- *Corresponding Author:
- M. Yao
Department of Orthopedic Surgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, 150086, China
E-mail: yaom79151640@163.com
| This article was originally published in a special issue, "Clinical and Experimental Studies on Drug and Intervention Repurposing in China |
| Indian J Pharm Sci 2019:81(4)spl issue1;58-64 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this study was to establish a new rat model of lumbar disc herniation for minimally invasive interventional surgery. Sixty Sprague Dawley rats were selected and randomly divided into three groups of 20 in each group, group A the new model group, group B model control group and group C the sham operation group. Group A sample was taken from the nucleus pulposus of the caudal vertebra and transplanted to the root of the sciatic nerve. The group B sample was taken from the nucleus pulposus of the caudal vertebra and transplanted to the L5 spinal nerve side to establish a model. The C group was only sutured after the sciatic nerve was separated, and no other treatment was performed. The mechanical pain threshold and thermal pain threshold were measured before and after 1, 3, 5, 7 and 14 days of operation, and the lower limb temperature was measured. Half of the rats in each group were sacrificed on the day 7 and 14 after operation. The expression levels of IL-8 and TNF-α were detected by immunohistochemistry in the L5 dorsal root ganglia and sciatic nerve tissues. The results showed that compared with group C, group A and group B had lower mechanical pain threshold and thermal pain threshold at 1, 3, 5, 7 and 14 days after operation and lower average limb temperature. The number of IL-8 and TNF-α positive cells in L5 dorsal root ganglia and sciatic nerve tissue increased at 7 days after operation. The number of positive cells decreased at 14 days, but it was still higher than that of group C. The difference between the two groups was significant (p>0.05); compared with group B, the mechanical pain threshold and thermal pain threshold and the temperature of lower limbs of group A tend to be consistent at 1, 3, 5, 7, and 14 d after operation, and the number of IL-8 and TNF-α positive cells tends to be consistent. There is no significant difference between the two groups (p<0.05). It could be concluded that the new rat model of lumbar disc herniation in this experiment can perfectly replicate the etiology of lumbar disc herniation. The procedure is simple and the injury is small. It provides a favorable and stable experimental model for further study of the mechanism and treatment of lumbar disc herniation in the future.
Keywords
Lumbar disc herniation, rat model, sciatic nerve, IL-8, TNF-α
As a common disease, lumbar disc herniation seriously affects the quality of life of patients. In order to study the pathophysiological mechanism of lumbar disc herniation and explore new treatment methods, researchers at home and abroad use various methods to establish animal models to simulate the process of disease development. However, the various animal models are produced in a variety of ways, and the nature and duration of the pain obtained are not the same. At present, animal models commonly used for disc herniation can be divided into 3 categories, a simple mechanical compression model, a simple inflammatory stimulation model, and a compound model combining compression and inflammatory stimuli. These 3 models have different application, and the compound animal model combining compression and inflammatory stimulation is more in line with the pathological mechanism of the disease, and is also the typical animal model at present[1,2]. Sasaki et al. simulated the inflammatory stimulation and mechanical compression of human lumbar disc herniation appropriately through the transplantation of intervertebral disc tissue and carry through mechanical compression[3]. Such a model can better induce changes in neurobehavior in rats, but the modelling operation is difficult and the normal anatomical structure of the rats is greatly damaged. Therefore, this study intended to transplant the nucleus pulposus at the sciatic nerve and conduct cerclage, to observe the advantages and disadvantages of the model.
Materials and Methods
An optical microscope (Nikon 80i, Nikon, Japan), twostep immunohistochemistry detection kits, SV0002- rabbit IgG, rabbit antitumor necrosis factor α and rabbit antiIL8 all were obtained from Wuhan Boster Biological Engineering Co., Ltd.
Experimental animals and grouping:
Sixty healthy male Sprague Dawley rats (200~250 g) were selected and randomly divided into 3 groups, with 20 rats in each group. Group A is the model group, group B served as the control group and group C the sham operated group. For group A, rats were anesthetized with 10 % chloral hydrate (Shanghai Fourth Pharmaceutical Co., Ltd.), and the skin was cut in the outer edge of the femur in the middle of one thigh to expose the main sciatic nerve. A longitudinal incision was made in the proximal part of the rat tail, and the nucleus pulposus of the rat's tail disc (about 0.4 mg) was taken out and covered on the main stem of the exposed sciatic nerve. The fibre loop was placed on the nucleus pulposus by about 0.3 mm. Three silk thread (4th size) knots spaced about 1 mm apart were evenly placed on the sciatic nerve trunk. Each ligation showed slight twitching on the ipsilateral hind leg, forming a slight compression.
Group B rats were anesthetized with 10 % chloral hydrate. The skin was cut in the medial longitudinal direction with the L5 spinous process as the center, and the bilateral L4 and L5 lamina were exposed, and the right nerve roots of L4 and L5 were revealed. The sampling of the nucleus pulposus of the rat tail is the same as above. It was then placed at the nerve root and circulated the L4 and L5 nerve roots with a 4th silk thread. The tightness of the ligation should produce a slight twitch on the ipsilateral hind leg, forming a slight compression. The sciatic nerve was isolated after anesthesia in the group C rats in the same manner, and the incision was sutured gently layer by layer without causing damage.
Detection of pain-related neurobehaviors:
Three days before surgery, the threshold of mechanical stimulation of the hind legs and the latency of thermal contracture reflex were measured, and the difference was calculated as a normal control. Neurobehavioral tests were performed on each group of animals at 1, 3, 5, 7, and 14 d after surgery.
For mechanical stimulation of hyperalgesia (PWT), von Frey fibres (Von Frey Hairs, USA) were placed against the posterior part of the right hind limb of the rat for 4 to 5 s, and the rats were lifted. Avoidance, lameness, licking footing and the like were considered positive reactions. The average value was obtained from measurements of 5 times.
Paw withdrawal thermal latency (PWL) was tested using a smart hot plate tester (Shandong, YLS-6B), and the temperature of the hot plate was set to 52°. When the rat quickly lift the foot, the time from the start to the contraction was recorded, which is PWL. Each test interval was at least 5 min, and the measurement was averaged 5 times.
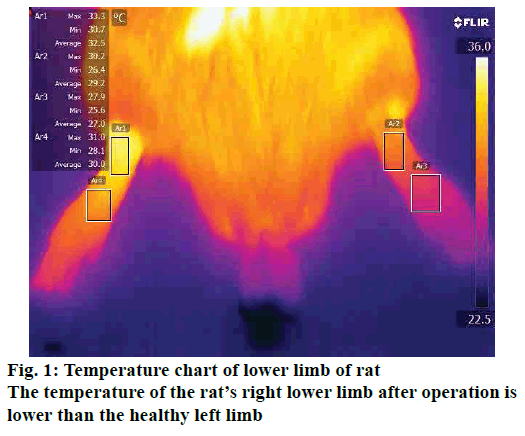
For detecting the average temperature change of lower limbs, temperature measurement of the lower limbs of both sides of the rats was measured on days 1, 3, 5, 7, 14, and 21 d after surgery. The rats were placed in a prone position and the lower limbs were placed in a naturally straight state, and the lower limbs were photographed using an infrared camera (FLIR T650sc, USA) and the average temperature was recorded.
Immunohistochemical detection of spinal ganglia and nerve roots:
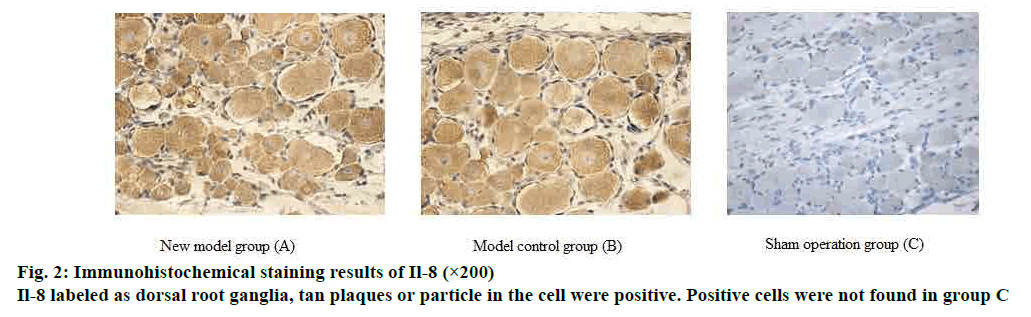
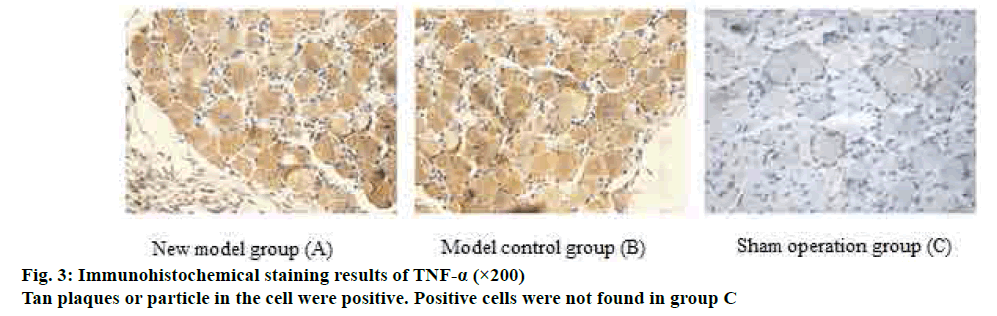
The expression levels of IL-8 and tumour necrosis factor (TNF-α) in the L5 dorsal root ganglia and sciatic nerve tissues of the rats were detected by immunohistochemistry. Fifteen rats were randomly selected and sacrificed from each group on d 7 and d 14 after surgery. L5 dorsal root ganglia and sciatic nerve were surgically removed, fixed, embedded, and sliced. Hematoxylin-eosin (HE) staining was performed, and brownish yellow particles were positive reactants.
IL-8 and TNF-α staining was measured by flaky or granular brown-yellow staining in the cytoplasm and on the nuclear membrane. Only the blue color of the nuclei of the cells was negative. The results under the microscope were graded as follows. A- staining intensity: no staining was 0 points; light yellow particles in the cytoplasm that is significantly higher than the background was 1 point; blue particles were 2 points; brown yellow particles were 3 points. B- percentage of positive (10 fields of view were randomly observed), 0 points if the number of positive cells counted was negative; ≤10 % of the number of positive cells was 1 point; 11-50 % was 2 points; 51-75 % was 3 points; >75 % was 4 points. 0-1 A+B scores was negative (-); 2 score for weak positive (+); 3-4 were positive (++); 5-7 were strongly positive (+++).
Statistical analysis:
The data were statistically processed using SPSS18.0 statistical software. The data were expressed as mean±standard deviation. One-way ANOVA was used to compare the measurement data between groups and within the group. P<0.05 was considered to be statistically significant.
Results and Discussion
In both the experimental group and the control group, the behavioural manifestations of licking foot and dangling were observed in the right hind paw with mild exstrophy. There was no significant difference in the mechanical pain threshold between the groups at 1 d before surgery (p>0.05). Except for the sham operation group, the mechanical pain threshold of the other groups showed a downward trend from 1 to 5 d after operation, and reached the lowest at 5 d after operation. Compared with before operation and group C, the difference of mechanical pain threshold was significantly lower in each group (p<0.05). Compared with group C, the mechanical pain thresholds of group A and group B increased at 7 d and 14 d after operation, but the difference was still statistically significant (p<0.05). The detailed data was shown in Table 1.
| Group | -1d | 1d | 3d | 5d | 7d | 14d |
|---|---|---|---|---|---|---|
| Group A | 5.93±0.16 | 5.24±0.18 | 4.58±0.44*? | 3.67±0.56*? | 3.92±0.55*? | 4.47±0.45*? |
| Group B | 5.94±0.15 | 5.35±0.14 | 4.83±0.41*? | 3.92±0.62*? | 4.08±0.47*? | 4.37±0.35*? |
| Group C | 5.96±0.09 | 6.00±0.09 | 6.00±0.10 | 6.03±0.08 | 6.00±0.10 | 6.05±0.07 |
| Group | -1d | 1d | 3d | 5d | 7d | 14d |
| Group A | 6.22±0.73 | 4.85±0.69 | 3.96±0.58*? | 3.14±0.63*? | 3.43±0.49*? | 3.86±0.45*? |
| Group B | 6.41±0.51 | 5.19±0.29 | 4.30±0.26*? | 3.53±0.31*? | 3.72±0.30*? | 4.18±0.27*? |
| Group C | 6.10±0.41 | 5.84±0.57 | 6.07±0.46 | 6.12±0.55 | 6.01±0.63 | 5.99±0.54 |
Note: *p<0.05 compared with before operation; ?p<0.05 compared with group C
Table 1: Mechanical pain and thermal pain threshold in each group rats of different time points (MEAN ± SD)
There was no significant difference in the thermal pain threshold between the rats in each group at 1 day before surgery (p>0.05). The thermal pain threshold of rats in all groups except the sham operation group showed a downward trend from 1 to 5 d after operation and reached the lowest at 5 d after operation. Compared with pre operation and group C, the difference of thermal pain threshold was significantly lower after modelling in each group (p<0.05). There was a statistically significant difference in the thermal pain threshold between group A and group B at 7 and 14 d after surgery (p<0.05). The detailed data was shown in Table 1.
There was no significant difference in the average temperature of lower limbs between the groups at 1 d before surgery (p>0.05). The lower limb temperature of the rats in the other groups except the sham operation group showed a downward trend from 1 to 5 d after operation and reached the lowest at 5 d after operation. There was a statistically significant difference in the lower limb temperature between the rats in each group after modeling (p<0.05). The detailed data was shown in Table 2, fig. 1.
| Group | -1d | 1d | 3d | 5d | 7d | 14d |
|---|---|---|---|---|---|---|
| left side of Group A | 29.20±0.07 | 29.36±0.22 | 29.39±0.24 | 29.26±0.48 | 29.29±0.06 | 29.32±0.02 |
| right side of Group A | 29.23±0.21 | 27.55±0.16*? | 27.24±0.08*? | 26.88±0.24*? | 27.24±0.39*? | 27.92±0.23*? |
| left side of Group B | 29.23±0.43 | 29.17±0.37 | 29.10±0.65 | 29.05±0.55 | 29.19±0.63 | 29.16±0.13 |
| left side of Group B | 29.21±0.33 | 27.45±0.38*? | 27.10±0.37*? | 26.82±0.52*? | 27.18±0.57*? | 27.83±0.51*? |
Note: mean±SD, *p<0.05 vs preoperation; ?p<0.05 vs the contralateral limb
Table 2: Temperature of lower limb in each group rats of different time points
On d 5 after operation, the number of IL-8 and TNF-α positive cells in L5 dorsal root ganglia and sciatic nerve tissue increased, and the number of positive cells decreased on d 14, but still higher than the sham operated group. The difference between the two groups was significant (p>0.05); there was no significant difference in cell positive rate between the model group and the control group from 5 and 14 d (p>0.05). The detailed data was shown in figs. 2 and 3.
Lumbar disc herniation is a common and recurrent disease and one of the main causes of sciatica[4]. Due to the high incidence rate, it has become a hot topic in current clinical research. Lumbar disc herniation is a disease caused by acute injury, accumulated strain and other factors leading to rupture of the annulus fibrosus and compression of the nerve root or spinal cord by nucleus pulposus evagination on the basis of degeneration of the intervertebral disc. Studies have shown that there are two main causes of root pain caused by herniated disc, mechanical compression of nerve roots and the physiological or biochemical activity of intervertebral disc tissue on nerve roots[5].
At present, the rat model of lumbar disc herniation used is mainly divided into three categories: simple mechanical compression model, simple inflammatory stimulation model and combining model of mechanical compression and inflammatory stimulation. Early studies have shown that mechanical compression of the spinal nerve is the cause of sciatica. Wang et al. established a chronic nerve root injury model to simulate the compression of the intervertebral disc nerve root compression model by compressing the L5 nerve roots of rats with micro-silica balls[6]. However, it was found clinically that imaging studies often show that there is no protrusion or less protrusion of disc tissue but symptoms of sciatica, suggesting that mechanical compression may not be the only cause of sciatica[7]. Animal experiments have also confirmed that nucleus pulposus can cause pain even without mechanical compression[8]. Based on the previous single factor model, Kim et al. designed a model of sciatica caused by the combination of mechanical compression and nucleus pulposus inflammatory stimuli[9]. Transplantation of the SD rat tail intervertebral disc tissue to the L5 dorsal root ganglion (DRG) directly compresses the nerve root, which simulated the pathophysiology of the disease. This model has become a well-recognized one that is commonly used at this stage. However, this model has disadvantages such as complicated operation, great damage to the normal anatomy of rats, and difficulty in surgical intervention in the later stage. The new model developed in this investigation is an improvisation on the basis of this model. Since rat L4 and L5 were constantly transferred into the sciatic nerve, the sciatic nerve was used for nucleus pulposus transplantation and compression[10]. In addition, this study was based on the CCI model. The classical CCI model uses a chromic catgut for cerclage, and this experiment used silk thread for cerclage to prevent the catgut from interfering with the behavioural thermal pain threshold[11]. A new rat model of lumbar disc herniation was established using a method that was simple, easy to expose nerves, and is less disruptive to normal anatomical structures. It can obtain larger sciatic nerves, which is easy to identify, and is simple to bundle, and the lesions can be quickly found by late surgical intervention.
Mechanical pain threshold and thermal pain threshold are the reliable indicators of the stability of sciatica caused by lumbar disc herniation[12]. Compared with the classic model, the results of the mechanical pain threshold and the thermal pain threshold of the new model group began to decline on the first day after surgery until the fifth day, which was then basically stable, at least until the 14th day after surgery. It can be observed from Tables 1 and 2 that the mechanical pain threshold and the thermal pain threshold were significantly reduced from the first day, and the comparison with the sham operation group was statistically significant. Until the 14th d, there was still statistical significance compared with the sham operation group. There was no statistically significant difference between the new model group and the control group. This proved that the new model is stable and reliable, early in pain sensitivity and long in duration. It is a new and reliable model of lumbar disc herniation, which can guarantee further experiments.
With the development of technology, the detection of lower limb temperature has become a relatively new indicator[13]. Lumbar disc herniation is found in the clinic to cause changes in the temperature of the lower limbs, which can assist in the diagnosis of the disease by measuring the lower limbs of the patient[14]. Therefore, this experiment compared the surgical side and healthy side temperature of the rat leg to prove that the new model is consistent with the classical model and conforms to the pathophysiological changes of lumbar disc herniation. In this experiment, it was found that the new model group and the model control group in Table 2 showed lower temperature of the surgical side at the first day after surgery until the fifth day. Compared with the sham operation group, there was a statistical difference between the body temperature of surgical side and the healthy side from the first day after surgery. There was no significant difference between the new model group and the control group. This proved that the new model can comprehensively replicate the pathological process of the disease. Decreased limb temperature may be associated with less blood flow caused by compression on concomitant vessel blood vessels, which still needs further investigation.
Numerous studies have shown that lumbar disc herniation is caused by a combination of chemical and mechanical factors. Inflammatory mediators play an important role in the development and progression of herniated disc pain. The behavioral symptoms of pain caused by nucleus pulposus in the intervertebral disc are mainly due to the inflammatory response mediated by pro-inflammatory factors in the nucleus pulposus of the intervertebral disc[15]. The expression of some cytokines such as IL-1, IL-6, IL-8, TNF-α and cyclooxygenase-2 is closely related to ganglion injury induced by nucleus pulposus and related nerve root pain[16]. In this study, IL-8 and TNF-α were selected for immunological analysis. IL-8 is a CXC chemokine, its main role is for the chemotaxis of neutrophils and lymphocytes, and is closely related to some intervertebral disc degeneration pain and chronic inflammatory pain[17]. TNF-α played an important role in inflammatory pain by regulating central sensitization. Studies have shown that TNF-α interacts with the neuroinflammatory system through peripheral or central mechanisms to regulate neuropathic pain[18]. TNF-α causes the continuous up-regulation of primary sensory afferent DRG and mechanical hyperalgesia through autocrine stimulation stimulated by peripheral nerve stimulation, which may indicate that up-regulation of TNF-α on DRG plays an important role in the formation of pathological pain[19]. Since the role of DRG in pain management and regulation is very important, DRG and peripheral sciatic nerve were selected for immunological testing[20]. In this study, immunological assays were performed on IL-8 and TNF-α in the sciatic nerve and DRG. It was found that the number of IL-8 and TNF-α positive cells in the inflammatory mediators of peripheral nerve tissue and DRG increased significantly at 7 days after modeling, and the difference was statistically significant compared with the sham operation group. The expression of positive cells decreased at 14 d after surgery, but the difference from the sham group was still significant. There was no statistically significant difference between the new model group and the model control group. It shows that the new model group can simulate the inflammation development process of disc herniation as well as the model control group.
Because the model control group is a classic animal model of lumbar disc herniation, the animal model designed by this experiment can simulate the pathological development process of disc herniation as well as the classic animal model through the comparison of behavioural, limb temperature changes and immunohistochemistry results. In addition, the new animal model has the following advantages: firstly, the operation is simple. The sciatic nerve is superficial and easy to find. The nerve is thick, so it is easy for free, cerclage, and it can reduce the pulling damage to the nerve during cerclage. Secondly, there is very little bleeding. There is no need to remove the bone when exposing the nerve, and there is almost no obvious bleeding. Thirdly, the symptoms appear early. The lower limb symptoms appeared on the first day after surgery. Fourthly, the model is stable. By the 14th d after surgery, the behavioral and immunological indicators of rats were stable. Fifthly, it is convenient for further experimental intervention. The purpose of the model was to further perform surgical and therapeutic interventions. In the classic model, the nerve roots were relatively small, the tissue adhesion was heavier, and the operation was difficult when reoperation was performed. In the new model group, the nerves were thick and superficial, and the surrounding muscles were lightly attached, and the reoperation was easy.
In conclusion, the rat model of lumbar disc herniation established in this investigation is simple, minimally invasive, and stable in mechanical pain sensitivity and heat sensitivity. The inflammatory response of the sciatic nerve and DRG is accurate, which provides a good and stable experimental model for further exploration of the mechanism and treatment of lumbar disc herniation in the future.
Acknowledgement:
This work was supported by the Education Department of Heilongjiang Province.
References
- Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine 1998;23:2538-544.
- Olmarker K, Brisby H, Yabuki S, Nordborg C, Rydevik B. The effects of normal, frozen, and hyaluronidase-digested nucleus pulposus on nerve root structure and function. Spine 1997;22:471-7.
- Sasaki N, Sekiguchi M, Kikuchi S, Konno S. Anti-nociceptive effect of bovine milk-derived lactoferrin in a rat lumbar disc herniation model. Spine 2010;35:1663-7.
- Motiei-Langroudi R, Sadeghian H, Seddighi AS. Clinical and magnetic resonance imaging factors which may predict the need for surgery in lumbar disc herniation. Asian Spine J 2014;8(4):446-52.
- Matsuyama Y, Sakuma Y, Suzuki M, Orita S, Yamauchi K, Inoue G. Evaluation of Behavior and Expression of Receptor Activator of Nuclear Factor-Kappa B Ligand in Dorsal Root Ganglia after Sciatic Nerve Compression and Application of Nucleus Pulposus in Rats. Asian Spine J 2014;8(5):557-64.
- Wang YJ, Zhou CJ, Shi Q, Smith N, Li TF. Aging delays regeneration process after sciatic nerve injury in rats. J Neurotrauma 2007;24:885-94.
- Kim ES, Oladunjoye AO. Spontaneous regression of herniated lumbar discs. Clin Neurosci 2014;21(6):909-13.
- Cho HK, Cho YW, Kim EH, Sluijter ME, Hwang SJ, Ahn SH. Changes in pain behaviour and glial activation in the spinal dorsal horn after pulsed radiofrequency current administration to the dorsal root ganglion in a rat model of lumbar disc herniation: laboratory investigation. J Neurosurg Spine 2013;19(2):256-63.
- Kim SJ, Park SM, Cho YW, Jung YJ, Lee DG, Jang SH, et al. Changes in expression of mRNA for interleukin-8 and effects of interleukin-8 receptor inhibitor in the spinal dorsal horn in a rat model of lumbar disc herniation. Spine 2011;36:2139-46.
- Wei M, Mo SL, Nabar NR, Chen Y, Zhang JJ, He QL, et al. Modification of rat model of sciatica induced by lumber disc herniation and the anti-inflammatory effect of osthole given by epidural catheterization. Pharmacology 2012;90:251-63.
- Wang W, Feng ZG, Ma T, Chen N. Efficiency of CCI models made with chromic gut and silk thread. ChinPLA Postgrad Med School 2010;31(5):480-2.
- Sasaki T, Fassler R, Hohenester E. The crux of basement membrane assembly. J Cell Biol 2004;164:959-63.
- ?ebkowski WJ, Koz?owski A, ?yso? T. The superficial skin temperature of low extremities in patients with sciatica. Rocz Akad Med Bialymst 2001;46:153-7.
- Liu Y, Wei G, Yang W, Wang C, Huang R. Anterior lumbar interbody fusion with self-locked cage for treatment of central type lumbar intervertebral disc protrusion and recessive lumbar segmental instability. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2014;28(1):69-73.
- Zhu X, Cao S, Zhu MD, Liu JQ, Chen JJ, Gao YJ. Contribution of chemokine CCL2/CCR2 signaling in the dorsal root ganglion and spinal cord to the maintenance of neuropathic pain in a rat model of lumbar disc herniation. J Pain 2014;15(5):516-26.
- Schistad EI, Espeland A, Pedersen LM, Sandvik L, Gjerstad J, Røe C. Association between baseline IL-6 and 1-year recovery in lumbar radicular pain. Eur J Pain 2014;18(10):1394-401.
- Kuelling FA, Foley KT, Liu JJ, Liebenberg E, Sin AH, Matsukawa A, et al. The anabolic effect of plasma-mediated ablation on the intervertebral disc: stimulation of proteoglycan and interleukin-8 production. Spine J 2014;14(10):2479-87.
- Ohtori S, Miyagi M, Eguchi Y, Inoue G, Orita S, Ochiai N, et al. Epidural administration of spinal nerves with the tumor necrosis factor-alpha inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: a prospective randomized study. Spine 2012;37:439-44.
- Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Norton HJ, Hanley EN Jr. Production and expression of RANTES (CCL5) by human disc cells and modulation by IL-1-β and TNF-α in 3D culture. Exp Mol Pathol 2014;96(2):133-8.
- Lin ML, Lin WT, Huang RY, Chen TC, Huang SH, Chang CH, et al. Pulsed radiofrequency inhibited activation of spinal mitogen-activated protein kinases and ameliorated early neuropathic pain in rats. Eur J Pain 2014;18(5):659-70.