- *Corresponding Author:
- Thacheril Sukumaran
Department of Botany, University of Kerala, Kariavattom, Thiruvananthapuram, Kerala 695581, India
E-mail: swapnats@yahoo.com
| Date of Received | 14 September 2021 |
| Date of Revision | 14 August 2022 |
| Date of Acceptance | 07 February 2023 |
| Indian J Pharm Sci 2023;85(1):135-144 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Cancers are the uncontrolled growth of cells or tumors, and it is one of the leading causes of death all over the world. Currently, available synthetic drugs such as cisplatin, doxorubicin and fluorouracil are reported to have notable side effects in living tissues (skin irritations, dry and cracked skin, and mouth sores). So researchers are scrutinizing for alternative medications to overcome these side effects. Plants are one of the vital sources of alternative medicines with less toxicity and may overcome these side-effects of synthetic drugs. The study evaluated cytotoxicity and antioxidant potential on methanolic and aqueous extract of Pittosporum dasycaulon leaves. In the present study of cytotoxicity, the selected extracts were screened against two cancer cell lines (human liver cancer cells and Lymph cancer cell-Dalton's lymphoma ascites ascites). Two normal cells, intestinal epithelial-6, and Rat spleen were used to test cytotoxicity by using the trypan blue exclusion method and 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide assay. The antiradical activity was also evaluated with its half maximal inhibitory concentration. The content of total phenolic, flavonoid and saponins was also estimated. The present study displayed leaves methanolic extract found to have a significant rate of cell death against cancer cell lines (Dalton's lymphoma ascites and human liver cancer cells) with lethal concentration 50 values ranging from 8.21±0.3 to 55.65±0.11 μg/ml. However, the same extract had very less cytotoxicity even in higher doses against non- cancerous intestinal cell lines (intestinal epithelial-6) and rat spleen cell lines ranging from 389.50±0.6 to 1800.00±0.1 μg/ml. The quantitative analysis of leaf methanolic extract showed a high level of saponins and phenolic compounds. Therefore, leaves of Pittosporum dasycaulon Miq are a promising candidate for natural antioxidant as well as anticancer components. Hence, further extensive study urges to evaluate the mechanism as well as to investigate of individual components responsible for cytotoxicity and antiradical potential.
Keywords
Pittosporum dasycaulon, anticancer, antioxidant, saponins, Hep-G2
The species Pittosporum dasycaulon (P. dasycaulon) belongs to the family Pittosporaceae. The family consists of about nine genera with 200 species. Among these only one genus with eleven species is represented in India[1]. The members of the genus include trees, large shrubs and climbers, which grow up to 2-30 m tall, commonly with lenticels or resin canals. Leaves are simple, entire, rarely lobed and spirally arranged around the stem. Flowers are hermaphrodite, complete, actinomorphic and solitary and found in raceme as corymb or umbel inflorescence. Sepals and petals are five each and the flowers are sweetly scented. The fruit is a capsule with many seeds that are enveloped by resinous pulp. Several species of the genus are aromatic with fragrant flowers and hence are cultivated in gardens[2].
Several phytochemical studies emphasized the genus Pittosporum revealed its wide range of pharmacological potentials such as antioxidant, anticancer, anti-biofilm and hepatoprotective activity. Traditionally local tribes depend on the species P. dasycaulon as a remedy against tumor and an antidote to snake venom. Hence the species are locally known as 'Analivengam'[3]. Secondary metabolites are non-nutritive phytoconstituents along with several versatile applications and clinical bustles[4]. Phytochemicals are categorized in groups based on their structure and executed functions. Among them, phenolics are the most dominant and diverse group in the plant kingdom with several pharmacological activities[5].
Based on the current state of research, cancer is one of the leading reasons of death worldwide and is expected to become the primary cause of death within the upcoming years[6]. There are 700 000 deaths reported annually due to liver cancer which is considered the second leading cause of cancer-related death[7-9]. Liver cancer was prevalent in Africa and Asia[10,11]. The data published by Surveillance Epidemiology, there were 75 000 persons who suffered due to lymphoma cancer in the United States alone in 2011[12], and cancer deaths are expected to rise to 20.3 million by 2026[13]. Patients with liver cancer reported to below 9 % survival rate for at least a 5 y[14]. Treatment like chemotherapy was available for cancer treatment, but the existing chemotherapeutic drugs like cisplatin, doxorubicin, fluorouracil do not have a great effect on liver cancer and are reported to have exhibit toxicity towards normal tissues and can induce undesirable side effects including cardio toxicity[15-17]. Overuse of drugs can lead to drug resistance and fail to effective dose when an anticancer drug can be administered[18]. So the above-summarized problems urge the requirement of new treatment strategies for cancer patients that are more effective for cancer and less toxic to normal tissues. Antioxidants activity is connected with anticancer activity as antioxidant compound have the ability to reduce cancer induction by scavenging free radicals[19]. Therefore, the drug candidate with anti- oxidants will favor good anticancer drugs[20].
The scientific world is always scrutinizing novel antioxidants and compounds with fewer side effects. Antioxidants can obstruct or prevent the formation of free radicals by providing unpaired electrons[21]. The most important free radical ions, such as super Oxide anions (O2•), Hydroxyl ions (•OH), Nitric oxide (NO) and Hydrogen peroxide (H2O2) which, are formed during the normal metabolism of oxygen and are exogenous factors all these were disrupts the normal metabolic pathways by damaging vital biomolecules[22,23]. This immoderate formation of radical ions interrupts the immune system of the body, which can also induce certain types of cancer due to excessive oxidation of cellular substrates, said to be oxidative stress[24]. In the present scenario, Propyl Gallate (PG), Tetra Butylhydroquinone (TBHQ), Butylated Hydroxyanisole (BHA) and Butylated Hydroxytoluene (BHT) are the most common synthetic chemicals used as antioxidants[25]. Recently efficacy and safety of these antioxidants have been questioned because of their toxicity. Some reports suspected that BHA and BHT have been responsible for hepatic damage and carcinogenesis[26].
Interestingly, plants are one of the important reservoirs of versatile compounds that produce antioxidants and anticancer properties with less toxicity and can also overcome the side effects of synthetic drugs[5,18,27]. The currently available information about the species P. dasycaulon Miq. is much limited. Hence, the necessity to exploration of the phytochemicals evaluation, anticancer and antioxidant potentials of the taxon is very much important.
Materials and Methods
Chemicals:
Chemicals used for the study are Aluminium chloride, Ammonium acetate, Ammonium molybdate, Ammonium solution, Ascorbic acid, chloroform, 2,2-Diphenyl-1-Picrilhydrazyl Acid (DPPH), Dimethyl Sulphoxide (DMSO), Ethylenediaminetetraacetic Acid (EDTA), Ethanol, Fetal Bovine Serum (FBS), Ferrous ammonium sulphate, Folin Ciocalteu reagent, Gallic acid, Glacial acetic acid, Hydrochloric acid, Magnesium, Mercuric chloride, 3-(4, 5-Dimethylthiazolyl-2)-2, 5-Diphenyltetrazolium Bromide (MTT), Nitro Blue Terazoleum (NBT), Penicillin, Phenolphthalein, Phosphate Buffer Saline (PBS), Potassium hydroxide, Potassium iodide, Pyridine, Quercetin, Roswell Park Memorial Institute (RPMI) Medium, Saponin, Sodium carbonate, sodium nitrite, Sodium nitroprusside, Sodium hydroxide (NaOH), streptomycin, methanol, Sodium phosphate buffer, Trichloro Acetic Acid (TCA), trypan blue, Trypsin, sodium phosphate, Sulphuric acid (H2SO4), vanillin.
Collection of plant and preparation of the extract:
The plant parts, such as leaves of P. dasycaulon Miq. were collected from the southern part of Western Ghats (Wayanadu), Kerala, India, in 2019 is shown in fig. 1. The plant was identified by the experts from the University of Calicut and authenticated with the help of Flora. The voucher has been deposited in the University Kerala herbarium at Kariavattom (Voucher No; KUBH10250). The leaves were washed thrice with distilled water to remove dust particles, shade dried for more than 2 w and the dried leaves were ground in an Electrical Mixture Grinder (BL Platinum 750 W- MG 139). The powdered crude extract of leaves was stored in an airtight container until further use.
P. dasycaulon (25 g) in crude powdered form were extracted with 300 ml of various organic solvents (hexane, chloroform, methanol and water) and subjected to successive serial extraction using a Soxhlet apparatus. After each extraction, the corresponding solvent was separated in a Rotary Evaporator (Rotavap-Superfit PBU-6). Then, the crude extract was air-dried overnight for the complete evaporation of the extraction solvent before the subsequent extraction with another solvent. Each dried extract was weighed to determine the percentage yield of soluble constituents using the formula[28].
Percentage yield=(Weight of individual extract)/ (Weight of crude extract)×100
The dried extracts of leaves were stored at 4° for further evaluation in vitro studies, including anticancer, antioxidant properties and quantitative estimation of secondary metabolites. Due to the lack of knowledge about phytoconstituents in P. dasycaulon leaves dried extract was subjected to preliminary phytochemical analysis. By considering the yield and polarity of extract, methanolic and aqueous extract were mainly selected for further quantitative analysis of biologically potent secondary metabolites, which are further used to evaluate in vitro anticancer and antioxidant potential.
Quantitative estimation of secondary metabolites:
Determination of total saponin content: Vanillin H2SO4 assay is used to determine the total saponin content of the extract[29]. To 0.5 ml of an aqueous solution of the sample, 0.5 ml of freshly prepared vanillin of 8 % and then 5 ml of H2SO4 of 72 % were added and thoroughly mixed in an ice water bath. The mixture was then boiled in a warm water bath at 60° for 10 min and then it cooled again in ice-cold water. Absorbance at 535 nm was recorded against the blank reagent along with standard saponin using a spectrophotometer (Ultra Violet (UV)-1700-(E) 23OEC-Shimadzu).
Determination of total phenolic content: The total amount of phenolic content was determined using the Folin-Ciocalteu reagent. To 0.1 ml of the extract, added 3.9 ml of distilled water and 0.5 ml of Folin's reagent[30]. The solution was kept at room temperature for 3 min for incubation and 2 ml of 20 % sodium carbonate solution was added to the mixture. The solution was kept in a water bath for 1 min, cool and then read absorbance at 650 nm with gallic acid equivalents against the blank.
Determination of total flavonoid content: Total flavonoid content was estimated by using the Aluminium chloride method[31]. To 1 ml of the sample, add 75 µl of 5 % sodium nitrite. After 6 min of incubation, 150 µl of 10 % aluminum chloride solution was added and kept the solution undisturbed for 5 min. Add 0.5 ml of 1 M NaOH and made up to 2.5 ml by adding distilled water. Mix the solution well and read absorbance at 510 nm with standard quercetin. The result expressed as mg of flavonoid as quercetin equivalents.
Anticancer study:
Cell lines and culture medium: Dalton's Lymphoma Ascites (DLA) cells were used for short-term in vitro cytotoxicity experiments. These cell lines were maintained as ascites tumors in Swiss albino mice. Normal spleen cells were procured from rats by providing carbon dioxide anesthesia. Human- Hepatocellular Liver Carcinoma Cell Line (HepG2) and Intestinal Epithelial-6 cell (IEC-6) were procured from National Centre for Cell Sciences, Pune, India. Stock cells were cultured in Dulbecco’s modified eagle medium supplemented with 10 % inactivated FBS, penicillin (100 IU/ml-1), streptomycin (100 μg/ ml-1), and amphotericin B (5 μg/ml-1) in a humidified atmosphere of 5 % Carbon dioxide (CO2) at 37° until confluent. The cells were dissociated with trypsin phosphate versene glucose solution (0.2 % trypsin, 0.02 % EDTA, 0.05 % glucose in PBS). The stock cultures were grown in 25 cm2 culture flasks (Tarsons India Pvt. Ltd., Kolkata, India).
Trypan blue dye exclusion technique: The tumor cells (DLA) aspirated from the peritoneal cavity of tumor-bearing mice was washed thrice with PBS or normal saline. Cell viability was as per the trypan blue exclusion method determined by Asirvatham et al.[32]. The cell viability should be above 98 % and cell suspension (1×106 cells in 0.1 ml) was added to tubes containing various concentrations of the test compounds and the volume was made up to 1 ml using PBS. The control tube contained only cell suspension. This assay mixture was incubated for 3 h at 37°. Later, the cell suspension was mixed with 0.1 ml of 1 % trypan blue and kept for 2-3 min, and loaded on a hemocytometer. Dead cells take up the blue color of the trypan blue, while live cells do not take up the dye. The number of stained and unstained cells was countered separately.
In vitro, short term cytotoxicity was also done by using normal rat spleen cells. For this study, the rat was sacrificed using carbon dioxide anesthesia and the spleen tissue was dissected. It was then smashed to single-cell suspension in RPMI complete medium containing antibiotics and filtered using mesh cloth. The collected cells were washed thrice and suspended in a known volume of RPMI complete medium containing antibiotics and counted. Viable cell suspension (1×106 cells in 0.1 ml) was added to tubes containing various concentrations of the test compound, and the volume was made up to l ml using RPMI media. Control tubes contained only cell suspension (without additives).
These tubes were incubated for 3 h at 37°. At the end of incubation, cell suspension in the tubes was mixed with 0.1 ml of 1 % trypan blue and kept for 2-3 min, and loaded on a hemocytometer. Dead cells take up the blue color of trypan blue, while live cells do not take up the color of dye[33]. The number of stained cells was counted separately, and calculated the percentage of cytotoxicity using the formula[34].
Percentage cytotoxicity=(No. of dead cell)/(No. of live cell+No of dead cell)×10
Long term in vitro cytotoxicity by MTT assay: The ability to determine the cells to survive against a test compound is the basis of most cytotoxic assays. This assay was carried out according to the method based on the assumption that dead cells or their products do not reduce tetrazolium[35,36]. The cells were seeded in a 96- well flat-bottom plate (5000 cells/well) and permitted to adhere for 24 h at 370° with a 5 % CO2 atmosphere. Different drug concentration was added and incubated further for 48 h. Before 4 h of the completion of incubation, 20 μl of MTT (5 mg/ml) was added. Dead cell percentage was determined using an enzyme- linked immunosorbent assay plate reader set to record absorbance at 570 nm. The percentage growth inhibition was calculated using the formula given below[37].
Percent growth inhibition=(Optical Density (OD) value of control-OD value of sample)/(OD of control)×100
Determination of antioxidant property:
DPPH radical scavenging assay: The DPPH is the stable nitrogen centered free radical of violet color, get reduced to yellow colored chemical compound diphenylpicrylhydrazine by the activity of plant extract, which can be measured colorimetrically; thus the chemical compound which is capable of performing this reaction can be considered as antioxidant or radical scavengers[38]. Here, a free radical scavenging assay was done[39]. Briefly, 1 ml of 0.1 mM DPPH solution in methanol was mixed with 1 ml of a plant extract with different concentrations (20 µg-100 µg) of the sample prepared in methanol and made up to 2 ml the mixture which was shaken vigorously and left to stand for 30 min under dark in room temperature and the absorbance read at 517 nm (UV-1700-(E) 23OEC-Shimadzu). The free radical scavenging ability (%) of DPPH radical was determined using the following formula.
Free radical scavenging ability (%) = [(A0-A1)/A0]
Where A0 and A1 represent the absorbance values of the control and of the test sample, respectively, and ascorbic acid was used as a standard drug.
Superoxide radical scavenging assay: This method of superoxide radical scavenging assay was described by Kunchandy et al.[40]. The reaction mixture containing 0.1 ml of NBT (mg/ml solution in DMSO), various concentrations (20-100 µg) of plant extract and standard ascorbate dissolved in DMSO and made up to 100 µl by adding DW. An amount of DMSO (1 ml DMSO containing 5 mM NaOH in 100 µl DW) was added to produce a final volume of 1.4 ml, and the absorbance was read at 560 nm using a spectrophotometer (UV-1700- (E) 23OEC- Shimadzu). The decreasing wavelength and percentage of inhibition were calculated.
Determination of hydroxyl radical scavenging potential: Hydroxyl radical scavenging activity of the extracts was determined according to the method by Klein et al.[41]. The reaction mixture contained 1.0 ml of different concentration of extracts (20-100 µg/ml), 1.0 ml of ferric iron-EDTA solution (0.13 % ferrous ammonium sulphate 0.26 % EDTA), 0.5 ml of 0.018 % EDTA, 1.0 ml of DMSO (0.85 % in 0.1 mol/l phosphate buffer pH 7.4) and 0.5 ml of 0.22 % ascorbic acid. The tubes were capped tightly and heated in a water bath at 80°-90° for 15 min and the reaction was terminated by adding 1.0 ml of ice-cold TCA (17.5 %). To the above reaction mixture, 3.0 ml of Nash reagent (75.0 g of ammonium acetate, 3.0 ml of glacial acetic acid and 2.0 ml of acetyl acetone) was mixed, and distilled water was added to a total volume of 1 l) and incubated at room temperature for 15 min for color development. The intensity of the yellow color formed was measured at 412 nm against a reagent blank. Ascorbic acid was used as standard, and the percentage of inhibition was calculated.
Statistical analysis:
Data were expressed as Mean±Standard Error (SE) (n=3). Results of the antioxidant and anticancer activity were determined by using one-way Analysis of Variance (ANOVA). Values differ significantly at p<0.05 were inspected using Statistical Package for the Social Sciences (SPSS) software.
Results and Discussion
The dried powdered leaves (25 g) of P. dasycaulon were serially extracted with hexane, chloroform, methanol and aqueous (non-polar-polar) for 6 h, and the percentage yield was 3.036, 4.523, 8.804 and 7.93 respectively. The differences in the extraction of yield depend on the nature of the sample. The high yield was observed in methanolic extract followed by an aqueous extract of leaves. So these two extracts were selected for further quantitative studies.
The total amount of phenolics, flavonoids, tannin and saponin content of selected extracts was depicted in Table 1. The phenolic (31.43 % and 25 %) and tannin (2.25 % and 1.63 %) content was observed higher in Pittosporum dasycaulon Methanol Extract (PDME) followed by Pittosporum dasycaulon Aqueous Extract (PDAQ), but flavonoid content was comparatively higher in LFAQ (20.4 %) than LFME (16 %). Several studies supported saponins having remarkable cytotoxicity against cancer cell lines and phenolics well known for natural antioxidant activities due to its redox potential[42].
| Secondary metabolites mg/g | Methanolic extract | Aqueous extract |
|---|---|---|
| TSC | 465. 32±3.1 | 335.67±9.4 |
| TPC | 314.35±0.66 | 250.33±3.6 |
| TFC | 160.89±0.7 | 204.40±1.9 |
| TTC | 22.35±1.06 | 16.35±0.18 |
Note: Values are expressed as mean±SE of triplicates; TSC: Total Saponins Content; TPC: Total Phenolic Content; TFC: Total Flavonoid Content and TTC: Total Tannin Content is expressed mg of saponin, gallic acid, quercetin and tannic acid equivalents/gm of extract)
Table 1: Quantitative Estimation of Secondary Metabolites From P. dasycaulon
| LC50 values (μg/ml) | ||||
|---|---|---|---|---|
| Trypan blue method | MTT method | |||
| Cancer cell line | Normal cell line | Cancer cell line | Normal cell line | |
| Plant samples | DLA | Spleen | HepG2 | IEC-6 |
| LFME | 8.21±0.3 | 1800.00±0.1 | 55. 65±0.11 | 389.50±0.6 |
| LFAQ | 141.57±01 | - | 399.92±0.28 | - |
Note: Results are expressed as mean±SE (n=3); all statistical analysis was expressed using one way ANOVA. Values differ significantly at p<0.05 were inspected using SPSS software, LC50 is described test compound needed to kill 50 % cells
Table 2: IN VITRO Cytotoxicity Study against different Cancer Cell Lines
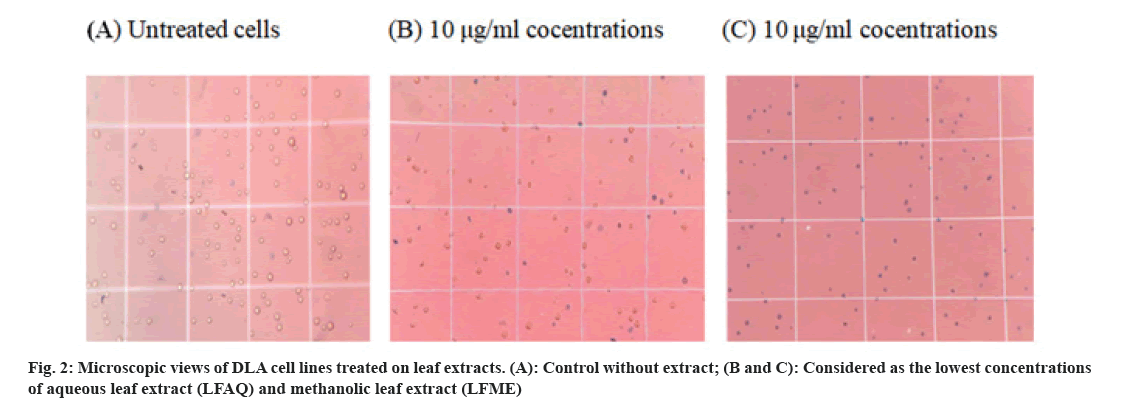
The result of the cytotoxicity study on DLA and spleen cells was evaluated by the short term method using trypan blue dye. Any cytotoxic compound can impair cell proliferation or ultimately kill the cells. The dye trypan blue can penetrate only the dead cells. So we can count the number of viable cells and dead cells. Cytotoxicity of LFME and LFAQ on lymphoma cancer cells (DLA) is shown in fig. 2. The Lethal Concentration (LC50) values of the LFME and LFAQ extracts tested against DLA ascites ranged from 8.21±0.3-141.57±0.1 µg/ml. However, interestingly LFME extract displayed LC50=1800 µg/ml when it was treated against a rat spleen (non-cancerous) cell line (Table 2).
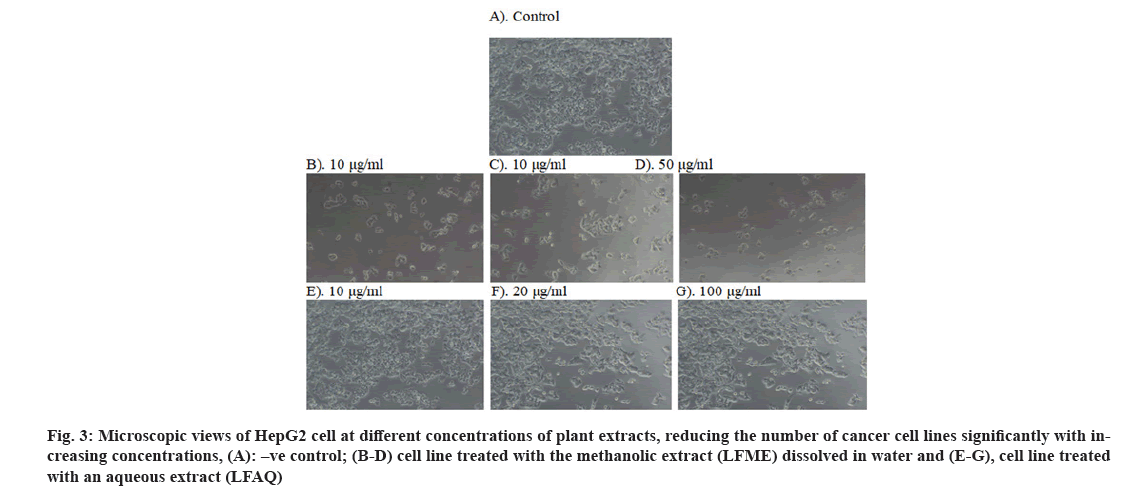
The cytotoxicity of the extract can be identified when it is stained with trypan blue (fig. 2), and the number of dead cells can be counted with the use of a hemocytometer and calculated the percentage of cytotoxicity. The result of cytotoxicity through the MTT method was also described in Table 2 and shown in fig. 3. The LFME and LFAQ tested against HepG2 and normal IEC-6 are summarized in Table 2. The study revealed that 50 % of cell death takes place in the HepG2 cell line at the concentration of 55.65±0.11 μg/ ml of LFME, where 399.92±0.28 µg/ml is needed for aqueous LFAQ. Interestingly note that strong anticancer LFME exhibited a higher LC50 value=389.50±0.6 μg/ ml against non-cancerous IEC-6 cell line.
Fig 3: Microscopic views of HepG2 cell at different concentrations of plant extracts, reducing the number of cancer cell lines significantly with increasing concentrations, (A): –ve control; (B-D) cell line treated with the methanolic extract (LFME) dissolved in water and (E-G), cell line treated with an aqueous extract (LFAQ)
The present study of DPPH assay revealed that the LFAQ and leaf LFME showed the highest DPPH radical scavenging activity, with an inhibition rate of 50 % (Half Maximal Inhibitory Concentration (IC50)) at 61.50±0.2 µg/ml and 91.25±1.12 µg/ml concentrations respectively, whereas standard vitamin C exhibited 50 % inhibition at a concentration of 8.10±0.91 µg/ml concentration. In the superoxide radical scavenging assay, the ability of both LFAQ and LFME was almost similar (89.45±0.25 and 89.045±0.27 µg/ml), and standard vitamin C possessed the IC50 value at a concentration of 19.23±0.11 µg/ml. The hydroxyl radical scavenging capacity indicates that 50 % of the generated hydroxyl radical was scavenged by LFAQ at the concentrations of 88±0.54 µg/ml, and the value of LFME was found to be 91.661±0.0 µg/ml. Here, the selected species P. dasycaulon leaf exhibited the highest inhibition performance. The free radical scavenging potential of two selected extracts and positive control was illustrated in Table 3. It was interestingly noted that all the studied assays displayed IC50 value of less than 100 μg.ml-1 (lower IC value).
| Plant samples | DPPHa | O2.-b assay | •OHc assay |
|---|---|---|---|
| LFME | 91.25±1.12 | 89.04±0.27 | 91.661±0.0 |
| LFAQ | 61.50±0.2 | 89.45±0.25 | 88±0.54 |
| Ascorbic acid | 8.10±0.91 | 19.23±0.11 | 29.23±1.00 |
Note: Results are expressed as mean±SE (n=3); all statistical analysis was expressed using one way ANOVA. Values differ significantly at p<0.05 were inspected using SPSS software. DPPHa; O2.-b assay and •OHc assay
Table 3: Antioxidant Capacity of Aqueous and Metahnolic Extract of Leaves P. dasycaulon
According to the American Cancer Institute (ACI), plant crude extract is toxic to cells when the LC50 is less than 30 µg/ml [43] . Thus, the studied anticancer extract did not exhibit notable toxicity to selected non-cancerous cell lines (Spleen and IEC). Hence, P. dasycaulon leaf methanolic extract exhibited noteworthy anticancer activity against selected cancer cells (DLA and HepG2), with LC50 values ranging from 8.21±0.3- 5.65±0.11 µg/ml. The present study also showed P. dasycaulon has a high content of saponins 465.32±3.1 mg/ml as depicted in Table 1. The remarkable cytotoxic and antiproliferiative activity of saponins was already reported[44]. The above-summarized results could be supported by several studies among the different species of Pittosporum described as saponin rich [45,46].
There are a number of triterpenoid saponins, along with strong anticancer activity was reported among the different species of the genus Pittosporum. Trihydroxy oleanolic acid (pentacyclic triterpenoid saponin) was reported from the leaves of an Indian species Pittosporum tetraspermum[47]. In a study [48], trihydroxy oleanolic acid is reported for reducing the overexpression of AKR1B10 (a protein potential target for cancer therapy) in human liver cancer cells described as HepG2[49]. It was also reported to inhibit mutagenicity caused by aflatoxin B1[48]. Remarkable cytotoxicity was displayed by two novel triterpenoid estersponins isolated from the Pittosporum tobira[50]. Steroid saponins such as Uvaol and erythrodiol from the leaves of Pittosporum resiniferum[51] showed notable anti-proliferiative activity against human colon adenocarcinoma cells-HT29[52]. A recent study reported pittangretoside saponins from Pittosporum angustifolium exhibited anticancer activity against the human urinary bladder cell line[53]. Saponins have the ability to interact with the membrane, enhance membrane permeability by disturbing ionic homeostasis, induce mitochondrial membrane depolarization, promote nuclear condensation and ultimately kill the cells. This could be the reason for saponins as a cytotoxic or antiproliferiative effect[54].
There are a number of in vitro methods used to evaluate the free radical scavenging ability of natural compounds. Here, the radical scavenging capacity of plant extracts was determined using the DPPH, Superoxide (SO), Hydroxyl (OH) assay. Free radicals are known reasons for deoxyribonucleic acid damage, which results in the initiation of carcinogenesis. Hence, inhibition of free radicals could potentially aid in preventing carcinogenesis[55,56]. Among these selected assays, DPPH is the most common method to evaluate the free radical scavenging ability of natural compounds. Superoxide anion radical is formed by the single electron reduction of molecular oxygen of the primary oxidase of Reactive Oxygen Species (ROS). These kinds of frequently generated ROS in the living cell can be scavenged by Superoxide Dismutase (SODs) known to be considered the first line of defense[57]. In addition, the enzymes like Glutathione peroxidase catalyzes and converts superoxide to water and molecular oxygen, mostly in peroxisomes. This reaction can inhibit the conversion of the superoxide anion to hydroxyl radical and other toxic ROS[58]. High levels of these radicals can induce cell death and tissue damage[59]; thus, the search for a new natural antioxidant compound with anticancer potential is currently investigating by the scientific community for better health. The anti- radical property of an extract or compound is inversely proportional to its IC50 value, meaning the lower the IC50 value, the stronger the antioxidant activity . A study done by Mani and Dennis et al.[3] defined the antioxidant activity of P. dasycaulon using different assays (DPPH, and superoxide) and reported good antioxidant activity of methanolic and aqueous extracts of bark with IC50 values ranging from 151.60 to 154.5 ug/ml, and 25.50 to 29 mg/ml respectively. In the present study, the leaf extracts of P. dasycaulon exhibited stronger antioxidant activity than the bark extracts in both DPPH and superoxide radical scavenging assay. Both LFAQ and LFME of P. dasycaulon exhibited high content of phenolic compounds, as described in Table 1. Additionally, several studies reported phenolic compounds to act as a promising antioxidant agent among the secondary metabolites[60,61].
However, antioxidant ability differs among the different classes of phenolic compounds. General antioxidant activity of saponins was less reported by Zhao et al.[62] Interestingly note that water extract displayed more scavenging activity than methanolic extract and lower than standard vitamin C in all described assays. So, these above results summarized that the good antioxidant potential could be the synergetic effect of phenolic compounds or individual phytoconstituent among the phenols.
The present study of P. dasycaulon leaves was shown a good level of antioxidant potential as well as a high level of anticancer potential even in the crude extract. Saponins and phenolics content were strongly correlated with the anticancer and antioxidant activity of the crude extract. So this study provides some credence value to the traditional use of this plant against cancer treatment. So P. dasycaulon leaf is recommended as a more promising source of an anticancer drug than antioxidants. Thus, further phytochemical investigations to isolate and evaluate bioactive compounds and their activities will continue from the plant P. dasycaulon, due to the high anticancer potential and free radical scavenging activity. This is the first report on the anticancer, antioxidant potential and evaluation of secondary metabolites from the leaves of P. dasycaulon.
Acknowledgment:
The first author is CSIR-SRF, thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi for financial assistance. We acknowledge Mr Salim Pichan for his help during plant collection..
Conflict of interests:
The authors declared no conflict of interests.
References
- Singh RK. Lectotypification of three Indian endemic taxa of Pittosporum (Pittosporaceae). Turk J Bot 2016;40(5):557-60.
- Gopalakrishnan KK, Thomas TD. Reproductive biology of Pittosporum dasycaulon Miq.,(Family Pittosporaceae) a rare medicinal tree endemic to Western Ghats. Bot Stud 2014;55(1):15.
[Crossref] [Google Scholar] [PubMed]
- Remesh M, Manilal K S , Muktesh Kumar M S. Ethnobotanical aspects of trees of Palakkad District, Kerala, India. Devagiri J Sci 2016;2(1):32-51.
- Bariş Ö, Güllüce M, Şahin F, Özer H, Kiliç H, Özkan H, et al. Biological activities of the essential oil and methanol extract of Achillea biebersteinii Afan.(Asteraceae). Turk J Biol 2006;30(2):65-73.
- King AM, Young G. Characteristics and occurrence of phenolic phytochemicals. J Am Diet Assoc 1999;99(2):213-8.
[Crossref] [Google Scholar] [PubMed]
- Unger C. New therapeutic approaches in cancer treatment. Drugs Future 1997;22(12):1337-45.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55(2):74-108.
[Crossref] [Google Scholar] [PubMed]
- Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma–epidemiological trends and risk factors. Dig Dis 2009;27(2):80-92.
[Crossref] [Google Scholar] [PubMed]
- Ismail N, Abdel–Mottaleb Y, Ahmed AA, El-Maraghy NN. Novel combination of thymoquinone and resveratrol enhances anticancer effect on hepatocellular carcinoma cell line. Future J Pharm Sci 2018;4(1):41-6.
- Levrero M. Viral hepatitis and liver cancer: The case of hepatitis C. Oncogene 2006;25(27):3834-47.
[Crossref] [Google Scholar] [PubMed]
- Hacioğlu B, Kuş G, Kutlu HM, Kabadere S. The effect of R547, a cyclin-dependent kinase inhibitor, on hepatocellular carcinoma cell death. Turk J Biol 2020;44(1):24-33.
[Crossref] [Google Scholar] [PubMed]
- Gupta A, Singh AK, Kumar R, Ganguly R, Rana HK, Pandey PK, et al. Corilagin in cancer: A critical evaluation of anticancer activities and molecular mechanisms. Molecules 2019;24(18):3399.
[Crossref] [Google Scholar] [PubMed]
- Castillo JJ, Mull N, Reagan JL, Nemr S, Mitri J. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: A meta-analysis of observational studies. Blood J Am Soc Hematol 2012;119(21):4845-50.
[Crossref] [Google Scholar] [PubMed]
- S Darvesh A, B Aggarwal B, Bishayee A. Curcumin and liver cancer: A review. Curr Pharm Biotechnol 2012;13(1):218-28.
[Crossref] [Google Scholar] [PubMed]
- Tyagi AK, Agarwal C, Chan DC, Agarwal R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB468 cells. Oncol Rep 2004;11(2):493-9.
[Google Scholar] [PubMed]
- Kettenbach J, Stadler A, Katzler IV, Schernthaner R, Blum M, Lammer J, et al. Drug-loaded microspheres for the treatment of liver cancer: Review of current results. Cardiovasc Intervent Radiol 2008;31:468-76.
[Crossref] [Google Scholar] [PubMed]
- El-Sayyad HI, Ismail MF, Shalaby FM, Abou-El-Magd RF, Gaur RL, Fernando A, et al. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int J Biol Sci 2009;5(5):466.
[Crossref] [Google Scholar] [PubMed]
- Jeena K, Liju VB, Kuttan R. Antitumor and cytotoxic activity of ginger essential oil (Zingiber officinale Roscoe). Int J Pharm Pharm Sci 2015;7(8):341-4.
- Slaga TJ. Inhibition of the induction of cancer by antioxidants. Nutr Biotechnol Heart Dis Cancer 1995:167-74.
- Madikizela B, McGaw LJ. In vitro cytotoxicity, antioxidant and anti-inflammatory activities of Pittosporum viridiflorum Sims and Hypoxis colchicifolia Baker used traditionally against cancer in Eastern Cape, South Africa. South Afr J Bot 2019;126:250-5.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biol Med 2010;49(11):1603-16.
[Crossref] [Google Scholar] [PubMed]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 2010;48(12):909-30.
[Crossref] [Google Scholar] [PubMed]
- Lung MY, Tsai JC, Huang PC. Antioxidant properties of edible basidiomycete Phellinus igniarius in submerged cultures. J Food Sci 2010;75(1):E18-24.
[Crossref] [Google Scholar] [PubMed]
- Meng JF, Fang YL, Qin MY, Zhuang XF, Zhang ZW. Varietal differences among the phenolic profiles and antioxidant properties of four cultivars of spine grape (Vitis davidii Foex) in Chongyi County (China). Food Chem 2012;134(4):2049-56.
[Crossref] [Google Scholar] [PubMed]
- Sun B, Fukuhara M. Effects of co-administration of butylated hydroxytoluene, butylated hydroxyanisole and flavonoids on the activation of mutagens and drug-metabolizing enzymes in mice. Toxicology 1997;122(1-2):61-72.
[Crossref] [Google Scholar] [PubMed]
- Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J Funct Foods 2015;18:820-97.
- Dai J, Mumper RJ. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010;15(10):7313-52.
[Crossref] [Google Scholar] [PubMed]
- Pavithra K, Vadivukkarasi S. Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn. Food Sci Hum Wellness 2015;4(1):42-6.
[Crossref] [Google Scholar] [PubMed]
- Hiai S, Ho TN. Color reaction of some saposenins with vanillin and sulfuric acid. Plant Mdica 1976;29:116-22.
- Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Altern Med 2010;10(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 2002;10(3):178-82.
- Asirvatham R, Christina A. Anticancer activity of Drosera indica L., on Dalton and #8217;s Lymphoma Ascites (DLA) bearing mice. J Int Ethnopharmacol 2013;2(1):9.
- Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett 1985;29(2):197-202.
[Crossref] [Google Scholar] [PubMed]
- Shrivastava S, Ganesh N. Tumor inhibition and cytotoxicity assay by aqueous extract of onion (Allium cepa) & Garlic (Allium sativum): An in vitro analysis. Int J Phytomed 2010;2(1):80-4.
- Campling BG, Pym J, Baker HM, Cole SP, Lam YM. Chemosensitivity testing of small cell lung cancer using the MTT assay. Br J Cancer 1991;63(1):75-83.
[Crossref] [Google Scholar] [PubMed]
- Khairunnisa K, Karthik D. Evaluation of in vitro apoptosis induction, cytotoxic activity of Hymenodictyon excelsum (Roxb) Wall in Dalton’s lymphoma ascites (DLA) and Lung fibroblast-Mouse L929 cell lines. J Appl Pharm Sci 2014;4(8):011-7.
- Hajighasemi F, Mirshafiey A. Propranolol effect on proliferation and vascular endothelial growth factor secretion in human immunocompetent cells. J Clin Immunol Immunopathol Res. 2010;2(2):22-7.
- Dehpour AA, Ebrahimzadeh MA, Fazel NS, Mohammad NS. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas y Aceites. 2009;60(4):405-12.
- Aramwit P, Bang N, Srichana T. The properties and stability of anthocyanins in mulberry fruits. Food Res Int 2010;43(4):1093-7.
- Kunchandy E, Rao MN. Oxygen radical scavenging activity of curcumin. Int J Pharm 1990;58(3):237-40.
- Klein SM, Cohen G, Cederbaum AI. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systems. Biochemistry 1981;20(21):6006-12.
[Crossref] [Google Scholar] [PubMed]
- Mishra SL, Sinhamahapatra PK, Nayak A, Das R, Sannigrahi S. In vitro antioxidant potential of different parts of Oroxylum indicum: A comparative study. Indian J Pharm Sci 2010;72(2):267.
[Crossref] [Google Scholar] [PubMed]
- Steenkamp V, Gouws MC. Cytotoxicity of six South African medicinal plant extracts used in the treatment of cancer. South Afr J Bot 2006;72(4):630-3.
- Melzig MF, Bader G, Loose R. Investigations of the mechanism of membrane activity of selected triterpenoid saponins. Planta Med 2001;67(1):43-8.
[Crossref] [Google Scholar] [PubMed]
- Rosakutty PJ, Roslin AS. Isolation and characterization of an antimicrobial compound from the traditional medicinal plant Pittosporum tetraspermum Wight and Arn. Int J Med Aromatic Plants 2012;2(1):141-50.
- Manase MJ, Mitaine-Offer AC, Miyamoto T, Tanaka C, Delemasure S, Dutartre P, et al. New triterpenoid estersaponins from the root barks of Pittosporum verticillatum subsp. Verticillatum and evaluation of cytotoxicities. Fitoterapia 2013;91:231-5.
[Crossref] [Google Scholar] [PubMed]
- Takemura M, Endo S, Matsunaga T, Soda M, Zhao HT, El-Kabbani O, et al. Selective inhibition of the tumor marker aldo-keto reductase family member 1B10 by oleanolic acid. J Nat Prod 2011;74(5):1201-6.
[Crossref] [Google Scholar] [PubMed]
- Zu X, Yan R, Ma J, Liao DF, Cao D. AKR1B10: A potential target for cancer therapy. Biosci Hypotheses 2009;2(1):31-3.
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 1995;49(2):57-68.
[Crossref] [Google Scholar] [PubMed]
- El Dib RA, Eskander J, Mohamed MA, Mohammed NM. Two new triterpenoid estersaponins and biological activities of Pittosporum tobira ‘Variegata’(Thunb.) WT Aiton leaves. Fitoterapia 2015;106:272-9.
[Crossref] [Google Scholar] [PubMed]
- Alimboyoguen AB, Cruz-De Castro KA, Van Altena IA, Ragasa CY. Triterpenes from Pittosporum resiniferum Hemsl. Int J Toxicol Pharmacl Res 2016;8(4):261-2.
- Kazakova OB, Giniyatullina GV, Tolstikov GA, Baikova IP, Zaprutko L, Apryshko GN. Synthesis and antitumor activity of aminopropoxy derivatives of betulin, erythrodiol, and uvaol. Russian J Bioorg Chem 2011;37(3):369-79.
[Crossref] [Google Scholar] [PubMed]
- Bäcker C, Jenett-Siems K, Siems K, Niedermeyer TH, Wurster M, Bodtke A, et al. Taraxastane-type triterpene saponins isolated from Pittosporum angustifolium Lodd. Sect B J Chem Sci 2015;70(6):403-8.
- Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: A review. Phytochem Rev 2010;9:425-74.
[Crossref] [Google Scholar] [PubMed]
- Twilley D, Langhansová L, Palaniswamy D, Lall N. Evaluation of traditionally used medicinal plants for anticancer, antioxidant, anti-inflammatory and anti-viral (HPV-1) activity. South Afr J Bot 2017;112:494-500.
- Oroian M, Escriche I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res Int 2015;74:10-36.
- Kehrer JP, Klotz LO. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for health. Crit Rev Toxicol 2015;45(9):765-98.
[Crossref] [Google Scholar] [PubMed]
- Messner DJ, Murray KF, Kowdley K V. Mechanisms of Hepatocyte Detoxification. 1st ed. Elsevier Inc; 2012.
- Gupta A, Marquess AR, Pandey AK, Bishayee A. Jackfruit (Artocarpus heterophyllus Lam.) in health and disease: A critical review. Crit Rev Food Sci Nutr 2022:1-35.
[Crossref] [Google Scholar] [PubMed]
- Petti S, Scully C. Polyphenols, oral health and disease: A review. J Dent 2009;37(6):413-23.
[Crossref] [Google Scholar] [PubMed]
- Jdey A, Falleh H, Jannet SB, Hammi KM, Dauvergne X, Ksouri R, et al. Phytochemical investigation and antioxidant, antibacterial and anti-tyrosinase performances of six medicinal halophytes. South Afr J Bot 2017;112:508-14.
- Zhao X, Sun H, Hou A, Zhao Q, Wei T, Xin W. Antioxidant properties of two gallotannins isolated from the leaves of Pistacia weinmannifolia. Biochim Biophy Acta 2005;1725(1):103-10.
[Crossref] [Google Scholar] [PubMed]