- *Corresponding Author:
- Sri Sakthi Priyadarsini
Department of Pharmacognosy, Sri Ramaswamy Memorial College of Pharmacy, Faculty of Medicine and Health Sciences, Sri Ramaswamy Memorial Institute of Science and Technology, Kanchipuram, Chennai 603203, India
E-mail: sakthivendan@gmail.com
| Date of Received | 25 May 2020 |
| Date of Revision | 05 January 2022 |
| Date of Acceptance | 04 October 2022 |
| Indian J Pharm Sci 2022;84(5):1197-1202 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In the present study, the shade dried powdered leaves of Rhinacanthus nasutus (L.) Kurz., was subjected to maceration with ethyl acetate, concentrated and evaporated to dryness. The ethyl acetate extract was subjected to anion exchange column chromatography and the isolated rhinacanthin enriched extract was analyzed by thin layer chromatography and fourier-transform infrared studies. In the present study, the in vitro cytotoxic activity of the rhinacanthin rich extract of Rhinacanthus nasutus (L) Kurz., over SH-SY5Y human neuroblastoma cell line was assessed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide assay and compared with the standard drug doxorubicin. The preliminary phytochemical screening revealed the presence of alkaloids, quinones, glycosides, carbohydrates, amino acids and tannins. The thin layer chromatography profiling showed the presence of secondary metabolites in various mobile phase systems. Fourier-Transform Infrared analysis of rhinacanthin enriched extract confirmed the presence of functional groups including 1, 4-quinone carbonyl component. The results of in vitro cell line studies revealed a dose dependent cytotoxic effect on human neuroblastoma cell lines with an IC50 value of 88.9 μg/ml. Thus the results validate the potential of rhinacanthin enriched extract against neuroblastoma cancer and based on this initial screening, further studies at elucidation of its molecular mechanism as cancer therapeutics can be undertaken.
Keywords
Anion exchange chromatography, fourier-transform infrared analysis, naphthoquinones, neuroblastoma
Neuroblastoma stands as the common malignant tumor in infants with tumor invasion and metastasis being the two major causes[1,2]. It is found to be derived from the primitive sympathetic neural precursor cells of peripheral nervous system[3]. Most neuroblastoma develop in adrenal medulla but also a few from Para spinal sympathetic ganglia of neck, chest, abdomen or pelvis[3,4]. Early detection and diagnosis followed by drugs targeting at molecular level play a major role in the survival and quality of life of patients with neuroblastoma[5].
Rhinacanthus nasutus (R. nasutus) (L.) Kurz, commonly known as ‘Nagamalli’ in Tamil holds a promising and proven traditional claims which was found due to the presence of varied active metabolites including naphthoquinones, flavonoids, coumarins and phytosterols[6,7]. The plant leaves has been traditionally used in the treatment of cancer[6]. Rhinacanthins were the naphthoquinones reported in R. nasutus (L.) Kurz with potential cytotoxic activity[8,9]. Studies report the neuroprotective role of R. nasutus (L.) Kurz which was attributed to the antioxidant activity of the plant extracts[10,11]. Hence the present study was aimed at investigating the antitumor effect of rhinacanthin enriched extract from the leaves of R. nasutus (L.) Kurz., in SH-SY5Y neuroblastoma cell lines.
Leaves of R. nasutus (L.) Kurz were collected from Chengalpattu District, Tamil Nadu in a fine dry weather during October 2019. The plant was identified and authenticated by Plant Anatomy Research Centre, Chennai, No. PARC/2020/4280. Amberlite IRA-67 and Doxorubicin HCl used in this study were purchased from Sigma-Aldrich. All the chemicals and reagents were purchased from certified suppliers and were of highest analytical grade. SH-SY5Y human neuroblastoma cell lines were obtained from National Centre for Cell Sciences, Pune, India (NCCS). The cells were maintained in Dulbecco Modified Eagle Medium (DMEM) supplemented with 10 % inactivated Fetal Bovine Serum (FBS), penicillin (100 U/ml) and streptomycin (100 µg/ml) in a humidified atmosphere of 5 % Carbon dioxide (CO2) at 37°.
The plant material was dried in a hot air oven at 50° and were coarsely powdered and stored in an air tight container for further studies[12]. Extraction was performed by cold maceration of coarsely powdered leaves of R. nasutus (L.) Kurz with the solvent ethyl acetate. The extract obtained was then concentrated by distilling the solvent and evaporated to dryness. The percentage yield of the ethyl acetate extract was found to be 3.78 % w/w (Table 1).
| Extract | Method of extraction/isolation | Physical nature | Colour | Yield |
|---|---|---|---|---|
| Ethyl acetate extract | Cold maceration | Sticky | Greenish black | 3.78 % w/w |
| Rhinacanthin enriched extract | Anion resin exchange column chromatography | Semisolid mass | Reddish brown | 0.6 % w/w |
Table 1: The Percentage Yield of Ethyl Acetate Extract And Rhinacanthin Enriched Extract of Leaves of R. nasutus.
Fractionation of ethyl acetate extract was carried out by anion resin exchange chromatography using the method followed by Panichayupakaranant et al.[12] with some modifications. The resin Amber lite IRA-67 (100 gm) was treated with 50 ml methanol, gently stirred and allowed to settle for 15 min. The mixture was then decanted and the treated resin was washed twice with distilled water. The slurry was allowed to stand in methanol for further 10 min. A glass column (5×35 cm) was packed with the treated resin slurry and the excess methanol was drained followed by subsequent addition of fresh solvent. The ethyl acetate extract was treated with methanol and filtered. This is loaded on the top of the resin and the column was allowed to run at a flow rate of 1.5 ml/min. After the green pigments if any, were eluted, the 10 % acetic acid in methanol was added to the column at a flow rate 2 ml/min[12]. These fractions were collected and subjected to ‘sulphuric acid test’ for quinones[13] followed by Thin Layer Chromatography (TLC) using Methanol:Acetic acid (9:1) solvent system. The fractions with positive response for quinones giving a red color with concentrated sulphuric acid were pooled together, evaporated to dryness which yielded 0.6 % w/w of rhinacanthin enriched extract and was subjected to further studies (Tables 1 and 2).
| Eluent | Fraction | Appearance | Phytoconstituents |
|---|---|---|---|
| Methanol | 1 | Colourless | Negative for quinones |
| 2-11 | Slight green | Negative for quinones | |
| 10 % acetic acid in methanol | 12-17 (F1) | Yellow sticky mass | Positive for quinones |
| 18-22 (F2) | Brown semisolid mass | Positive for quinones | |
| 23-37 (F3) | Bright red semisolid mass | Positive for quinones |
Table 2: Isolation of Rhinacanthin Rich Extract by Anion Exchange Column Chromatography.
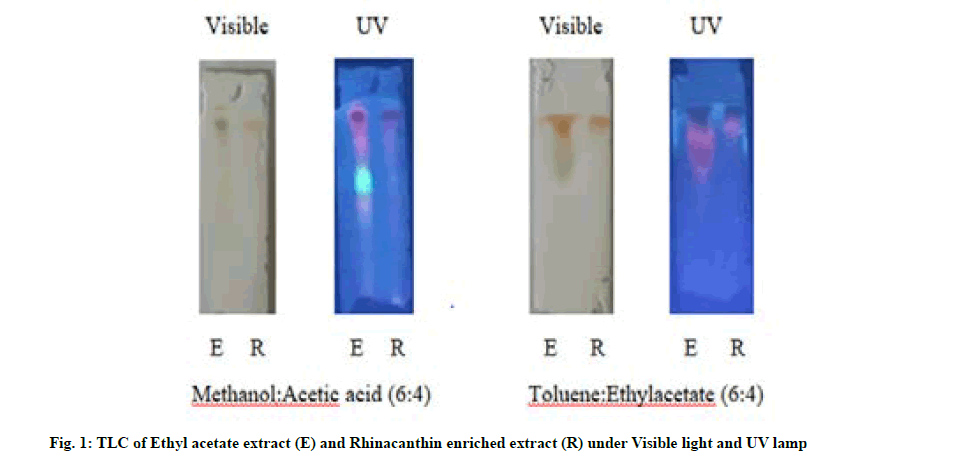
Qualitative preliminary phytochemical analysis of the leaf powder, ethyl acetate extract and rhinacanthin enriched extract was carried out with various chemical detecting agents and their chemical nature was recorded[13,14]. TLC profiling of ethyl acetate extract of R. nasutus (L.) Kurz and rhinacanthin enriched extract was carried out on TLC plates (20×20) precoated with silica gel G. TLC was analyzed with various solvent systems in the literature at different ratios by trial and error method. The plates were visualized both under normal and Ultraviolet (UV) lamp and the spots were observed and Retardation Factor (Rf) values were calculated[14].
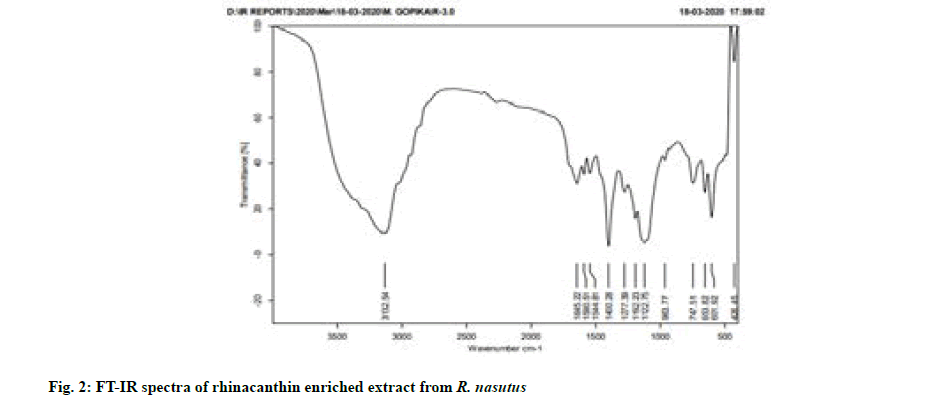
Fourier-Transform Infrared (FT-IR) spectroscopy was carried out using FT-IR spectrometer (Version 7.0 Bruker Optic). The rhinacanthin enriched extract obtained by column fractionation was mixed with 200 mg Potassium bromide (KBr), pressed into a pellet and FT-IR spectra were recorded.
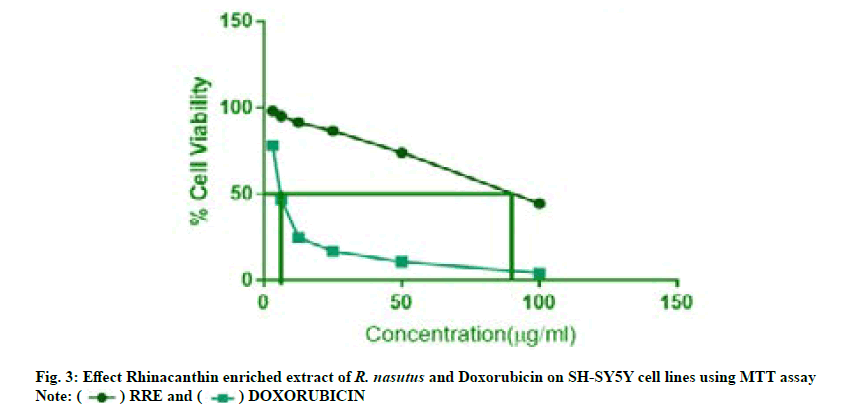
Cytotoxicity studies of rhinacanthin enriched extract on neuroblastoma cell lines were carried out using 3-(4,5-Dimethyl-2-Thiazolyl)-2,5-Diphenyl-Tetrazolium Bromide (MTT) assay. The assay is based on the ability of the metabolically active cells to convert yellow tetrazolium salt MTT to purple formazan crystals. Cells (1×105/100 μl) were seeded in a 96-well flat bottomed plate and incubated with various concentrations of rhinacanthin enriched extract of R. nasutus (L.) Kurz. After 24 h, the sample was treated with phosphate-buffered saline (pH 7.4). Then 100 µl/well (5 mg/ml) of 0.5 % MTT was added to each well and incubated for 4 h at 37°. The formazan crystals were then solubilized by adding 100 µl of Dimethyl Sulfoxide (DMSO). UV-Spectrophotometer was used to measure the absorbance at 570 nm with DMSO as control blank and the percentage cell viability was calculated. Graphs were plotted using the percentage cell viability at Y-axis and concentration of the sample in X-axis and the concentration required for a 50 % Inhibition (IC50) was determined graphically[15-17].
All data were presented as the mean±Standard Deviations (SD). The experiment was repeated at least in triplicates. Unpaired t-test was included to compare the difference between the groups using Graph pad prism. Any value of p<0.01 was considered as statistically significant. The results of the qualitative phytochemical screening revealed that the powdered plant material showed the presence of carbohydrates, amino acids, steroids, glycosides, tannins and quinones. Carbohydrates, amino acids, quinones were present in the ethyl acetate extract and with quinones and carbohydrates in the rhinacanthin enriched extract.
TLC was run one dimensionally in the mobile phase solvent systems, methanol:Acetic acid (6:4) and toluene:ethyl acetate (6:4) and the Rf values were tabulated in Table 3. 2 spots were found to be active in ethyl acetate extract (0.82, 0.93) whereas rhinacanthin enriched extract exhibited a single spot of Rf values 0.94 in the solvent system methanol:acetic acid (6:4). TLC run in the mobile phase system, toluene:ethylacetate (6:4) revealed the presence of 4 spots with Rf values, 0.96, 0.93, 0.72, 0.67 whereas the rhinacanthin enriched extract showed a single spot with similar Rf value of 0.96 in correlation with that obtained in ethyl acetate extract (fig. 1).
| Solvent system | Extract | Number of spots | UV detection Rf value | |
|---|---|---|---|---|
| Near UV | Far UV | |||
| Methanol:acetic acid (6:4) | Ethyl acetate extract | 2 | No UV active compounds | 0.82, 0.93 |
| Rhinacanthin Rich extract | 1 | No UV active compounds | 0.94 | |
| Toluene:ethylacetate | Ethyl acetate extract | 4 | No UV active compounds | 0.96, 0.93, 0.72, 0.67 |
| (6:4) | Rhinacanthin Rich extract | 1 | No UV active compounds | 0.96 |
Table 3: TLC of Ethyl Acetate Extract and Rhinacanthin Enriched Extract of Leaves of R. nasutus.
FT-IR analysis was based on the vibrations of the functional groups present in the sample at specific wave numbers. The Infrared (IR) absorption bands at 1644 cm-1 showed the presence of 1, 4-quinone carbonyl group. The presence of a broad alcoholic hydroxyl group was indicated by an IR absorption band between 3200 cm-1 and 3400 cm-1 (fig. 2), thereby confirming the rhinacanthins in rhinacanthin enriched extract based on the previous literature studies[8,18].
The anti-tumor effect of rhinacanthin enriched extract from the ethyl acetate leaf extract of R. nasutus (L) Kurz., on neuroblastoma cell lines (SH-SY-5Y) was tabulated in Table 4. SH-SY5Y human neuroblastoma cells were used to assess cytotoxic potential by MTT assay (fig. 3). Doxorubicin HCl was used as the standard drug. The results of the in vitro cytotoxicity studies of rhinacanthin enriched extract revealed a dose dependent decline in cell viability at the end of 24 h with IC50 value of 88.9 µg/ml in SH-SY5Y cells. At the concentration level of 100 μg/ml, the rhinacanthin enriched extract exhibited 45 % of cytotoxic activity against SH-SY5Y cells (Table 4). The standard drug Doxorubicin showed an IC50 value of 6.5 μg/ml. Even though doxorubicin exhibited higher cytotoxicity compared to the rhinacanthin enriched extract, the development of resistance resulting in increased malignancy remains as a major concern[19].
| Concentration (µg/ml) | Percentage cell viability | |
|---|---|---|
| Rhinacanthin enriched extract | Doxorubicin | |
| Control (DMSO) | 100 | 100 |
| 3.12 | 98.16±0.02** | 78.23±0.01** |
| 6.25 | 95.21±0.01** | 46.92±0.01** |
| 12.5 | 91.40±0.01** | 24.85±0.02** |
| 25 | 86.51±0.01** | 16.84±0.01** |
| 50 | 74.05±0.02** | 10.73±0.01** |
| 100 | 44.58±0.02** | 4.21±0.02** |
Note: All data were presented as the mean±SD. The experiment was repeated at least in triplicates. Unpaired t-test was included to compare the difference between the groups using Graph pad prism. **Any value of p<0.01 was considered as statistically significant
Table 4: Cytotoxic Activity of Rhinacanthin Enriched Extract and Doxorubicin on SH-SY5Y Human Neuroblastoma Cell Line.
A varied array of chemical constituents including naphthoquinones, lignans, benzenoids, anthroquinones, triterpenoids, flavonoids, sterols, coumarins, glycosides were reported in R. nasutus[20]. Several literatures were reported, investigating the cytotoxic potential and the underlying mechanisms exhibited by the bioactive constituents of R. nasutus, particularly, the rhinacanthins, on different cancerous cell lines. In a study carried out by Wu et al.[8], among the two isolated naphthaquinones, rhinacanthin-A and rhinacanthin-B from the methanolic root extracts of R. nasutus, the rhinacanthin-B was found to exhibit cytotoxic potential with an ED50 value of 3.0 μg/ml in KB human epidermis carcinoma cell lines.
In another study carried out by Wu et al.[21], rhinacanthin-Q along with several known compounds from the methanolic root extracts of R. nasutus were investigated for the antiplatelet and cytotoxic effect against KB, P388, A549, HT29 and HL60 cell lines. Gotoh et al.[9] reported the anti-proliferative activity of the ethanolic root extract and aqueous leaf extracts in comparison to the chemically synthesized rhinacanthin C against human cervical carcinoma HeLa cell lines, Hyr100-6, multidrug resistant sub line of HeLa, PC-3 human prostate carcinoma cell line and T24 human bladder cell lines. The key findings revealed the bioactive potential of rhinacanthin C, particularly in R. nasutus roots. Further in vivo anti-proliferative study on sarcoma-180 bearing Institute of Cancer Research (ICR) mice showed a significant tumor inhibition exhibited by both the extracts[9]. In a study by Siripong et al.[22], yet another isolated bioactive compound, rhinacanthone showed an apoptotic cell death in HeLa cervical cancer cell lines by multiple pathways, primarily, through mitochondria-dependent signaling pathway. Moreover, antimetastatic activity of liposomal rhinacanthin-N, isolated from the roots of R. nasutus was reported on B16F10 melanoma cells induced pulmonary metastasis in C57BL/6 mice[23]. Horii et al.[24] reported the diversified biological activities of rhinacanthin C. Upon investigation of five isolated components rhinacanthin C, G, N, Q and rhinacanthone from the ethyl acetate fraction of methanolic extract of R. nasutus, the rhinacanthin C proved to be highly tumor specific and induced non-apoptotic cell death. Additionally, rhinacanthin C also inhibited RANKL-stimulated osteoclast formation in RAW 2647 cells[24]. Moreover, synergistic approach ensuring the phytoconstituents to combat in a coordinated manner could help in further phytotherapeutic research against neuroblastoma. Thus the current study concludes that rhinacanthins present in the enriched extract may possess cytotoxic potential against malignant neuroblastoma cells and further in vivo studies can be considered to focus on the exact molecular mechanisms involved in the anticancer activity.
Conflict of interests:
The authors declared no conflict of interest.
References
- Wu PY, Liao YF, Juan HF, Huang HC, Wang BJ, Lu YL, et al. Aryl hydrocarbon receptor downregulates MYCN expression and promotes cell differentiation of neuroblastoma. PloS One 2014;9(2):e88795.
[Crossref] [Google Scholar] [PubMed]
- Boubaker A, Delaloye AB. Nuclear medicine procedures and neuroblastoma in childhood: Their value in the diagnosis, staging and assessment of response to therapy. Q J Nucl Med Mol 2003;47(1):31-40.
[Google Scholar] [PubMed]
- Brodeur GM. Neuroblastoma: Biological insights into a clinical enigma. Nat Rev Cancer 2003;3(3):203-16.
[Crossref] [Google Scholar] [PubMed]
- Maris JM. Recent advances in neuroblastoma. N Engl J Med 2010;362(23):2202-11.
[Crossref] [Google Scholar] [PubMed]
- Tsubota S, Kadomatsu K. Origin and initiation mechanisms of neuroblastoma. Cell Tissue Res 2018;372(2):211-21.
[Crossref] [Google Scholar] [PubMed]
- Irfan AK, Atiya K, editors. Herbal medicine for human diseases. Hyderabad: Ukaaz Publications; 1995. p. 150-1.
- Ken Fern. Useful Tropical Plants Database. 2020.
- Wu TS, Tien HJ, Yeh MY, Lee KH. Isolation and cytotoxicity of rhinacanthin-A and-B, two; Naphthoquinones, from Rhinacanthus nasutus. Phytochemistry 1988;27(12):3787-8.
- Gotoh A, Sakaeda T, Kimura T, Shirakawa T, Wada Y, Wada A, et al. Antiproliferative activity of Rhinacanthus nasutus (L.) K URZ extracts and the active moiety, rhinacanthin C. Biol Pharm Bull 2004;27(7):1070-4.
[Crossref] [Google Scholar] [PubMed]
- Brimson JM, Brimson SJ, Brimson CA, Rakkhitawatthana V, Tencomnao T. Rhinacanthus nasutus extracts prevent glutamate and amyloid-β neurotoxicity in HT-22 mouse hippocampal cells: Possible active compounds include lupeol, stigmasterol and β-sitosterol. Int J Mol Sci 2012;13(4):5074-97.
[Crossref] [Google Scholar] [PubMed]
- Brimson JM, Tencomnao T. Medicinal herbs and antioxidants: Potential of Rhinacanthus nasutus for disease treatment? Phytochem Rev 2014;13(3):643-51.
- Panichayupakaranant P, Charoonratana T, Sirikatitham A. RP-HPLC analysis of rhinacanthins in Rhinacanthus nasutus: Validation and application for the preparation of rhinacanthin high-yielding extract. J Chromatogr Sci 2009;47(8):705-8.
[Crossref] [Google Scholar] [PubMed]
- María R, Shirley M, Xavier C, Jaime S, David V, Rosa S, et al. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J King Saud Univ Sci 2018;30(4):500-5.
- Harborne JB. Phytochemical method. A guide to modern techniques of plant analysis: 2nd ed. London: Chapman and Hall; 1973. p. 4-34.
- Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods 1986;94(1-2):57-63.
[Crossref] [Google Scholar] [PubMed]
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 1988;48(3):589-601.
[Google Scholar] [PubMed]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65(1-2):55-63.
- Sendl A, Chen JL, Jolad SD, Stoddart C, Rozhon E, Kernan M, et al. Two new naphthoquinones with antiviral activity from Rhinacanthus nasutus. J Nat Prod 1996;59(8):808-11.
[Crossref] [Google Scholar] [PubMed]
- Kotchetkov R, Cinatl J, Blaheta R, Vogel JU, Karaskova J, Squire J, et al. Development of resistance to vincristine and doxorubicin in neuroblastoma alters malignant properties and induces additional karyotype changes: A preclinical model. Int J Cancer 2003;104(1):36-43.
[Crossref] [Google Scholar] [PubMed]
- Bukke S, Raghu PS, Sailaja G, Kedam TR. The study on morphological, phytochemical and pharmacological aspects of Rhinacanthus nasutus.(L) kurz (A review). J Appl Pharm Sci 2011;1(8):26-32.
- Wu TS, Hsu HC, Wu PL, Teng CM, Wu YC. Rhinacanthin-Q, a naphthoquinone from Rhinacanthus nasutus and its biological activity. Phytochemistry 1998;49(7):2001-3.
[Crossref] [Google Scholar] [PubMed]
- Siripong P, Hahnvajanawong C, Yahuafai J, Piyaviriyakul S, Kanokmedhakul K, Kongkathip N, et al. Induction of apoptosis by rhinacanthone isolated from Rhinacanthus nasutus roots in human cervical carcinoma cells. Biol Pharm Bull 2009;32(7):1251-60.
[Crossref] [Google Scholar] [PubMed]
- Siripong P, Yahuafai J, Piyaviriyakul S, Kanokmedhakul K, Koide H, Ishii T, et al. Inhibitory effect of liposomal rhinacanthin-N isolated from Rhinacanthus nasutus on pulmonary metastasis in mice. Biol Pharm Bull 2012;35(7):1197-200.
[Crossref] [Google Scholar] [PubMed]
- Horii H, Suzuki R, Sakagami H, Tomomura M, Tomomura A, Shirataki Y. New biological activities of Rhinacanthins from the root of Rhinacanthus nasutus. Anticancer Res 2013;33(2):453-9.
[Google Scholar] [PubMed]
 .
.



