- *Corresponding Author:
- M. Alkhaled
Department of Biology, College of Science, University of Jeddah, Jeddah 21589, Saudi Arabia
E-mail: malkhaled@uj.edu.sa
| This article was originally published in a special issue,“New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “42-47” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Natural killer cells are specific innate lymphoid cells which have therapeutic ability in adoptive cell transferprimarily based on most cancers immunotherapy that has been installed throughout a variety of earlyphase clinical trials. Natural killer cells to be used in adoptive transfer treatments are received from diverse resources. These cells have attracted widespread interest as adoptive immunotherapy for most cancers because of their preventive antitumor properties. In order to study the efficacy of adoptive natural killer cell immunotherapy in a preclinical manner with clinical translation potential, a reliable ex vivo natural killer cell expansion platform may be required. As a preclinical study, we have designed a humanized mouse model using NOD scid gamma mice, human leukaemia cells and expanded natural killer cells. At the beginning, peripheral blood mononuclear cells from patients with different thalassemia subtypes were co-cultured with irradiated, genetically engineered K562-mb15-41BBL cells in the presence of interleukin-2 for 14 d. Humanized NOD scid gamma mice with human leukaemia were treated with different approaches of natural killer cells, gamma delta cells and clusters of differentiation 20 immunoglobulin G1 antibody. In vivo results show that our strategy of immunotherapy with expanded natural killer cells has extended survival of mice. Flow cytometry results from the peripheral blood mononuclear cells of the treated mice showed that the expanded natural killer cells have potential ability to effectively kill the leukaemia cells. Based on these findings, adoptive transfer of expanded and activated natural killer cells ex vivo is gaining similar clinical evaluation as a potential new therapeutic alternative for patients with tumors and other immunological diseases.
Keywords
Thalassemia, immunotherapy, natural killer cells, NOD scid gamma mice
During the last decade, there was excellent interest in making use of Natural Killer (NK) cells adaptive immune treatment for numerous tumors [1-5]. The natural potential of NK cells to kill tumor cells without prior sensitization [6], their central role in anticancer reaction and immune monitoring vs. most cancers [7], in conjunction with the findings that NK cells did not induce Graft Versus Host Disease (GVHD) after Hematopoietic Cell Transplantation (HCT) [8], at the same time as preservation transplant vs. tumor impact have all been critical to the general enchantment of NK cells for adaptive immune treatment for most cancers [9]. Even so, the development of NK cell immunotherapy for most cancers has been sluggish, especially because of the challenge in acquiring enough quantity of NK cells (NKs) for strong preclinical and medical critiques. In regular human Peripheral Blood Mononuclear Cells (PBMCs), the NK cells include 1 %-32.6 % of PBMCs [10]. Using immunotherapy has extended extensively during the last 25 y. The new findings in fundamental biology of NKs, collectively with developing scientific experience inside the establishment of HCT, have positioned NKs alongside a direction of translational refinement. A better knowledge of the mutual influences among stimulated and suppressive immune cells that respond to infected cells have antitumor roles in establishing NK therapy for cancer, essential clinical advances encompass using cytokines to prompt NKs in vivo [11-13], redirecting or modifying NK cell signatures using monoclonal antibodies [4-6], adoptive transfer of T cells or NK cells with anti- tumor interest [14,15] and crucially NKs are engineered to engraft with synthetic receptors specific for cancer cells unique antigens [16,17]. In addition to NKs there are the gamma delta cells (γδ cells), γδ cells are a little part of lymphocytes cytotoxic T cells. The γδ cells do not request antigenic presentation by Major Histocompatibility Complex (MHC) molecules for recognition and activation [18,19]. Many studies showed that the expansion of γδ cells in vitro or in vivo is realistic and functional [20,21].
Materials and Methods
Ex vivo expansion of NK cells:
Peripheral blood was obtained from patients and healthy donors after informed written consent. PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation. Cells were incubated with irradiated (100 Gy) artificial antigen expressing K562 modified to express a membrane-bound form of Interleukin (IL)-15 and 4-1BB Ligand (K562-mb15- 41BBL) cells at a ratio of 1:1.5 (PBMC:irradiated feeder cells) in Roswell Park Memorial Institute (RPMI) 1640 medium (Life Technologies, Darmstadt, Germany) supplemented with 10 % human AB serum (Department of Transfusion Medicine, University Hospital Tuebingen, Germany), L-glutamine (Life Technologies) and 100 IU/ml IL-2 (Proleukin) (Novartis, Basel, Suisse). The K526-mb15-41BBL cell line which was genetically modified to express membrane-bound IL-15 and 4-1BB (Cluster of Differentiation (CD) 137) ligand was kindly provided by Dario Campana, Department of Paediatrics, Center for Translational Medicine, National University of Singapore, Singapore. Half of the medium changes were done every 2-3 d with fresh IL-2 containing medium. Cell culture was maintained under the above described conditions for 14 d. Expanded NK cells were purified by CD56 positive cell selection with antibody-conjugated immunomagnetic microbeads (Milteny Biotec, Bergisch Gladbach, Germany) followed by CD3 positive cell depletion using Dynabeads (Life Technologies, Darmstadt, Germany). Phenotyping of PBMCs of treated NOD Scid Gamma (NSG) mice cells was performed using directly conjugated monoclonal antibodies or appropriate isotype controls against: CD16 (clone 3G8), CD25 (clone M-A251) FITC, CD56 (clone B159) PE-Cy7, CD19 (clone IB19 RUO), CD10 (clone HI10a) (Becton Dickinson, Heidelberg, Germany).CD3 (clone BW264/56) VioBlue (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were analyzed on a LSRII flow cytometer (Becton-Dickinson, Heidelberg, Germany) using FACSDiva (Becton Dickinson) and FCS Express software (de novo Software, Los Angeles, USA). Mean Fluorescence Intensity (MFI) ratios and percent positive cells were calculated for each cell surface antigen mice.
NSG mice:
NSG mice (JAX mouse strain name NOD. Cg- PrkdcscigdIl2rgtm1Wjl/SzJ; Jackson Laboratory, USA) were housed in air-flow cages under special pathogen-free conditions at the Laboratory Animal Facility of the University Children’s Hospital Tubingen, Germany. All animal procedures were evaluated by the Animal Care Committee of the University of Tubingen (No. K06/11).
Humanization of NSG mice:
Human CD34 (HuCD34) stem cells were obtained from peripheral blood stem cells mobilized with excess Granulocyte-Colony Stimulating Factor (G-CSF) from parental donors which were deficient in T-cells due to CD34 selection (CliniMACS, Miltenyi, Germany). The cells were added in a proportion of 1:2 to a solution of Dimethyl Sulfoxide (DMSO)/5 % Human Serum Albumin (HSA) (20 %/80 %) and a SY-LAB Ice cube device and a regulated freezing rate was used to cytopreserve them. Cells were stained using Trypan blue following thawing and a Neubauer cell count chamber was used to determine their numbers. All donors provided their informed consent to scientifically use surplus cells according to the Declaration of Helsinki. There was an additional increase in the purity of the CD34 Complex (C) population to more than 99.99 % through a second round of CD3C depletion following thawing (LS Magnetic-activated cell sorting (MACS), Miltenyi). All of the stem cell donors were Human Leukocyte Antigen (HLA) mismatched to the human Rhabdomyosarcoma cell lines (RMS A204). The HuCD34C cells (1X106 cells in 100 ml prewarmed Phosphate Buffered Saline (PBS)) were added to the tail vein of sublethally irradiated (250 centigray (cGy)) NSG mice. Engraftment was supported by applications of 20 mg FcIL-7 (Merck, Germany) on a weekly basis. In every Nance-Horan syndrome (NHS)-IL 12 (IL-12 was complexed with NHS76) treatment group, long-term NHS-IL 12 cytokine treatment was given to 4 animals with FcIL-7 or IL- 2MAB602 for 15 w at most (100 d).
Tumor implantation and measurement:
Human leukaemia cells were obtained from patient blood and implantation was done into engrafted NSG mice. For each mouse,1X106 leukaemia cells were transplanted directly into the tail vein of NSG mice.
Treatment with expanded NK cells:
Two times every week for each mouse 5X106 expanded NKs or 2.5X106 expanded NKs+2.5X106 expanded γδ cells with and without using antibody (concentrations of 0.4 µg/ml of CD20 Immunoglobulin G 1 (IgG1) antibody; Mabthera; Roche, Basel, Switzerland) were injected in the tail vein of mice. Treatment was started 24 h after tumor implantation. The control group was injected with Primary Biliary Cholangitis (PBC). Treatment was terminated after 3 w.
Statistical analysis:
GraphPad Prism version 5 (GraphPad Software Inc, La Jolla, United States of America (USA)) was used to carry out statistical analysis. Data expression was either in the form of median and range or as mean±Standard Error of the Mean (SEM). Two- tailed t-test was carried out to find out the significance levels and p values of 0.05 or lower was deemed to be statistically significant.
Results and Discussion
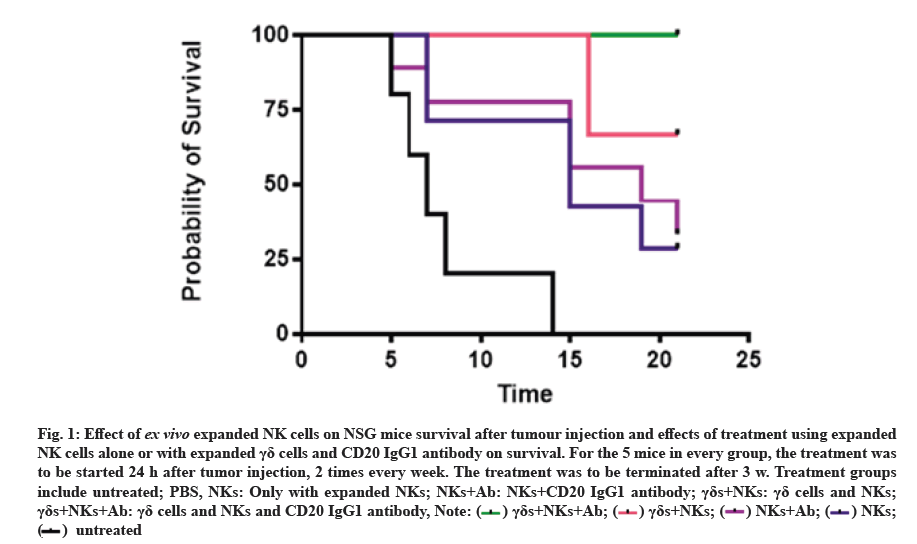
Treatment using ex vivo expanded NK cells increased the survival rate of mice. To confirm the cytotoxicity of NK cells after ex vivo expansion against human leukaemia cells, we tested this toxicity in vivo using humanized NSG mice. 24 h after injection of leukaemia cells we started the treatment with ex vivo expanded NK cells alone or with γδ cells and CD20 IgG1 antibody, we analyzed the effect of treatment on the survival of mice. The survival curves are shown in fig. 1. The cumulative percentage survival was significantly higher after treatment with NK cells, γδ cells and CD20 IgG1 antibody in comparison with the untreated mice (67.1 %). In general, treatment with NK cells increased survival rate of mice (fig. 1).
Fig. 1:Effect of ex vivo expanded NK cells on NSG mice survival after tumour injection and effects of treatment using expanded NK cells alone or with expanded γδ cells and CD20 IgG1 antibody on survival. For the 5 mice in every group, the treatment was to be started 24 h after tumor injection, 2 times every week. The treatment was to be terminated after 3 w. Treatment groups include untreated; PBS, NKs: Only with expanded NKs; NKs+Ab: NKs+CD20 IgG1 antibody; γδs+NKs: γδ cells and NKs; γδs+NKs+Ab: γδ cells and NKs and CD20 IgG1 antibody,

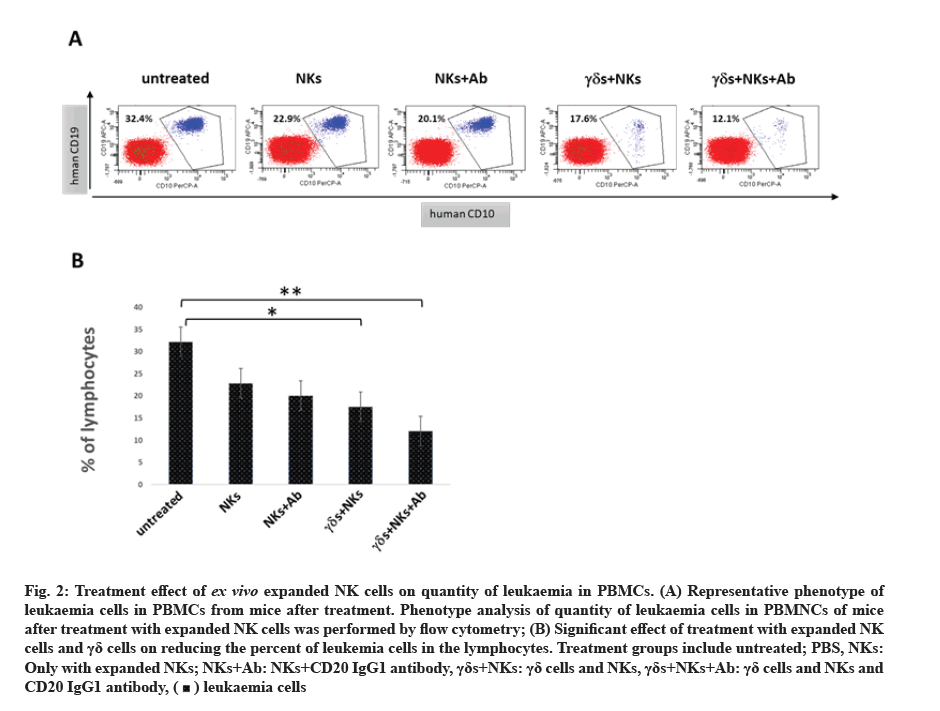
Treatment using ex vivo expanded NK cells reduced the proliferation rate of leukaemia in PBMCs of mice. To understand the effect of the treatment using ex vivo expanded NK cells on the survival rate of mice, we analysed the quantity of leukaemia cells in PBMCs from mice after treatment. The flow cytometry rate shows that the proliferation rate of leukaemia cells was reduced by 10 % after the treatment with expanded NK cells alone, while the treatment in combination between expanded NK cells and γδ cells and CD20 IgG1 has significantly reduced the proliferation rate of leukaemia cells by 20 % (fig. 2).
Fig. 2:Treatment effect of ex vivo expanded NK cells on quantity of leukaemia in PBMCs. (A) Representative phenotype of leukaemia cells in PBMCs from mice after treatment. Phenotype analysis of quantity of leukaemia cells in PBMNCs of mice after treatment with expanded NK cells was performed by flow cytometry; (B) Significant effect of treatment with expanded NK cells and γδ cells on reducing the percent of leukemia cells in the lymphocytes. Treatment groups include untreated; PBS, NKs: Only with expanded NKs; NKs+Ab: NKs+CD20 IgG1 antibody, γδs+NKs: γδ cells and NKs, γδs+NKs+Ab: γδ cells and NKs and CD20 IgG1 antibody,
The results of this study indicate that NK cells can be efficiently ex vivo expanded and activated despite functional impairment. Co-culture with irradiated K562 cells genetically modified to express membrane-bound IL-15 and 4-1BB ligand shifted the NK cell receptor balance towards activation, resulting in enhanced cytotoxicity against tumor leukemia cells. A main fence in cancer immunotherapy has been the several mechanisms by which cancer induce malfunction or tolerance of immune cells [22]. The application of NK cells as therapeutic beginning for the treatment of cancer, an inversion of phenotypic and functional failure is of utmost importance. Before ex vivo expansion, NK cells from patients showed reduced expression of activating receptors and no cytotoxic activity against tumor cell lines. This tumor-associated NK cell phenotype is a common phenomenon in patients with different kinds of malignancies. Moreover, in recent decades, the monoclonal antibodies have been better used to carry out the increase in recognition of the cancer cells by immune cells [23-25]. There are several mechanisms of monoclonal antibodies functions which enclose supplement fixation, induction of Antibody-Dependent Cellular Cytotoxicity (ADCC) and induction of aberrant signaling. The isotype IgG1 subclass is best often used for antibody treatment since it has approved and extremely forceful at stimulating and activating Fragment crystallizable (Fc) receptors on NK cells, neutrophils and macrophages [26]. Medical application of NK cells has been inspired by acceptance of their powerful antitumor activity. Many studies at present shown a respectable foundation for evolution of future NK cell trials for immunotherapy as reduction risks and toxicities [14,20,27-29]. For the improvement of NK cell treatments, either additional study of basic NK biology as a further insight of interactions with other immune cells will be necessary. The aim of developing clinical studies in the future will be to overcome the patient’s immune barriers. In addition, new strategies should be designed to achieve better ex vivo expansion rates. Based on the data presented, ex vivo expansion and activation of NK cells deserves further clinical evaluation as a possible new treatment approach for patients with soft tissue leukaemia.
Author’s contributions:
All authors listed have made a substantial, direct and intellectual contribution to the work and approved it for publication.
Acknowledgements:
This work was funded by the University of Jeddah, Saudi Arabia, under grant No. UJ-08-18-ICP. The authors, therefore, acknowledges and thanks the university for technical and financial support.
Conflict of interests:
The authors declare that there is no conflict of interest.
References
- Buddingh EP, Schilham MW, Ruslan SE, Berghuis D, Szuhai K, Suurmond J, et al. Chemotherapy-resistant osteosarcoma is highly susceptible to IL-15-activated allogeneic and autologous NK cells. Cancer Immunol Immunother 2011;60(4):575-86.
[Crossref] [Google Scholar] [PubMed]
- Alici E, Sutlu T, Björkstrand B, Gilljam M, Stellan B, Nahi H, et al. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood 2008;111(6):3155-62.
[Crossref] [Google Scholar] [PubMed]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105(8):3051-7.
[Crossref] [Google Scholar] [PubMed]
- Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother 2010;59(12):1781-9.
[Crossref] [Google Scholar] [PubMed]
- Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 2010;28(6):955-9.
[Crossref] [Google Scholar] [PubMed]
- Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975;5(2):112-7.
- Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000;356(9244):1795-9.
[Crossref] [Google Scholar] [PubMed]
- Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 1999;285(5426):412-5.
[Crossref] [Google Scholar] [PubMed]
- Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010;115(21):4293-301.
[Crossref] [Google Scholar] [PubMed]
- Pittari G, Fregni G, Roguet L, Garcia A, Vataire AL, Wittnebel S, et al. Early evaluation of natural killer activity in post-transplant acute myeloid leukemia patients. Bone Marrow Transplant 2010;45(5):862-71.
[Crossref] [Google Scholar] [PubMed]
- Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, et al. Safety (toxicity), pharmacokinetics, immunogenicity and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood 2011;117(18):4787-95.
[Crossref] [Google Scholar] [PubMed]
- Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: Implications for immunotherapy. Immunity 2001;14(2):105-10.
[Crossref] [Google Scholar] [PubMed]
- Becknell B, Caligiuri MA. Interleukin-2, interleukin-15 and their roles in human natural killer cells. Adv Immunol 2005;86:209-39.
[Crossref] [Google Scholar] [PubMed]
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007;7(5):329-39.
[Crossref] [Google Scholar] [PubMed]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008;8(4):299-308.
[Crossref] [Google Scholar] [PubMed]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725-33.
[Crossref] [Google Scholar] [PubMed]
- June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: Tools, trials and tribulations. Nat Rev Immunol 2009;9(10):704-16.
[Crossref] [Google Scholar] [PubMed]
- Legut M, Cole DK, Sewell AK. The promise of γδ T cells and the γδ T cell receptor for cancer immunotherapy. Cell Mol Immunol 2015;12(6):656-68.
[Crossref] [Google Scholar] [PubMed]
- Silva-Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol 2015;15(11):683-91.
[Crossref] [Google Scholar] [PubMed]
- Fournié JJ, Sicard H, Poupot M, Bezombes C, Blanc A, Romagné F, et al. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol 2013;10(1):35-41.
[Crossref] [Google Scholar] [PubMed]
- Marcu-Malina V, Garelick D, Peshes-Yeloz N, Wohl A, Zach L, Nagar M, et al. Peripheral blood-derived, γ9δ2 t cell-enriched cell lines from glioblastoma multiforme patients exert anti-tumoral effects in vitro. J Biol Regul Homeost Agents 2016;30(1):17-30.
[Google Scholar] [PubMed]
- Shi J, Tricot G, Szmania S, Rosen N, Garg TK, Malaviarachchi PA, et al. Infusion of haplo?identical killer immunoglobulin?like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol 2008;143(5):641-53.
[Crossref] [Google Scholar] [PubMed]
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010;10(5):317-27.
[Crossref] [Google Scholar] [PubMed]
- Romagné F, André P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti–KIR receptor therapeutic antibody that augments natural killer–mediated killing of tumor cells. Blood 2009;114(13):2667-77.
[Crossref] [Google Scholar] [PubMed]
- Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science 2013;341(6151):1192-8.
[Crossref] [Google Scholar] [PubMed]
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012;12(4):278-87.
[Crossref] [Google Scholar] [PubMed]
- Akhter N, Shiba K, Ogawa K, Tsuji S, Kinuya S, Nakajima K, et al. A change of in vivo characteristics depending on specific activity of radioiodinated (+)-2- [4-(4-iodophenyl) piperidino] cyclohexanol [(+)-pIV] as a ligand for sigma receptor imaging. Nucl Med Biol 2008;35(1):29-34.
- Rosenberg SA, Dudley ME, Restifo NP. Cancer immunotherapy. N Engl J Med 2008;359(10):1072.
- Tsuchida R, Das B, Yeger H, Koren G, Shibuya M, Thorner PS, et al. Cisplatin treatment increases survival and expansion of a highly tumorigenic side-population fraction by upregulating VEGF/Flt1 autocrine signaling. Oncogene 2008;27(28):3923-34.
[Crossref] [Google Scholar] [PubMed]