- *Corresponding Author:
- Bidisha Mondal
Department of Agriculture and Allied Sciences, The Neotia University, Sarisha, West Bengal 743368, India
E-mail: bidisha.mondal@tnu.in
| Date of Received | 03 October 2022 |
| Date of Revision | 14 May 2024 |
| Date of Acceptance | 29 October 2024 |
| Indian J Pharm Sci 2024;86(5):1590-1600 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Piper betle (betel) plants are profusely grown in different states of India. The conventional greenhouse structure known as barejas (baroj) produces best quality betel leaves with huge export potential. The creeper is grown vegetatively from stem cutting and the green foliage produces high quality essential oil that is being utilized in the medicinal, pharmaceutical and aroma industry. The vegetative mode of propagation of this plant species under semi-in vitro condition inside baroj structure generated a number of cultivars due to diverse epigenetic regulation. The cultivars respond separately in nonidentical habitat generating epi-variants. The aroma compound expresses differential seasonal, morpho-anatomical, chemotypic profile. This distinction may aid the farmers and oil manufacturercum exporters to utilize the creeper for diverse applications. The chemotypic variation of leaves and non-leafy segments made this plant a potential bio-prospecting agent. This plant has potential to substitute a number of indigenous and exotic medicinal plants in extraction of important volatile and non-volatile compounds. The unsold, additional leaves along with non-leafy organ from betel-vines could be utilized for cultivar specific essential oil extraction. The oil extraction and purification may enrich the aroma-therapy sector and medicinal industry along with contribution towards agricultural waste management and additional income generation for betel farmers.
Keywords
Piper betle, epigenetic, betel leaf essential oil, multi-purpose use, cultivar, agri-waste-management, industrial niche
Indian Piper betle (P. betle) creepers produce high quality betel leaves in conventional greenhouse structure called Pan-baroj (pan-barejas). Most of the cultivars of Indian subcontinent shows high level of Betel Leaf Essential Oil (BLEO) recovery from fresh as well as partially degraded leaves. The highly perishable betel leaves causes huge post-harvest loss of farmers reaching up-to 70 % in selected cases. The waste leaves causes environmental pollution and ecological imbalance[1]. In glut season and erratic weather condition, farmers sell betel leaves in throw away prices, additionally, the temporary baroj structure requires annual maintenance. This high value crop has good export potential. If Betel Leaf Export Consignment (BLEC) shows a slight deviation from the standard betel advisory published by Agricultural Processing and Export Development Authority (APEDA) or Indian Phyto-sanitary authority that leads to rejection of the whole consignment. This problem is faced by the exporters and they lose interest in betel leaf export after multiple non-compliance related rejection. Betel leaves are highly perishable and domestic left-over and rejected export consignment remains unutilized, experiences in situ decays and harbors huge microbial load[2]. Both the on-farm wastage as well as export loss could be mitigated by utilization of the excess leaves in extraction and purification of BLEO. An in depth study of cultivar specific component profiling of oil will not only increase the efficient BLEO extraction rather reveal the ultimate market value of the plants in suitable pharmaceutical industry. The precise component profiling of Piper cultivars could assist in extraction of novel bioactive compounds without application of metabolic engineering or biotechnological interventions. The information on epigenetic variation of betel leaves could be a boon for the BLEO industry. The available Omic data on betel could be successfully exploited for estimating the prospect of different cultivars for domain specific industrial utilization[3]. The recent shift of the aroma industry towards natural plant based ingredient could play key role in utilization of this BLEO[4]. This plant could replace some indigenous and exotic aromatic plants showing similar chemo-profiles in herbal drug industry. Information on the aroma profile of betel creeper could be useful as alternative for high value endangered aroma germplasms of the world. In this paper an attempt is taken to unwrap the epigenetics of this miraculous creeper as a potential alternative of popular aromatic germplasms in phyto-pharmaceutical sector.
Factors affecting BLEO Production and Recovery
The high perishability of the leaves along with oscillating demand in domestic and international market causes great hindrance for the betel farmers and sellers. The rotten leaves causes severe bacterial and fungal infection[5]. The detection of any pathogen in betel consignment causes non-compliance and a total ban on betel leaf export of the whole country. Betel leaves could be stored for only 4 d-6 d in ambient environmental condition but BLEO could remain intact for more than 3 y[6]. The high storage life and lucrative cost of the BLEO is the reason behind its extraction. The vegetative mode of propagation inside a closed conventional greenhouse structure causes noticeable variation in the production of betel leaves and nature of Essential Oil (EO). The differential intercultural operation leads to variation in the performance of the cultivars. The variation in essential oil production is dependent on seasonal variation of compound accumulation[7], histological diversity[8] sexual dimorphism[9] and ecological[2] as well as climatic influence[10] on numerous betel leaf cultivars. This epigenetic regulation has potential for betel waste management, signature compound identification, extraction and securing the industrial niche of the plant.
Leaf bio marking
The EO recovery in betel germplasm depends on different varieties and the application of different processes. The fresh or partially decomposed leaves could be taken for oil extraction. The leaf colour of betel shows significant difference from light green, dark green, yellow, red to even black in some cultivars. The leaf colour may act as a morphological marker in cultivar identification[11] and during examination of chemotypic diversity of BLEO. The variable colour may have developed due to the presence of particular signature compound and could act as an excellent morphological identifier in taxonomic management of the germplasms. The leaf morphological studies are regarded as the cheapest and rapid method for plant diversity studies[12]. The organoleptic and microscopic evaluation of the leaves reveals, marker important for cultivar identification. The presence of abundant pearl like secretory cell in Bangla cultivar of betel leaves distinguishes it from other cultivars. In kapoori cultivar, multi-layered epidermis, cuticular hypodermis and presence of silicified cell in the upper leaf surface proclaims its drought tolerant nature[13]. In South Indian cultivar Siguramani the presence of cylindrical finger like glandular trichomes and 3-4 layered secretory cell distinguishes it from others. Sessile secretory glandular trichomes and single mucus canal is characteristic of Pachaikodi cultivar[14]. The ash value, total soluble solid content of the leaves could additionally provide information on epigenetic variation of the cultivar performance under different inter-cultural operation[15].
Variation in oil content in betel leaves:
The Indian betel leaf export industry mainly emphasizes the export of raw leaves for mastication purpose.In Indiaabout 10 % of the total production of betel leaves remain surplus and subjected to wastage every year particularly during the rainy season. To mitigate the problem the extraction of essential oil from well-demanded varieties of betel leaves could be taken up during monsoon when the export and domestic market demand experiences drastic reduction[16]. The average oil content in the Bangla cultivar was 1.7 % and in the Mitha cultivar it was 2.0 % whereas in the Sanchi it was only 0.8 % on dry weight basis[17]. In Sada Bangla cultivar 1.8 % oil recovery was noticed with the application of modified hydro-distillation method[10]. The information on BLEO recovery is not available for Kali bangla/Jhalpata, Kharibangla cultivars. Bagerhati, which is a prominent cultivar of West Bengal, India was also neglected and data is not available regarding the oil content. This oil, which is the major ingredient imparting particular aroma and medicinal properties to the betel leaves, can be preserved for more than three years.
The oil has a multidimensional potential for utilization in cottage industry for manufacturing of numerous commercial products like medicine, Gutkha (chewable mouth freshener), incense sticks, fragrant and flavoring agent etc. The establishment of a rural industry for extraction of essential oil from betel leaves could be initiated at a very reasonable initial investment of Rs. 30 000-40 000/-(400-500 USD) in bareja adjacent region[16]. This mission may minimize the wastage of surplus betel leaves besides increasing the agro-industrial employment opportunities in the betel leaf growing regions of India and other betel leaf producing countries.
Morpho-anatomical variation and sexual dimorphism of BLEO:
The leaf biomass determines the economic yield in betel trade and increased biomass increases the prospect of more oil recovery. Leaf biomass production and composition depends on gender, location, season, agronomic technologies, and propagation method of a cultivar[3]. Additionally the leaf oil content of petiole[18], inflorescence[19], rhizomes[20] and root[21] of betel leaf cultivars were regarded as promising inclusion for bio-medicine manufacturers and food additive sector. It is important to analyze the potential of all the parts of P. betle creeper to maximize the need based utilization of different plant organ and reduction in the waste generation during climatic adversities. The saline belt of Indian subcontinent produces good quality betel leaves but the vines are devastated by super-cyclones in every year. The Sagar Meetha and Sagar Bangla produces highest quantity of essential oil in comparison to other cultivars of India. In such condition the different parts of the uprooted creeper could be separated, sorted and utilized for need based oil utilization from separate organs.
Additionally, the sexual dimorphism influences biomass production and the ultimate constituent diversity of the said biomass. The sexual dimorphism affects the leaf length, width, weight regulating economic yield of commercial cultivars. Leaf yield regulates the economic turnover of the prominent cultivars. The male plants have narrowly ovate leaves and female possess cordate leaves[2]. In clonally propagated plants, in absence of flowering, the leaf morphology is usually utilized for discrimination of male and female plants. In a study of wild betel cultivar it is noticed that female plant produces more than 62.9 % monoterpenes while male produces 60.93 % sesquiterpenes hydrocarbons. The female plants under stress condition produces some excess metabolite than the male partners including monoterpenes and oxygenated monoterpenes[22]. Higher amount of phenol and other antioxidants were produced in females. A quantitative difference in acetylation of phenols was observed between genders. Gender associated qualitative and quantitative difference ultimately influence the metabolite production and drug efficacy, recovery and yield in specific taxons[23]. The clonal propagation also leads to change in ploidy level in sexes and may produce larger leaf in some varieties[24]. In West Bengal, India majority of the betel-vines display sexual dimorphism and indirectly influences bio-prospect of the cultivated germplasm.
Seasonal fluctuation of BLEO:
Seasonal oscillation regulates production of different bioactive compounds. Difference in temperature, rainfall, humidity regulates the production of different compounds in P. betle. The volatile and non-volatile compounds accumulate within the oil glands in differential amounts. Gas Chromatography-Mass Spectroscopy (GC-MS) based analysis of the leaf extract on the accumulation of various metabolites in Piper[22], detected maximum storage of chemical constituents in winter and lowest in monsoon. Cluster analysis of the 8 cultivated varieties on the basis of 45 non-polar components revealed seasonal variation of components, though some major components remain unaffected irrespective of season. The growers and medicine industry may focus on seasonal extraction of different phyto-compounds[23]. Additionally epigenetic variation led to the production of multiple volatile and non-volatile aroma constituents in different developmental stages of the plant[24]. The clonal propagation mechanism leads to perpetuation of mitotically transmissible epigenetic traits by allowing promiscuous movement of diffusible epigenetic factors through plasmodesmata[25].
Histological and seasonal variation in monoterpenes and sesquiterpenes were also noticed in this aromatic species. Unbiased analysis of the Piper metabolome could specify the distinct role of genotype, developmental stages and environmental effects on Secondary Metabolite (SC) production[26]. The increase in biomass could regulate the percentage of essential oil recovery and competitive production and distribution of bioactive compounds regulating ultimate aroma-architecture of the genotype. GC-MS study of volatile and non-volatile components along with Principle Component Analysis (PCA) shows eugenol and sesquiterpenoids were responsible for seasonal variation of BLEO[27]. The quantitative and qualitative chemotypic profile of cultivars showed noticeable variation and the variation of BLEO signifies the operation of epigenetic factors as an operative force in BLEO diversification[28]. In different orchards of a single locality the plant types perform differently leading to differential orchard performance. The in vivo diffusion of selective epigenetic compounds through plasmodesmata may be the influence behind this orchard to orchard variation of same genotype[29]. In Indian subcontinent betel leaf based business exhibits seasonal influence and extraction and exploitation of a broad spectrum of EO could possible and prospective with respect to season and demand of the pharmaceutical sector.
Geographical variation of BLEO:
The epigenetic control mechanism affects the omic-architecture of clonally propagated plants affecting the chemo-diversity. In Piper significant variation of secondary metabolites are noticed pertaining to geographical variation. The varietal performance deviates in different geographical region mainly due to the accumulation of different secondary metabolites. The P. betle might have originated in the South-Eastern region of Asia in Indonesia or Thailand but in India all the cultivars of P. betle shows the presence of eugenol and chavibetol as major constituent of BLEO[30-34]. In Philippine and Malaysia the major compound was identified as chavibetol and its acetate[35,36]. The betel leaves of Kampuchea shows the presence of Alpha (α)-pinene and sabinene as major component[20]. The Taiwan type shows presence of chavibetol, safrole and myrcene[37]. Presence of saponin and glycoside is noticed in black betel of Africa[38]. Safrole followed by chavibetol are the major BLEO constituents of Srilanka. Malabulath cultivar of Srilanka shows unique presence of allylpyrocatecholdiacetate[39]. The major compound in Malabulath oil is allylpyrocatecholdiacetate which is regarded as the third major compound in common betel oil of Lanka. This chemical composition of the BLEO of Lanka shows proximity with Indian cultivar Deshawari[40]. The presence of different varieties could be recognized and marked by their chemo-diversity. In India the Northern, Eastern and Southern varieties shows distinct difference in overall chemical composition[41].
The popular cultivars of our state of West Bengal such as Bangla, Mitha and Sanchi, Sadabangla, kalibangla has number of valuable components but the signatory compound remain present in trace quantity and unique to a cultivar[3]. The discriminatory component could play significant role in cultivar biomarking. The presence of estragole and anethole provides the sweet fragrance to the ‘Meetha’ cultivar and is unique to the Meetha cultivar of West Bengal[42]. In Bangla cultivar of Bangladesh, the signatory compound were oxophorone and 9-epi-β-caryophyllene but in Sanchi the presence of H-indole was prominent[43]. The study on trace element is rare and requires replicated confirmatory study establishing the validity of the signature compound for authentic biomarking of promising cultivars. The cultivar could be classified on the basis of the presence of signature compounds in the betel plant types (Table 1).
| Country | Signature compound | Reference |
|---|---|---|
| Philippine | Hydroxychavicol | Iiao et al.[35] |
| Malaysia | Chavibetol, eugenol acetate | Jantan et al.[36] |
| Taiwan | Safrole, myrcene | Chang et al.[37] |
| Africa | Saponin, glycoside | Junairiah et al.[38] |
| Sri Lanka | Safrole, allylpyrocatechol, eugenol | Mohottalage et al.[39] |
| Indonesia | 4-allyl phenylacetate | Alighiri et al.[7] |
| Vietnam | Isoeugenol, isoeugenol acetate | Thann et al.[20] |
| Bangladesh | Eugenol, α-celinene, oxophorone, 9-epi-β-caryophyllene | Islam et al.[43] |
| Thailand | Chavicol, chavibetol | Nagabhushan et al.[44] |
| Nepal | Chavibetol, chavibetol acetate | Satyal and Setzar et al.[45] |
Table 1: Signature Compounds of Different Chemotypes of Betel Leaves of Asia
Performance of non-leafy organs for oil recovery:
P. betle plant is a store house of essential chemicals, though the performance of the leafy part is being evaluated by several researchers, the BLEO recovery from non-foliage tissues are scanty. The petiole, inflorescence, rhizome contains different valuable components and could be utilized for oil extraction. In betel leaf storage, the removal of petiole increases the longevity of the leaf. This segments could be evaluated for commercial oil extraction as it is removed for long term storage of betel leaves the excised petiole could be utilized for essential oil extraction. The petiole shows presence of methyl undecanote, dodecanoic acid, 3-methyl-butyl-dodecanoate and lauryl alcohol, that has great potential in flavor and cosmetics industry[18]. A novel hydroxychavicol dimer, 2-(g'-hydroxychavicol)-hydroxychavicol was isolated from the roots of P. betle along with five known compounds, hydroxychavicol, aristololactam A II, aristololactam B II, piperolactam A and cepharadione A[44-46]. The structures of these isolated compounds were elucidated by spectroscopic methods. Compounds 1 and 2 exhibited inhibitory effects on the generation of superoxide anion and the release of elastase by human neutrophils.
The extract of Indian P. betle roots furnished novel aristololactam A-II and a phenyl propene, characterized as 4-allyl resorcinol, with the petroleum-ether extract yielded a diketosteroid, viz. stigmast-4-en-3, 6-dione[47]. Diverse alkaloids and lignans present in stems of P. betle were elucidated by spectral analysis as piperine, pellitorine, N-isobutyl-2E, 4E-dodecadienamide, dehydropipernonaline, piperdardine, piperolein-B, guineensine, (2E, 4E)-N-isobutyl-7-(3', 4'-methylenedioxyphenyl)-2, 4-heptadienamide, syringaresinol-O-beta-D-glucopyranoside, pinoresinol. The compounds were isolated as novel types for stem[48]. The rhizome oil of P. betle contains 40 compounds with δ-cadinene, α-cadinol, T-cadinol and T-muurolol as major constituents[19]. The extraction and identification of several novel compounds from betel non-leafy parts bear prospect of enhancement of natural drug extraction. The plant organs may act as bio-factory for drug, therapeutic and organoleptic studies.
Prominent Betel Leaf Chemotypes of West Bengal with Agro-Industrial Potential
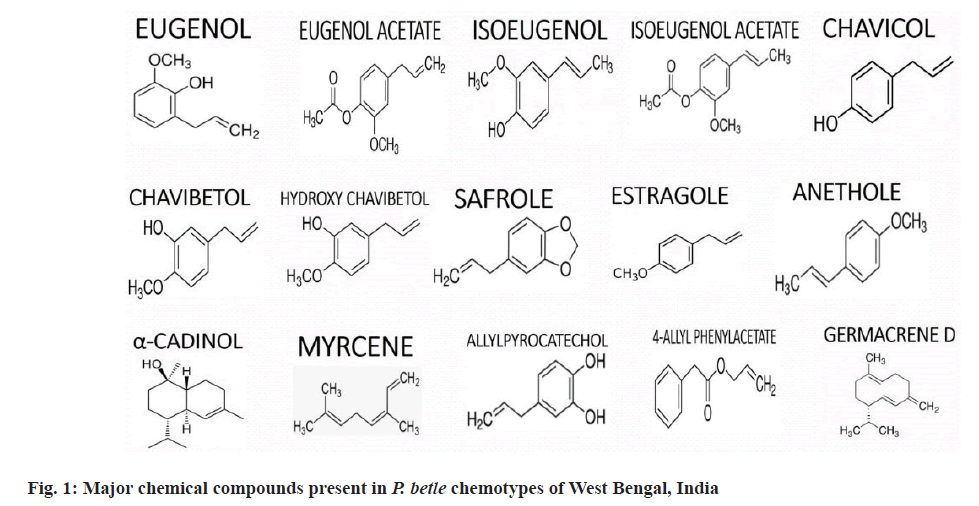
In P. betle the chemical polymorphism is well established leading to formation of eight distinct chemotypes predominantly on the basis of essential oil present in leaves. The prominent cultivars of the state of West Bengal, India are Bangla, Meetha. Kalibangla and Bagerhati. Those cultivars could be utilized for extraction of Chavicol, eugenol, eugenol acetate and anethole respectively. Sanchi, Haldia, Ghanegate and Manikdanga also shows promise as expensive medicinal cultivar[16,3]. The signature compounds of all the eight cultivar holds enormous potential to be used in a spectrum of pharmaceutical and aroma-industry and assist in boosting local business and enhancing indigenous economy. Additionally the rhizome oil may form another significant biomarker. The available metabolomics data could be used to identify the transcriptomics and genetic ontogeny of volatile compounds[49,50]. The molecular genetic study and information on genomics is less in Piper but the metabolomic data could be utilized for exploring the unexplored territory of epigenomic details of betel plants. The aroma-omic of betel-leaf has potential medicinal uses. The details of pre-clinical trials with betel leaf extract and essential oil in human diseases could throw light on the epigenomic performance and intricacies of this prominent creeper (Table 2 and fig. 1)[51-64].
| Clinical area | Type of action | Reference |
|---|---|---|
| Anti-breast cancer | Significant suppression and migration of cell lines MCF-7 at a dose of 25 µg/ml of leaf extract | Boontha et al.[51] |
| Human colorectal adeno carcinoma | 200 µg/ml leaf extract triggers micro-tubule polymerization and cell cycle arrest | Looi et al.[52] |
| Anti-cancer property | 100 µg/ml of leaf extract inhibited treated KB cells with 43.42 % viability | Veettil et al.[53] |
| Anti-diabetic property | Anti-diabetic assay by α-amylase and α-glucosidase enzyme inhibition (IC50) at 26.2 µg/ml and 96.8 µg/ml by methanolic extract of Kapoori Meetha cultivar | Satapathy et al.[54] |
| Anti-thrombin activity | Thrombin substrate III and thrombin inhibition (IC50) at 165.5 µg/ml by methanolic leaf extract | |
| Anti-oxidant activity | The ethyl acetate leaf extract shows highest anti-proliferative activity against MCF-7 cell lines in RPMI 1640 medium with IC50 value of 65 µg/ml | Abrahim et al.[55] |
| Anti-candida activity | The disc-diffusion assay using Candida albicans showed a MIC value of 0.1-2 mg/ml with a liquid formulation of 5 % BLEO | Shekhar et al.[56] |
| Episiotomy effect | A topical combination of 2 ml cinnamon oil and 20 % red betel leaf extract accelerate wound healing and reduce perineal pain in episiotomy incision model in Swiss mice. | Alfiana et al.[57] |
| Anti-estrogenic activity | 1 g/kg/day aqueous and methanolic leaf extract showed significant decrease in the duration of proestrus and estrus and reduction in the number of estrous cycle in female albino rats. | Biswal, et al.[58] |
| Anti-acne property | 15 % ethanolic extract of betel leaves shows strongest inhibitory zone of 15.35 mm in pure Propionibacterium culture equivalent to Clindamycin gel | Meinisasti et al.[59] |
| Healing of Stomach ulcer | 120 mg/kg of leaf extract shows 78.28±2.8 % protection in indomethacin induced stomach ulcerated rats | Bhattacharya et al.[60] |
| Anti-nociceptive activity | 100-200 mg/kg methanolic extract of leaf shows analgesic activity in hot-plate technique in Swiss-albino mice and Wister rats. Dose dependent variation in latency time was noticed with supra-spinal response of the test subjects. | Alam et al.[61] |
| Anti-inflammatory activity | 10-250 µg/ml of methanolic leaf extract shows anti-inflammatory activity with a maximum viability of 87.74 % in RAW 264.7 cells treated with 1 µg/ml of Escherichia coli LPS | Rintu et al.[62] |
| Immuno-protective property | Oral administration of aqueous leaf extract of 225 mg/kg 1 h before radiation exposure shows radio-protective activity in Swiss female mice with a reduction of 34.78 % of micro-nucleated cells in post-irradiation (24 h) bone-marrow samples. | Verma et al.[63] |
| Rheumatoid arthritis treatment | 200-400 mg/kg of methanolic leaf extract shows recovery from joint damage in male albino Wister rats in dose dependent manner. | Murugesan et al.[64] |
Note: MCF-7: Michigan Cancer Foundation-7; IC50: half-maximal inhibitory concentration; RPMI: Roswell Park Memorial Institute and LPS: Lipopolysaccharide
Table 2: Pre-Clinical Studies of BLEO as a Potent Pharmaceutical Agent
Epigenetic Control of Variability in BLEO Production
The spectrum of secondary metabolites and aroma-compound produced by P. betle under diverse environmental stimuli suggests the occurrence of a strong epigenetic control mechanism. The selective production of a compound in one season and then trace occurrence or total disappearance of the same compound in BLEO in next season suggests strong transcriptional as well as translational regulation. In plants methylation, acetylation/deacetylation controls gene repression and expression[65]. The cis and trans acting elements in cell controls the gene action and metabolite production. The covalent modification of conserved proteins in packaged Deoxyribonucleic Acid (DNA) controls epigenomics of different plants. Down regulation of genomic DNA in plants assist in conservation of energy to control biotic and abiotic stresses[66]. The less production of some compounds in selected season in P. betle may be a defense mechanism by resource storage. An array of non-coding Ribonucleic Acids (RNAs) might have played predominant role in performance of distinct landraces[67]. In several plants it has been noticed nitrogen assimilation, vernalization, pigmentation, photo-morphogenesis, male sterility, flowering, spore production, biotic and abiotic stresses were mediated by essential non-coding RNAs[68-73]. Histone acetylation and cytosine methylation regulates the promoter, regulator and enhancer action in post-transcriptional operations. The natural environmental factor or exogenous application of chemical compounds could assist in the recovery of commercially suitable epigenetic variants.
Prospect of Betel Creeper as Substitute of Aroma-Plants
Plant normally produces diverse bioactive compounds with different health benefits and nutritional productivity. These compounds are mainly related to defense mechanism of the plant or indirectly assist in successful propagation. Piper plant reserves a spectrum of monoterpenes and sesquiterpenes with more than 10 % additional products[74]. In Freesia hybrida the synergistic expression and hierarchical regulatory activity of transcription factors regulate the linalool production in different varieties of flowers. A regulatory gene showed two-fold activity in transcriptional modulation. It controls both the nature of secretory trichome formation in flower petals along with sequential expression of terpene biosynthesis gene[75]. In a similar manner in different betel cultivars the cascading activity of different transcription factor and associated cis acting elements activates/deactivates diverse candidate of terpenoid family. This event leads to histological, quantitative and qualitative variation in the composition of secondary metabolites in P. betle cultivars. Apart from P. betle several other species such as P. betle var. nigra[76], Piper crocatum[77], Piper sarmentosum[78] has potential to replace several aromatic plants as biophore for herbal drugs. Thus betel creeper could substantially replace a number of aromatic medicinal plant as biophore for extraction of multiple bioactive compounds. In this way the plant has potential to assist pharmaceutical industry as replacement of endangered indigenous or exotic plants in Indian subcontinent or in other countries as well (Table 3)[79-92].
| Scientific name | Name of the plant | Signature compound | Piper cultivar | Reference |
|---|---|---|---|---|
| Cymbopogon nardus | Citronella | Methyl eugenol | Bangla | Tan and Nishida et al.[79] |
| Syzygiumaromaticum | Clove | Eugenol | Meetha | Khalil et al.[80] |
| Petunia hybrid | Petunia | Isoeugenol | Vietnameze | Koeduka et al.[81] |
| Cinnamomumverum | Cinnamon | Acetyl eugenol | Maghai | Khalil et al.[80] |
| Acacia nilotica | Gum arabic | Eugenol acetate | Kalibangla | Abdulhamid et al.[82] |
| Ocoteapretiosa | Canelafuncho | Safrole | Sanchi | Lima et al.[83] |
| Burseracopallifera | Copal ancho | Germacrene-D | Siguramani | Noge and Becerra, et al.[84] |
| Pimpinellaanisum | Anise | Anethole | Meetha | Duta et al.[85] |
| Ocimum basilicum | Sweet basil | Chavibetol | Nepalese | Gang et al.[86] |
| Foeniculum vulgare | Bitter fennel | Estragole | Meetha | Sherif et al.[67] |
| Eletteriacardamomum | Cardamom | Terpinyl acetate | Kapoori | Vaiciulyte et al.[88] |
| Cannabis sativa | Cannabis | β-caryophyllene | Panama | Fiorenzani et al.[89] |
| Calendula officinalis | Calendula | Cadinol, δ-cadinene | Nepalese betel | Satyal and Setzer, et al.[45] |
| Pogostemoncablin | Patchouli | Trans-caryophyllene | Meetha | Basak and Guha, et al.[90] |
| Lavandula officinalis | Lavender | Geraniol | Tamluk Mitha | Basak and Guha, et al.[90] |
| Piper nigrum | Pepper | Sabinene | Siguramani I | Periyanayagam et al.[13] |
| Elettariacardamomum | Cardamom | Alpha-terpineol | South Indian betel | Kaliyaperumal, Nayaka et al.[91,92] |
Table 3: P. betle Creeper as Potential Alternative of an Array of Medicinal Plants
Conclusion
In P. betle the epigenetic and epigenomic studies could provide insight into the qualitative and quantitative variation in betel leaf constituent. The precise study of epigenetic regulation could substantiate the recovery of more essential oil, aroma compounds in the form of secondary metabolites. This review highlighted the value of understanding the morpho-anatomical variation, seasonal influence, sexual or ploidy analysis, ecological diversity in the exploitation of this high value plant for need based essential oil extraction. The plant could act as a bio-factory for domestic and export market of medicinal and therapeutic natural products. As the plant is vegetatively propagated through stem cutting in Indian subcontinent in semi-in vitro condition, a slight modification of agronomic techniques could alter the composition of essential oil in different cultivars. These modification could be exerted by natural or chemical alterations. These epigenetic variants as taxonomic cultivars could replace the expensive transgenic production or tissue culture based metabolic engineering. The epigenetics of BLEO is essential for understanding the industrial niche of the promising creeper.
Acknowledgement:
The author acknowledges and extends thanks to The Neotia University for granting of intramural fund on a project entitled “Estimation and characterization of essential oil from the surplus leaves of Betel vines (P. betle L.) for effective waste management and enhancement of rural employability & economy of South Bengal” (File No: R&D/2020/F6). Thanks are due to the Bishnupur Block I & II Offices under South 24 Parganas Agriculture department of West Bengal, India for collection of data and baroj visit for running the project as Principal Investigator.
Conflict of interests:
The authors declare no conflict of interests.
References
- Mondal B, Saha R, Samanta A. Improvement of Local Economy through the Maintenance of the Rural House Hold Bank (Piper betel Baroj) of South Bengal. Indian Horticulture J 2020;10(1 and 2):22-4.
- Mondal B. Extraction of betel leaf essential oil for the sustainable solution to betel business in west Bengal for effective economic gain: A review. Mysore J Agric Sci 2022;56(2):1-13.
- Mondal B. Conversion of metabolomic data to genomic marker for genetic characterization of Piper betle L. chemotypes: A review. Agric Rev 2022;43(2):162-9.
- Rathore R, Mathur A. Entrepreneurship development in medicinal and aromatic plants: Prospects and challenges. Int J Econ Plants 2018;5(1):32-5.
- McLauchlin J, Aird H, Andrews N, Chattaway M, De Pinna E, Elviss N, et al. Public health risks associated with Salmonella contamination of imported edible betel leaves: Analysis of results from England, 2011-2017. Int J Food Microbiol 2019;298:1-0.
[Crossref] [Google Scholar] [PubMed]
- Masud MZ, Islam MR, Imtiaz AA, Labiba T, Raihan MR, Eaty MN, et al. Morphology, prevalence and pathogenicity of fungi associated with diseased betel vine (Piper betle L.) in Bangladesh. Eur J Biol Biotechnol 2020;1(6):124.
- Alighiri D, Cahyono E, Eden WT, Kusuma E, Supardi KI. Study on the improvement of essential oil quality and its repellent activity of betel leaves oil (Piper betle l.) from Indonesia. Oriental J Chem 2018;34(6):2913.
- Dwivedi V, Tripathi S. Review study on potential activity of Piper betle. J Pharmacogn Phytochem 2014;3(4):93-8.
- Das S, Parida R, Sandeep IS, Nayak S, Mohanty S. Biotechnological intervention in betel vine (Piper betle L.): A review on recent advances and future prospects. Asian Pac J Trop Med 2016;9(10):938-46.
[Crossref] [Google Scholar] [PubMed]
- Mondal B. Cost effective essential oil extraction from surplus Betel (Piper betle L.) leaves. Asian J Biol Life Sci 2022;11(1):101.
- Patra B, Sahu A, Meena R, Pradhan SN. Estimation of genetic diversity in Piper betle L. based on the analysis of morphological and molecular markers. Lett Appl Nonobiosci 2021;10(2):2240-50.
- Roy SC, Sharma BD. Assessment of genetic diversity in rice (Oryza sativa L.) germplasm based on agro-morphology traits and zinc-iron content for crop improvement. Physiol Mol Biol Plants 2014;20:209-24.
[Crossref] [Google Scholar] [PubMed]
- Periyanayagam K, Jagadeesan M, Kavimani S, Vetriselvan T. Pharmacognostical and phyto-physicochemical profile of the leaves of Piper betle L. var Pachaikodi (Piperaceae)-valuable assessment of its quality. Asian Pac J Trop Biomed 2012;2(2):S506-10.
- Saini SA, Dhiman AN, Nanda SA. Pharmacognostical and phytochemical studies of Piper betle Linn. leaf. Int J Pharm Pharm Sci 2016;8(5):222-6.
- Sarma C, Rasane P, Kaur S, Singh J, Singh J, Gat Y, et al. Antioxidant and antimicrobial potential of selected varieties of Piper betle L.(Betel leaf). Agrarian Sci Acad Bras Cienc 2018;90:3871-8.
- Mondal B, Saha R, Samanta A. Post-Amphan management and rejuvenation of the ravaged betel (Piper betle) baroj in South Bengal. Farming Manag 2020;5(2):91-100.
- Guha P. Extraction of essential oil from betel leaf for manufacturing of food, medicinal and cosmetic products. Souvenir of the 19th Indian; 2007.
- Dwivedi BK, Kumar S, Nayak C, Mehta BK. Gas chromatography Mass Spectrometry (GC-MS) analysis of the hexane and benzene extracts of the Piper betle (leaf stalk) (Family: Piperaceae) from India. J Med Plants Res 2010;4(21):2252-5.
- Kemprai P, Bora PK, Mahanta BP, Sut D, Saikia SP, Banik D, et al. Edible source of betel‐scented sesquiterpene‐rich essential oil. Flavour Fragrance J 2020;35(1):70-8.
- Thanh L, Dũng NX, Bighelliand A, Casanova J, Leclercq PA. Combination of capillary GC, GC/MS and 13C‐NMR for the characterization of the rhizome oil of Piper betle L. (Piperaceae) from Vietnam. J Spectroscopy 1997;13(2):131-6.
- Lin CF, Hwang TL, Chien CC, Tu HY, Lay HL. A new hydroxychavicol dimer from the roots of Piper betle. Molecules 2013;18(3):2563-70.
[Crossref] [Google Scholar] [PubMed]
- Karak S, Acharya J, Begum S, Mazumdar I, Kundu R, De B. Essential oil of Piper betle L. leaves: Chemical composition, anti-acetylcholinesterase, anti-β-glucuronidase and cytotoxic properties. J Appl Res Med Aromat Plants 2018;10:85-92.
- El-Shemy H. Essential oils: Oils of nature. BoD–Books on Demand; 2020.
- Singroha G, Sharma P. Epigenetic modifications in plants under abiotic stress. Epigenetics 2019;10.
- Pikaard CS, Scheid OM. Epigenetic regulation in plants. Cold Spring Harb Perspect Biol 2014;6(12):a019315.
- Kapetanos C, Karioti A, Bojović S, Marin P, Veljić M, Skaltsa H. Chemical and principal‐component analyses of the essential oils of Apioideae taxa (Apiaceae) from Central Balkan. Chem Biodivers 2008;5(1):101-19.
[Crossref] [Google Scholar] [PubMed]
- Karak S, Bhattacharya P, Nandy A, Saha A, De B. Metabolite profiling and chemometric study for varietal difference in Piper betle L. leaf. Curr Metabolomics 2016;4(2):129-40.
- Ashapkin VV, Kutueva LI, Aleksandrushkina NI, Vanyushin BF. Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int J Mol Sci 2020;21(20):7457.
[Crossref] [Google Scholar] [PubMed]
- Chen XY, Kim JY. Transport of macromolecules through plasmodesmata and the phloem. Physiol Plantarum 2006;126(4):560-71.
- Patra B, Das MT, Dey SK, Das T. A review on Piper betle L. J Med Plants Stud 2016;4(6):185-92.
- Orlofsky EM, Kozhoridze G, Lyubenova L, Ostrozhenkova E, Winkler JB, Schroder P, et al. Sexual dimorphism in the response of Mercurialis annua to stress. Metabolites 2016;6(2):13.
[Crossref] [Google Scholar] [PubMed]
- Bajpai V, Pandey R, Negi MP, Bindu KH, Kumar N, Kumar B. Characteristic differences in metabolite profile in male and female plants of dioecious Piper betle L. J Biosci 2012;37:1061-6.
[Crossref] [Google Scholar] [PubMed]
- Samantaray S, Phurailatpam A, Bishoyi AK, Geetha KA, Maiti S. Identification of sex-specific DNA markers in betel vine (Piper betle L.). Genet Resources Crop Evolut 2012;59:645-53.
- Bajpai V, Sharma D, Kumar B, Madhusudanan KP. Profiling of Piper betle Linn. cultivars by direct analysis in real time mass spectrometric technique. Biomed Chromatogr 2010;24(12):1283-6.
[Crossref] [Google Scholar] [PubMed]
- Ilao S. Betel (Piper betle. L.) leaf has antibacterial property. PCARRD Monitor 2002;30(3).
- Jantan IB, Ahmad AR, Ahmad AS, Ali NA. A comparative study of the essential oils of five Piper species from Peninsular Malaysia. Flavour Fragr J 1994;9(6):339-42.
- Chang MJ, Ko CY, Lin RF, Hsieh LL. Biological monitoring of environment exposure to safrole and the Taiwanese betel quid chewing. Arch Environ Contam Toxicol 2002;43(4):432-7.
[Crossref] [Google Scholar] [PubMed]
- Junairiah NM, Nabilah IZ, Lilis S. Isolation and identification of secondary metabolites from black Betel (Piper betle var. nigrum). Jurnal Kimia Risat 2018;3(2):131-8.
- Mohottalage S, Tabacchi R, Guerin PM. Components from Sri Lankan Piper betle L. leaf oil and their analogues showing toxicity against the housefly, Musca domestica. Flavour Fragr J 2007;22(2):130-8.
- Arambewela LS, Arawwawala LD, Kumaratunga KG, Dissanayake DS, Ratnasooriya WD, Kumarasingha SP. Investigations on Piper betle grown in Sri Lanka. Pharmacogn Rev 2011;5(10):159.
[Crossref] [Google Scholar] [PubMed]
- Kaintura P, Bhandari M, Kumar R. Medicinal values of betel leaves and its application in food products: A review. Pharma Innov J 2020;9(6):344-8.
- Guha P, Nandi S. Essential oil of betel leaf (Piper betle L.): A novel addition to the world food sector. Essential Oil Res 2019:149-96.
- Islam MA, Ryu KY, Khan N, Song OY, Jeong JY, Son JH, et al. Determination of the volatile compounds in five varieties of Piper betle L. from Bangladesh using simultaneous distillation extraction and gas chromatography/mass spectrometry (SDE-GC/MS). Anal Lett 2020;53(15):2413-30.
- Nagabhushan M, Amonkar AJ, Nair UJ, D'Souza AV, Bhide SV. Hydroxychavicol: A new anti-nitrosating phenolic compound from betel leaf. Mutagenesis 1989;4(3):200-4.
[Crossref] [Google Scholar] [PubMed]
- Satyal P, Setzer WN. Chemical composition and biological activities of Nepalese Piper betle L. Int J Profes Holistic Aromather 2012;1(2):23-6.
- Liu J, Xu C, Zhang H, Liu F, Ma D, Liu Z. Comparative transcriptomics analysis for gene mining and identification of a cinnamyl alcohol dehydrogenase involved in methyleugenol biosynthesis from Asarum sieboldii Miq. Molecules 2018;23(12):3184.
[Crossref] [Google Scholar] [PubMed]
- Ghosh K, Bhattacharya TK. Chemical constituents of Piper betle Linn. (Piperaceae) roots. Molecules 2005;10(7):798-802.
[Crossref] [Google Scholar] [PubMed]
- Huang X, Yin Y, Huang W, Sun K, Cheng C, Bai L, et al. Alkaloids and lignans from stems of Piper betle. Zhongguo Zhong Yao Za Zhi 2010;35(17):2285-8.
[Google Scholar] [PubMed]
- Goyat S, Grewal A, Singh D, Katiyar RS, Tewari SK, Nainwal RC, et al. Evaluation of genetic diversity of Piper betle cultivars using ISSR markers. Inter J Adv Res 2016;4(1):571-9.
- Jaiswal S, Tomar RS, Vadukool K, Chopra M, Rathod M, Parakhia MV, et al. Transcriptome profiling of Indian sesame (Sissemum indicum L) and discovery of genetic region markers. Bhartiya Krishi Anusandhan Patrika 2020;35(3):151-8.
- Boontha S, Taowkaen J, Phakwan T, Worauaicha T, Kamonnate P, Buranrat B, et al. Evaluation of antioxidant and anticancer effects of Piper betle L (Piperaceae) leaf extract on MCF-7 cells, and preparation of transdermal patches of the extract. Tropic J Pharm Res 2019;18(6):1265-72.
- Looi ML, Wong AK, Gnapragasan SA, Japri AZ, Rajedadram A, Pin KY. Anti-migratory effects of Piper betle leaf aqueous extract on cancer cells and its microtubule targeting properties. J Zhejiang Univ Sci B 2020;21(9):745.
[Crossref] [Google Scholar] [PubMed]
- Veettil SR, Sunil EA, Mukunda A, Mohan A, John S, Pynadath MK. Anticancer effect of Piper betle leaf extract on KB cell lines-an in vitro study. Oral Maxillofacial Pathol J 2022;13(1).
- Satapathy BS, Biswal SK, Maharana L, Pattnaik S. Bioactive components of piper betel could be potential anticancer agents: A short review on pre-clinical inves-tigations and practical challenges. Curr Trends Biotechnol Pharm 2023;17(2):918-29.
- Shekhar R, Madhusudhan MC, Sanjay CJ, Prakash HS, Nagaraja G. In vitro anti-lipid peroxidation and anti-Candidal potentials of different betel leaf varieties. J Med Plants 2022;10(4):140-9. [Crossref]
- Alfiana RD, Kusumawardani N, Widyarini S. The episiotomy effect of topical combination of cinnamon oil and red betel on skin wound healing mechanism. 2nd Int Conference Contem Sci Clin Pharm 2021:144-51.
- Biswal S. Phytochemical analysis and a study on the antiestrogenic antifertility effect of leaves of Piper betel in female albino rat. Anc Sci Life 2014;34(1):16-22.
[Crossref] [Google Scholar] [PubMed]
- Meinisasti R, Muslim Z, Krisyanella K, Sunita S. The effectiveness test of betel leaf ethanol extract cream (Piper betle Linn) toward propionibacterium acnes bacterial growth. J Biomed Transl Res 2020;4(2):10-7.
- Bhattacharya S, Chaudhuri SR, Chattopadhyay S, Bandyopadhyay SK. Healing properties of some Indian medicinal plants against indomethacin-induced gastric ulceration of rats. J Clin Biochem Nutr 2007;41(2):106-14.
[Crossref] [Google Scholar] [PubMed]
- Abrahim NN, Kanthimathi MS, Abdul-Aziz A. Piper betle shows antioxidant activities, inhibits MCF-7 cell proliferation and increases activities of catalase and superoxide dismutase. BMC Complement Altern Med 2012;12:1-1.
[Crossref] [Google Scholar] [PubMed]
- Alam B, Akter F, Parvin N, Pia RS, Akter S, Chowdhury J, et al. Antioxidant, analgesic and anti-inflammatory activities of the methanolic extract of Piper betle leaves. Avicenna J Phytomed 2013;3(2):112-25.
[Google Scholar] [PubMed]
- Rintu D, Shinjini M, Kaustab M, Pramathadhip P, Umesh PS, Banerjee ER. Anti-oxidant and anti-inflammatory activities of different varieties of Piper leaf extracts (Piper betle L.). J Nutr Food Sci 2015;5(415):2.
- Verma S, Gupta ML, Dutta A, Sankhwar S, Shukla SK, Flora SJ. Modulation of ionizing radiation induced oxidative imbalance by semi‐fractionated extract of Piper betle: An in vitro and in vivo assessment. Oxid Med Cell Longev 2010;3(1):44-52.
[Crossref] [Google Scholar] [PubMed]
- Murugesan S, Ravichandran D, Lakshmanan DK, Ravichandran G, Arumugam V, Raju K, et al. Evaluation of anti-rheumatic activity of Piper betle L.(Betelvine) extract using in silico, in vitro and in vivo approaches. Bioorg Chem 2020;103:104227.
[Crossref] [Google Scholar] [PubMed]
- Kacmarczyk TJ, Fall MP, Zhang X, Xin Y, Li Y, Alonso A, et al. “Same difference”: Comprehensive evaluation of four DNA methylation measurement platforms. Epigenetics Chromatin 2018;11:1-6.
[Crossref] [Google Scholar] [PubMed]
- Latutrie M, Gourcilleau D, Pujol B. Epigenetic variation for agronomic improvement: An opportunity for vegetatively propagated crops. Am J Bot 2019;106(10):1281.
[Crossref] [Google Scholar] [PubMed]
- Fukuda M, Fujiwara T, Nishida S. Roles of non-coding RNAs in response to nitrogen availability in plants. Int J Mol Sci 2020;21(22):8508.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Yang Y, Jin L, Ling X, Liu T, Chen T, et al. Re-analysis of long non-coding RNAs and prediction of circRNAs reveal their novel roles in susceptible tomato following TYLCV infection. BMC Plant Biol 2018;18:1-6.
[Crossref] [Google Scholar] [PubMed]
- Pang J, Zhang X, Ma X, Zhao J. Spatio-temporal transcriptional dynamics of maize long non-coding RNAs responsive to drought stress. Genes 2019;10(2):138.
- Joshi M, Rajender S. Long non-coding RNAs (lncRNAs) in spermatogenesis and male infertility. Reprod Biol Endocrinol 2020;18(1):103.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Guo H, Hu W, Ji W. The emerging role of long non-coding RNAs in plant defense against fungal stress. Int J Mol Sci 2020;21(8):2659.
[Crossref] [Google Scholar] [PubMed]
- Xu S, Dong Q, Deng M, Lin D, Xiao J, Cheng P, et al. The vernalization-induced long non-coding RNA VAS functions with the transcription factor TaRF2b to promote TaVRN1 expression for flowering in hexaploid wheat. Mol Plant 2021;14(9):1525-38.
[Crossref] [Google Scholar] [PubMed]
- Azar AR, Maroufi A. Identification of long non-coding RNA transcripts in Glycyrrhiza uralensis. Iran J Biotechnol 2022;20(1):e2607.
[Crossref] [Google Scholar] [PubMed]
- Sanubol A, Chaveerach A, Sudmoon R, Tanee T, Noikotr K, Chuachan C. Betel-like-scented Piper plants as diverse sources of industrial and medicinal aromatic chemicals. Chiang Mai J Sci 2014;41(5/1):1171-81.
- Degenhardt J, Köllner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009;70(15-16):1621-37.
[Crossref] [Google Scholar] [PubMed]
- Damayanti AA, Trisnawati NL, Suyanto H. Wavelength identification of red betel leaf (Piper crocatum), green betel leaf (Piper betle L.) and black betel leaf (Piper betle v.) using Ultraviolet-Visible (UV-Vis) spectroscopy method coupled with Principal Component Analysis (PCA). SPIE 2021;11789:133-39.
- Madhumita M, Guha P, Nag A. Bio‐actives of betel leaf (Piper betle L.): A comprehensive review on extraction, isolation, characterization, and biological activity. Phytother Res 2020;34(10):2609-27.
[Crossref] [Google Scholar] [PubMed]
- Tagrida M, Benjakul S. Ethanolic extract of Betel (Piper betle L.) and Chaphlu (Piper sarmentosum Roxb.) dechlorophyllized using sedimentation process: Production, characteristics, and antioxidant activities. J Food Biochem 2020;44(12):e13508.
- Tan KH, Nishida R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J Insect Sci 2012;12(1):56.
[Crossref] [Google Scholar] [PubMed]
- Khalil AA, ur Rahman U, Khan MR, Sahar A, Mehmood T, Khan M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv 2017;7(52):32669-81.
- Koeduka T, Fridman E, Gang DR, Vassao DG, Jackson BL, Kish CM, et al. Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc the Natl Acad Sci 2006;103(26):10128-33.
[Crossref] [Google Scholar] [PubMed]
- Abdulhamid A, Awad TA, Ahmed AE, Koua FH, Ismail AM. Acetyleugenol from Acacia nilotica (L.) exhibits a strong antibacterial activity and its phenyl and indole analogues show a promising anti-TB potential targeting PknE/B protein kinases. Microbiol Res 2021;12(1):1-5.
- Lima LM. Safrole and the versatility of a natural biophore. Rev Virtual Quim 2015;7:495-538.
- Noge K, Becerra JX. Germacrene D, A common sesquiterpene in the genus Bursera (Burseraceae). Molecules 2009;14(12):5289-97.
[Crossref] [Google Scholar] [PubMed]
- Duta DE, Culetu A, Negoita M, Ionescu V. Quantification of anethole in fennel and anise essential oils using gas chromatography and 1H-NMR-spectroscopy. Bull UASVM Food Sci Technol 2019;76(2):105-13.
- Gang DR, Lavid N, Zubieta C, Chen F, Beuerle T, Lewinsohn E, et al. Characterization of phenylpropene O-methyltransferases from sweet basil: Facile change of substrate specificity and convergent evolution within a plant O-methyltransferase family. Plant Cell 2002;14(2):505-19.
[Crossref] [Google Scholar] [PubMed]
- Afifi SM, El-Mahis A, Heiss AG, Farag MA. Gas chromatography–mass spectrometry-based classification of 12 fennel (Foeniculum vulgare Miller) varieties based on their aroma profiles and estragole levels as analyzed using chemometric tools. ACS Omega 2021;6(8):5775-85.
[Crossref] [Google Scholar] [PubMed]
- Vaiciulyte V, Loziene K, Svediene J, Raudoniene V, Paskevicius A. α-Terpinyl acetate: Occurrence in essential oils bearing Thymus pulegioides, phytotoxicity, and antimicrobial effects. Molecules 2021;26(4):1065.
[Crossref] [Google Scholar] [PubMed]
- Fiorenzani P, Lamponi S, Magnani A, Ceccarelli I, Aloisi AM. In vitro and in vivo characterization of the new analgesic combination Beta‐caryophyllene and docosahexaenoic acid. Evid Based Complement Altern Med 2014;2014(1):596312.
[Crossref] [Google Scholar] [PubMed]
- Basak S, Guha P. Modelling the effect of essential oil of betel leaf (Piper betle L.) on germination, growth, and apparent lag time of Penicillium expansum on semi-synthetic media. Int J Food Microbiol 2015;215:171-8.
[Crossref] [Google Scholar] [PubMed]
- Ashokkumar K, Vellaikumar S, Murugan M, Dhanya MK, Ariharasutharsan G, Aiswarya S, et al. Essential oil profile diversity in cardamom accessions from southern India. Front Sustain Food Syst 2021;5:639619.
- Nayaka NM, Sasadara MM, Sanjaya DA, Yuda PE, Dewi NL, Cahyaningsih E, et al. Piper betle (L): Recent review of antibacterial and antifungal properties, safety profiles, and commercial applications. Molecules 2021;26(8):2321.
[Crossref] [Google Scholar] [PubMed]