard curves were established for comparison, and the sensitivity of the antibody to the sibutramine standard was the highest under the optimal combination of heterologous coating (the original coating was 0.03125 μg/ml, and the antibody dilution was 8000 times), with a semiinhibitory concentration of IC50 of 4.89 ng/ml and a detection limit of IC10 of 0.21 ng/ml. The detection range of IC20-IC80 was 0.68-35.21 ng/ml. Finally, the recovery experiment was carried out to evaluate the rapid detection method, and the recovery rate was between 88 % and 105 %, which could meet the requirements of daily rapid detection within a reasonable range, and could be used for the rapid detection of sibutramine content.
Acknowledgements:
This work was financially supported by national key research and development program of China (2023YFF1104200, 2023YFF1104700), Guangdong Marine Economic Development Program for Six Categories of Marine Industries (No: GDNRC (2023) 36), Guangdong Natural Science Foundation (2023A1515011834), generic technique innovation team construction of modern agriculture of Guangdong province (2023KJ130), Zhongshan science and technology innovation team (CXTD2022004), natural science research project of universities in Anhui Province (2023AH052271).
Conflict of interests:
The authors declared no conflict of interests.
References
- Praoboon N, Tangkuaram T, Kruefu V, Pookmanee P, Phaisansuthichol S, Kuimalee S, et al. Fabrication of a simple 3D-printed microfluidic device with embedded electrochemiluminescence detection for rapid determination of sibutramine in dietary supplements. Microchim Acta 2023;190(4):145.
[Crossref] [Google Scholar] [PubMed]
- Elbalkiny HT, Yehia AM, Tantawy MA. A trimodal detection paper chip for undisclosed drug “sibutramine” in nutraceuticals. Anal Methods 2023;15(28):3439-48.
- Kozhuharov VR, Ivanov K, Karcheva-Bahchevanska D, Prissadova N, Ivanova S. Development and validation of gas chromatography-mass spectrometry method for quantification of sibutramine in dietary supplements. Processes 2023;11(8):2337.
- Kongsuwan A, Chuenjitt S, Phua CH, Wangchuk S, Saichanapan J, Saisahas K, et al. Sibutramine detection in weight-loss products using a sodium/phosphorus dual-doped carbon nanotubes modified electrode. Microchem J 2023;190:108668.
- Fukiwake T, Oosawa S, Yoshino H, Shinozuka T, Nishimura M, Ito R, et al. Simultaneous detection of sibutramine and phenolphthalein in a diet jelly health food product that caused health problems. Anal Sci 2023;39(4):455-61.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Huang S, Chao M, Wang Y, Liu P, Li P, et al. Highly resistant and sensitive colorimetric immunochromatographic assay for Sibutramine (SBT) illegally adulterated into diet food based on PDA/AuNP labelling. Analyst 2023;148(20):5094-104.
[Crossref] [Google Scholar] [PubMed]
- Gui Y, Zhao Y, Liu P, Wang Y, Mao X, Peng C, et al. Colorimetric and reverse fluorescence dual-signal readout immunochromatographic assay for the sensitive determination of sibutramine. ACS Omega 2024;6:7975-84.
- Ulusoy Hİ, Ulusoy S. Use of Fe3O4@ MPTMS-dithizone magnetic nanoparticles as solid phase sorbent for sensitive analysis of sibutramine molecules in herbal slimming products. Curr Anal Chem 2023;19(6):472-81.
- Suparmi S, Yuliyanti S, Karyadini HW, Syamsudin AM, Gau EK. Toxicity of sibutramine hydrochloride-adulterated weight loss supplements in rats based on biochemical and organ weight parameters. J Pharm Pharmacogn Res 2024;12(2):363-70.
- Andawiyah RA, Satriawan NE. The fabrication of test strip for sibutramine HCL detection in slimming traditional herbal medicine. Jurnal Kimia Riset 2023;8(1):27-36.
- Fakhriah SN, Mahdiyah D. I identification of sibutramine hydrochloride compounds in slimming herbal medicine. Integr Proc 2023;1(1):166-71.
- Nachalaem P, Watlaiad K, Khamphikham P. Development of dried urine samples for simultaneous quantitative detection of sibutramine and its active metabolites by liquid chromatography/mass spectrometry. Sci Technol Asia 2023;82(1):206-16.
[Crossref] [Google Scholar] [PubMed]
- Yadav L, Agrawal B, Meshram R, Ahirwar DK, Sahu SK. Rational design, synthesis and characterization of novel sibutramine derivatives as anti-obesity activity. Int J Med Pharm Sci 2023;13(10):1.
- Nejdar P, Salunkhe S, Pishawikar S, Uchgaon K. Development of validated RP-HPLC method for determination of sibutramine applying QbD approach. Int J Innov Sci Res Technol 2023;8(6):2261-4.
- Wan R, Song H, Qu G, Ren L, Zhou X, Tian Q, et al. Cardiogenic shock in a 28 y old woman associated with sibutramine use. Int J Legal Med 2024;138(3):833-8.
[Crossref] [Google Scholar] [PubMed]
- Gajratovna RA. Sibutramine in the complex therapy of obesity in patients with Hashimoto's thyroiditis; 2023.
- Fabresse N, Micallef J, Corteggiani-Giraud C, Becam J, Richez M, Solas C, et al. Complements alimentaires adulteres: Enfin une phytotherapie efficace. Toxicol Anal Clin 2024;36(1):5.
- Boger BS, Queiroz NL, Noriega PE, Canuto MC, Stumpf MA, Cercato C, et al. Treatment with antiobesity drugs in weight regain after bariatric surgery: A retrospective cohort study. Obesity Surg 2023;33(9):2941-4.
[Crossref] [Google Scholar] [PubMed]
- Azminah A, Ahmad I, Fikri JA, Jumadil MI, Erza NA, Abdullah S, et al. Rapid detection of synthetic adulterants in Indonesian herbal medicines using ATR-FTIR spectroscopy combined with chemometrics. J Res Pharm 2023;27(1):184-95.
- Lim SY, Alshawsh MA, Alshagga M, Kong C, Fang CM, Pan Y. CYP gene expression and in vivo biological effects of khat ethanol extract, cathinone and cathine in Caenorhabditis elegans. Prog Microb Mol Biol 2023;6(1).
 Inhibition
Inhibition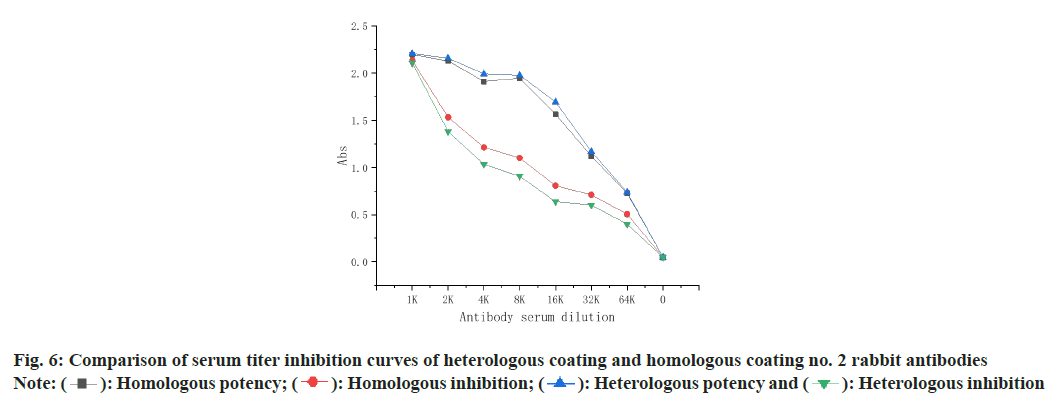
 Homologous inhibition;
Homologous inhibition;  Heterologous inhibition
Heterologous inhibition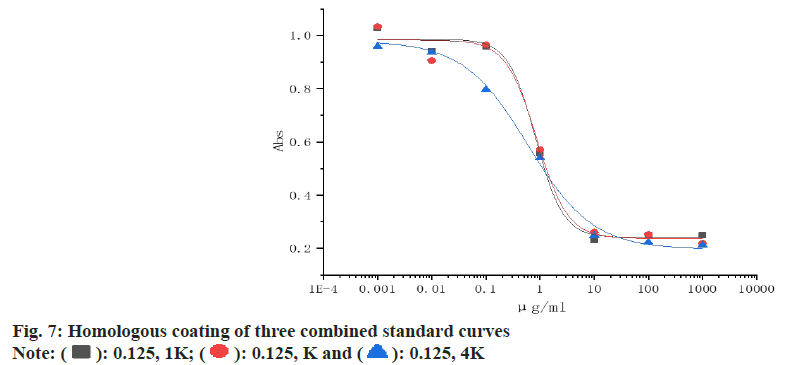
 0.125, 4K
0.125, 4K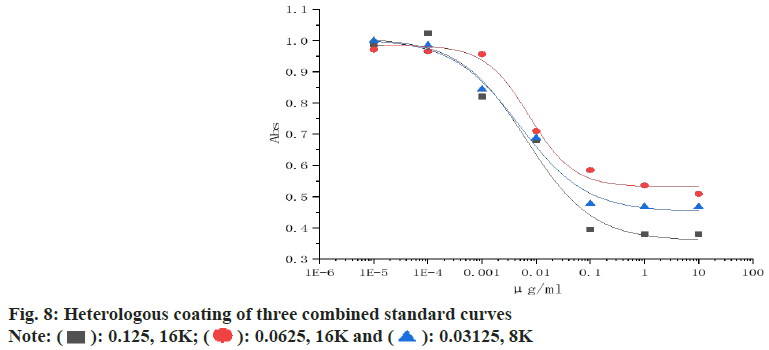
 0.03125, 8K
0.03125, 8K