- *Corresponding Author:
- Nora Vigasini
Department of Home Science, Women’s Christian College (Autonomous), Affiliated to the University of Madras, Chennai, Tamil Nadu 600006,India
E-mail: noravigasini267@gmail.com
| Date of Received | 24 May 2021 |
| Date of Revision | 22 August 2021 |
| Date of Acceptance | 11 May 2022 |
| Indian J Pharm Sci 2022;84(3):586-592 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The Borassus flabellifer fruit which is one of the summer fruits of India can be considered a promising therapeutic food as it is a good source of bioactive compounds. The aim of this study was to evaluate the antibacterial, anticancer and antidiabetic activity of the locally available aqueous freeze-dried seed powder extract of Borassus flabellifer. Antibacterial activity was evaluated using agar well diffusion assay against gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) and gram-negative bacteria (Escherichia coli and Klebsiella pneumoniae). Anticancer activity was studied using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay against HeLa and Vero cells. Antidiabetic activity of the sample was evaluated using alpha amylase and alpha glucosidase inhibition assays. Results revealed that the sample displayed maximum antibacterial activity against gram-negative bacteria (Escherichia coli and Klebsiella pneumoniae); showed satisfactory anticancer activity against the HeLa cells and exhibited potent alpha-amylase and alpha-glucosidase inhibitory activity, thus indicating the use of the Borassus flabellifer seed powder as a potential therapeutic agent for the development of drugs and functional foods.
Keywords
Antibacterial, anticancer, antidiabetic, Borassus flabellifer L., freeze-dried
Non-Communicable Diseases (NCDs) have turned out to be the foremost reason of human mortality and morbidity in many countries. NCDs comprise of diabetes, Cardiovascular Disease (CVD), cancer, chronic respiratory disease and mental health conditions; obesity and overweight being related to one or more of these NCDs. NCDs lead to chronic metabolic syndrome due to a combination of factors that include genetics, physiology, lifestyle, environment, food and behaviour patterns[1]. Bray et al.[2] predicted that, cancer would be the major cause of death in the 21st century. There are 36 different kinds of cancer affecting both men and women. Although there are conventional and modern methods to treat cancer, all of them have side effects[3]. On the other hand, Szablewski[4] reported that, India has the second largest diabetic population in the world. Diabetes is characterized by increased blood sugar levels and associated complications such as kidney and cardiovascular malfunction[5]. Although a number of hypoglycemic agents are available to treat diabetes, these synthetic drugs have many side effects such as gastrointestinal d6isorders, nausea and leave a metallic taste in the mouth[6]. Another major public health concern in recent times is antibiotic resistance. Bacterial infections are constantly developing and re-emerging, leading to an alarming fear and desperate need to treat them[7]. An analysis on global burden of diseases, has projected that, diets low in fruits and vegetables were accountable for 1.8 and 3.4 million deaths in 2013[8], showing that poor dietary habits lead to a range of NCDs.
Fruits occupy a major part in the functional food category owing to the high amounts of bioactive compounds. These compounds have the ability to lower the risk of a wide number of diseases [9]. Researchers are continuously exploring natural agricultural products for pharmaceutical purposes[10] since natural sources are widely available, lower in cost and have no side effects when compared to modern therapeutic drugs[11]. Secondary metabolites such as flavonoids are capable of exerting biological activity and thereby exhibit pharmacological properties such as antimicrobial, anti-inflammatory, antidiabetic, anticholesterolemic, antioxidant and anticancer. A high flavonoid content is directly associated with a high total phenolic content in foods[12]. Hence consuming phenolic-rich fruits can positively impact human health.
Among the different variety of fruits of South India, Borassus flabellifer (B. flabellifer) of Arecaceae family, is a plant native to Tamil Nadu and bears fruits commonly called “Nungu” in Tamil and “Palmyra palm” in English. These fruits are widely distributed and consumed throughout Tamil Nadu during summer season. This plant is highly versatile and capable of exerting a number of pharmacological benefits such as anti-inflammatory, anticancer, antioxidant, antidiabetic, antibacterial, antifungal, anthelmintic activity, hemolytic activity, analgesic activity[13], wound healing, immunomodulatory, diuretic and anti-malarial activity[14]. The tender seed found within the seed coat is fondly consumed because of its natural cooling property. In addition, the tender seed has the ability to treat dermatitis, nausea and vomiting and is known to possess antioxidant and anti-inflammatory potential[15].
Different parts of B. flabellifer are known to contain abundant phytochemicals17[16]. Muthulakshmi et al.[17] carried out a qualitative phytochemical analysis on the B. flabellifer seed coat and identified the presence of flavonoids, tannins, saponins, alkaloids, carbohydrates, phenolic compounds, terpenoids and glycosides, recommending its usage for drug formulation and medicinal application.
The thin outer covering of B. flabellifer called the seed coat is peeled off and often discarded as waste; this has been utilized in this study along with the tender seed to investigate the different therapeutic properties such as antibacterial, anticancer and anti- diabetic effects of B. flabellifer seed powder to obtain maximum medicinal benefits.
Materials and Methods
Procurement of materials:
B. flabellifer fruits were procured from a local farm in Kancheepuram district, Tamil Nadu during summer months. Fruits of the same size and maturity level were selected. Both seed and seed coat were utilized in this study. 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl-2H-tetrazolium bromide (MTT) was procured from Sigma-Aldrich. Other chemicals and reagents used in the study were procured from SRL chemicals, Sigma-Aldrich, MP Biomedicals, India, Research Laboratory Corporations, Pune and Himedia, Mumbai, India. The instruments used in the study were Carbon dioxide (CO2) incubator (BPN 50CH (UV)), multimode microplate reader (Synergy™ LX), refrigerated centrifuge (C-24 Plus), 96-well plate and serological pipette.
Preparation of extract:
The fibrous outer portion of the fruits was removed and the tender seed with seed coat found within, was used in the present study. The sample was prepared by subjecting the seeds and seed coats to washing with distilled water, pulping and freeze-drying in a lyophilizer (LSI 30). The sample was freeze-dried at
-20° and dried at 65°. The prepared sample was stored in aluminum pouches maintained at 4° for further analysis. The sample extract was prepared by dissolving 200 mg extract in 20 ml distilled water to arrive at a concentration of 10 mg/ml.
In vitro antibacterial activity:
Test microorganisms: Gram-positive strains such as Bacillus subtilis (MTCC 441), Staphylococcus aureus (MTCC 96) and gram-negative strains such as Escherichia coli (MTCC 443), Klebsiella pneumoniae (MTCC 109) were procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India.
Agar well diffusion assay: The antibacterial activity of aqueous B. flabellifer seed powder extract was analysed using agar well diffusion method[18]. About 25 ml of molten Mueller Hinton agar was poured into sterile petri plates and allowed to solidify. 100 µl of 18 h grown test organisms were streaked on the surface of the solidified agar medium using an L-rod spreader and was left to set for 5 min. 5 mm wells were made in all plates using a sterile cork borer. Different concentrations of the aqueous seed powder extract (50- 200 µg/well) were poured into the wells. Solvent saline loaded well served as negative control and azithromycin well (30 µg/ml) served as positive control. Finally, the inoculated plates were incubated at 37° for 24 h, after which the zone of inhibition diameter around the wells was measured using an antibiotic zone scale.
In vitro anticancer activity:
The aqueous B. flabellifer seed powder extract was evaluated for its cell growth inhibition property on African Green Monkey kidney cell line Vero and cervical carcinoma cell line HeLa.
Cell culture: HeLa and Vero cells were purchased from National Centre for Cell Science (NCCS), Pune. The cell lines were grown in T25 tissue culture flasks containing Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10 % Fetal Bovine Serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin obtained from Genetix Biotech, India and Sigma-Aldrich. All cell cultures were maintained at 37° in a humidified atmosphere of 95 % air and 5 % CO2. Cells were regularly passaged and maintained until commencement of analysis.
Cell growth inhibition by MTT assay: Cell proliferation (MTT) assay was performed according to the method described by Dantu et al.[19]. A monolayer of HeLa and Vero cells were separated and suspensions of single cell were prepared with trypsin-Ethylenediaminetetraacetic Acid (EDTA). Using a haemocytometer, the viable cells were counted and a cell suspension of final density of 1×105 cells/ml was prepared with Dimethylsulfoxide (DMSO) medium containing 5 % FBS. A density of 3000 cells/well (100 µl per well) of cell suspension were seeded in 96-well plates and incubated for attachment at 37° for 24 h. A working stock of aqueous B. flabellifer seed powder extract of 2× (25, 50, 100, 200, 300 and 400 µg/ml) concentration was prepared in complete DMEM supplemented with 10 % FBS and antibiotics. 100 μl volume of 2× stock was transferred to respective wells to maintain the final concentration range of 12.5, 25, 50, 100, 150 and 200 μg/ml and the plate was further incubated for 48 h at 37°, 5% CO2in the incubator. Standard mefloquine was used as positive control in the concentration similar to that of samples (12.5, 25, 50, 100, 150 and 200 µg/ ml). The medium without samples served as blank and triplicates were maintained for all concentrations.
After 48 h of incubation, 15 µl of MTT (5 mg/ml) in Phosphate Buffered Saline (PBS) was added to each well and incubated at 37° for 3 h. The medium that did not react with MTT was decanted, while the formazan crystals that were formed were solubilized in 100 µl of DMSO. The plates were read at 570 nm using Synergy HT Microplate Reader and the percentage growth was calculated using the formula
Growth percentage (%)= [(T-T0)/(C-T0)]×100
Where, T=optical density of test, C=optical density of untreated control and T0=optical density at time zero (at the time of compound addition).
From the percentage growth, a dose response curve was generated and Half Maximal Inhibitory Concentration (IC50) values were interpolated from the growth curves.
In vitro antidiabetic activity:
Alpha amylase inhibition assay:Alpha amylase inhibition assay was studied according to the method described by Telagari and Hullatti[20]. Different concentrations (100, 200, 300, 400 and 500 µg/ml) of the sample were added to 200 µl of 0.02 M sodium phosphate buffer. 20 µl of alpha amylase solution was added and incubated for 10 min at room temperature. Following this, 200 µl of soluble starch was added and incubated for 1 h. The enzymatic reaction was terminated by adding 400 µl of 3,5-Dinitrosalicylic acid (DNS) reagent and placing it in a boiling water bath for 5 min, followed by cooling and the addition of 15 ml distilled water. The color change was observed and the absorbance was read at 540 nm using a Ultraviolet-Visible (UV-Vis) spectrophotometer. Percent inhibition was calculated according to the formula
% Inhibition=Absorbance (control)-Absorbance (sample)/ Absorbance (control)×100
Alpha glucosidase inhibition assay: Alpha glucosidase inhibition assay was studied according to the method described by Watanabe et al.[21]. 0.7 U/ml of yeast alpha glucosidase was dissolved in 100 mm phosphate buffer (pH 7.0) and used as enzyme extract. P-nitrophenyl alpha- D-glucopyranoside (pH 7.0) was used as the substrate. 100 µl of different concentrations (100, 200, 300, 400 and 500 µg/ml) of the seed powder extract were mixed with 1000 µl of phosphate buffer at 37° for 5 min. Following this, 50 µl of substrate solution was added and incubated for another 5 min at 37°. The absorbance was read at 405 nm using a UV visible spectrophotometer and percentage inhibition was calculated according to the formula
% Inhibition=Absorbance (control)-Absorbance (sample)/ Absorbance (control)×100
Statistical analysis:
The study data was analysed using Statistical Package for the Social Sciences (SPSS) software version 7. Results are expressed as mean±Standard Deviation (SD) of the triplicate values. Mean values were tested for significance using one-way Analysis of Variance (ANOVA) followed by Tukey’s test. IC50 value was calculated using GraphPad Prism 5 software.
Results and Discussion
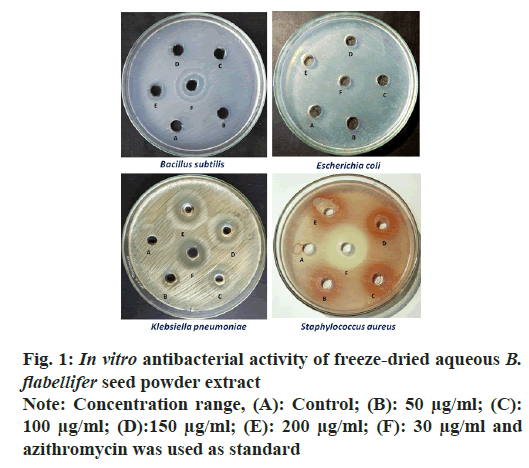
The antibacterial potential of the aqueous sample extract was evaluated by measuring the zone of inhibition against gram-positive (Bacillus subtilis and Staphylococcus aureus) and gram-negative (Escherichia coli and Klebsiella pneumoniae) bacteria as shown in fig. 1. The zone of inhibition was measured in mm and compared against the standard i.e., azithromycin (30 µg/ ml). Results indicated that the inhibitory activity of B. flabellifer seed powder was dose-dependent as shown in Table 1. As the concentration of the sample increased, the diameter of zone of inhibition also increased. The sample exhibited maximum inhibition against gram negative bacteria (Klebsiella pneumoniae-20 mm and Escherichia coli-16 mm) and minimum inhibition was recorded against gram-positive bacteria (Bacillus subtilis-10 mm and Staphylococcus aureus-10 mm).
| Bacterial pathogens | Zone of inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| 50 µg/ml | 100 µg/ml | 150 µg/ml | 200 µg/ml | Standard 30 µg/ml (azithromycin) | ||
| Gram positive | Bacillus subtilis | - | 6 | 8 | 10 | 20 |
| Gram negative | Escherichia coli | 8 | 9 | 14 | 16 | 26 |
| Gram negative | Klebsiella pneumoniae | 8 | 11 | 18 | 20 | 23 |
| Gram positive | Staphylococcus aureus | 6 | 7 | 8 | 10 | 25 |
Note: Values of single experiment
Table 1: Antibacterial activity of Freeze-Dried Aqueous B. Flabellifer Seed Powder Extract
It can be seen from Table 1 that, although there was an inhibitory effect against all bacteria, the aqueous freeze-dried seed powder extract exhibited maximum inhibitory activity against gram negative bacteria, Klebsiella pneumoniae at 200 µg/ml (20 mm) and Escherichia coli at 200 µg/ml (16 mm). Gram negative bacteria possess an outer membrane which act as a barrier and prevent the entry of antibiotics thus making it a challenge for antibiotics to pass through[22]. Antibiotic resistance has become a serious concern as certain bacterial strains have developed resistance to almost all available antibiotics. In 2017, the World Health Organization (WHO) published a list of priority pathogens that are antibiotic resistant, appealing for the development of new antibiotics to reduce mortality and morbidity worldwide. Forming a majority of the list was, gram-negative bacteria due to their distinctive structure[23]. Some severe infections like intra- abdominal infections, urinary tract infections, ventilator- associated pneumonia and bacteremia are caused by gram-negative bacteria[24]. One promising way to fight these infections and overcome antibiotic resistance is to identify natural sources exhibiting antibacterial effect and in this search, the B. flabellifer fruit has been identified as one such. This can be supported by a study conducted by Vijayakumari et al.[25] where ethanolic B. flabellifer fruit pulp powder extract was analysed for the presence of phytochemicals and it was found to contain flavonoids, saponins, phenolic compounds and glycosides.
The presence of these bioactive compounds was correlated to the antibacterial effect exhibited by the fruit pulp powder against pathogens such as Escherichia coli and Staphylococcus aureus. In another study, Gummadi et al.[26] observed significant antibacterial activity of methanolic B. flabellifer seed coat extract against both gram-positive and gram-negative bacteria. Since freeze-dried B. flabellifer seed powder showed good inhibitory activity against the gram-negative bacteria, the present study suggests that, it can be considered as a potential ingredient in the development of antibiotics.
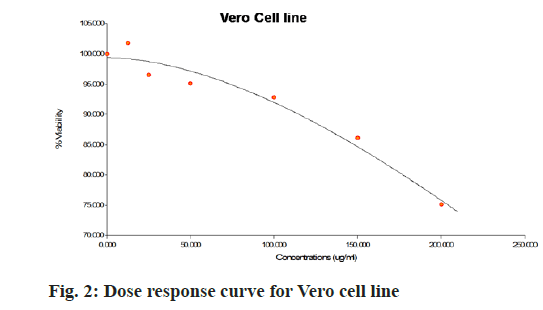
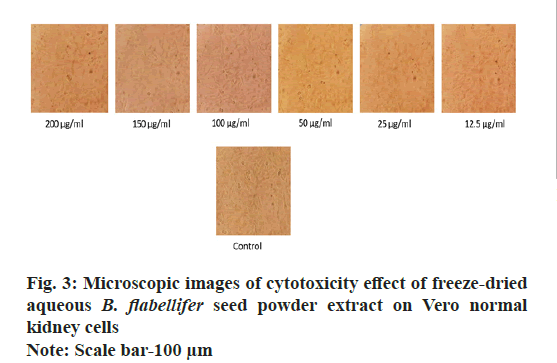
The aqueous freeze-dried B. flabellifer seed powder extract was tested against Vero cell line to evaluate its cell growth inhibition property and the results are presented in Table 2. Since the freeze-dried B. flabellifer seed powder can be considered as an essential ingredient in food product development, testing the safety of the compound and ruling out toxicity on normal cells is important. Thus, the cytotoxicity activity of the sample was tested on the Vero cells and a slight decrease in cell viability was seen with an IC50 value of 734 µg/ml. The dose response curve obtained for Vero cells was shown in fig. 2 and microscopic images of cytotoxicity activity of the sample on Vero cells was shown in fig. 3.
| Concentration (µg/ml) | % Viability of Vero cells | |
|---|---|---|
| Standard mefloquine | B. flabellifer seed powder extract | |
| 12.5 | 105.7±0.78a | 105.8±0.1a |
| 25 | 100.8±2.2b | 104.5±0.5a,b |
| 50 | 103.0±0.9c | 101.7±4.6c |
| 100 | 99.3±0.5d | 96.5±3.8d |
| 150 | 97.2±0.3d,e | 95.1±8.6d,e |
| 200 | 94.6±1.0f | 92.8±4.0f |
Note: Mean±SD of three independent estimations; a-f values followed by the different superscripts are significantly different within the column
Table 2: Cytotoxicity Effect of Freeze-Dried Aqueous B. flabellifer Seed Powder Extract on Vero Cell Line
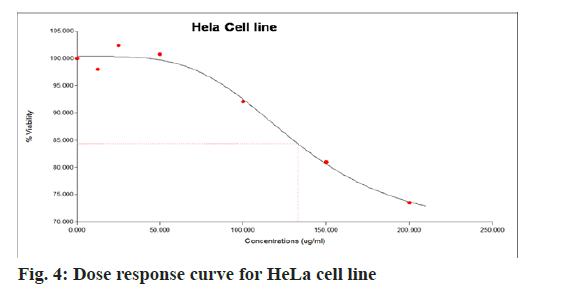
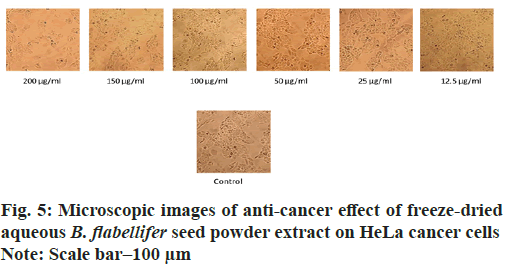
The aqueous freeze-dried B. flabellifer seed powder extract was tested against HeLa cell line to evaluate its cell growth inhibition property and the results are presented in Table 3. The anti-cancer effect of B. flabellifer seed powder on HeLa cells indicates that with increasing concentration of the sample, the viability percentage of cancerous cells significantly (p>0.05) decreased by 30 % (from 102.7±3.4 to 73.6±4.1) indicating that the sample has the ability to kill cervical cancer cells in vitro. On the other hand, the viability percentage reduced from (75.7±0.4 to 12.6±0.55) in the presence of the standard mefloquine. The Half maximal Effective Concentration (EC50) value for HeLa cell line was 137 µg/ml, which was calculated based on top-end bottom value along with the hill slope as shown in fig. 4. The EC50 value indicates the concentration of sample effective in inhibiting 50 % growth of cancer cells. The dose response curve obtained for HeLa cells was shown in fig. 4 and microscopic images of anti-cancer activity of the sample on HeLa cells was shown in fig. 5.
Results in Table 2 and Table 3 indicate that, cell viability was inversely proportional to the sample concentration because as the sample concentration increased, there was a decline in the cell viability percentage. The proliferation of normal Vero cells was minimal (Table 2) and this demonstrates the in vitro safety of the freeze- dried B. flabellifer seed powder suggesting that it can be used as an ingredient in food product development once it is clinically tested on animals and humans. The presence of phytochemicals could be the contributing factor to the anticancer potential of the sample and this is supported by the following studies. Mohan et al.[27], while evaluating the antimitotic activity of the methanolic B. flabellifer seed coat extract, identified the presence of phytoconstituents like carbohydrates, reducing sugars, triterpenoids, tannins and phenolic compounds and recommended its use for the treatment of cancer. Rao et al.[28] noticed significant cytotoxicity while testing the seed coat extract for its anticancer activity on the HeLa cell line and it was seen that, the seed coat extract inhibited cancer cell growth even at lower concentrations (32 µg/ml). It has been reported that approximately 1000 plant species on earth have anticancer properties[15] and the in vitro findings of the present study help us understand that the B. flabellifer seed powder could also be one among them.
| Concentration (µg/ml) | % Viability of HELA cells | |
|---|---|---|
| Standard mefloquine | B. flabellifer seed powder extract | |
| 12.5 | 75.7±0.4a | 102.7±3.4a |
| 25 | 62.09±0.6b | 100.8±9.6a,b |
| 50 | 44.0±0.25c | 98.0±6.6b,c |
| 100 | 28.9±0.9d | 92.1±5.3d |
| 150 | 20.2±2.4d,e | 81.2±2.4e |
| 200 | 12.6±0.55f | 73.6±4.1f |
Note: Mean±SD of three independent estimations; a-f values followed by the different superscripts are significantly different within the column
Table 3: Anticancer Effect of Freeze-Dried Aqueous B. Flabellifer Seed Powder Extract on HELA Cell Line
The aqueous freeze-dried B. flabellifer seed powder extract was evaluated for its antidiabetic activity using the alpha amylase and alpha glucosidase assays. The experiment was performed in triplicates and the results are presented in Table 4. The maximum alpha amylase inhibition was 30.39±0.02 % at 500 µg/ml and the IC50 was 822 µg/ml, while maximum alpha glucosidase inhibition was 32.42±0.02 % at 500 µg/ml and IC50 value was 777 µg/ml. The results in Table 4 indicate that the inhibition of both enzymes by the sample extract was dose-dependent (100-500 µg/ml) and there was a significant effect (p>0.05) of the plant extract on the enzyme activities.
| Concentration (µg/ml) | % Inhibition of alpha amylase | % Inhibition of alpha glucosidase |
|---|---|---|
| 100 | 10.29±0.02a | 13.65±0.01a* |
| 200 | 14.71±0.01a,b | 15.19±0.02a* |
| 300 | 24.51±0.03 c* | 18.26±0.01a,b |
| 400 | 26.47±0.02c | 26.45±0.02c |
| 500 | 30.39±0.02d | 32.42±0.02d* |
Note: Mean±SD of three independent estimations; a-dvalues followed by the different superscripts are significantly different within the column; *values are significantly different within the row, p<0.05
Table 4: Antidiabetic Activity of Freeze- Dried Aqueous B. Flabellifer Seed Powder Extract
Diabetes, which is a worldwide prevalent metabolic disorder can be controlled by maintaining glucose homeostasis[5] and one such way is by inhibiting the carbohydrate metabolizing enzymes namely alpha amylase and alpha glucosidase[12]. Although synthetic anti-diabetic drugs are available, there is a huge demand for natural, non-toxic anti-diabetic agents since the former is expensive and causes side effects. Medicinal fruits stand a great chance in controlling blood sugar levels and exerting antidiabetic activity due to the pharmacologically active phytoconstituents present in them[29]. While looking for natural options, researchers stumbled upon the ethanolic extract of B. flabellifer flowers and observed a decrease in the blood glucose levels when administered to alloxan induced diabetic rats[30].
This paved way for this plant to be identified as a potential antidiabetic source. On evaluating the in vitro anti-diabetic activity of the aqueous B. flabellifer seed powder extract, it was observed that the sample had the ability to inhibit both enzymes thereby delaying glucose absorption. This is an indication for the pharmaceutical industry to consider using this seed coat powder in formulating a potent, natural anti-diabetic drug.
In conclusion, the results of the present study indicate that freeze-dried B. flabellifer seed powder has the ability to exhibit inhibitory activity against gram- negative bacteria, thereby helping to reduce the incidence of bacterial infections; can inhibit the growth of cervical cancer cells and is capable of inhibiting alpha amylase and alpha glucosidase enzymes. This proves that B. flabellifer possesses in vitro antibacterial, anticancer and antidiabetic activities. However, to be considered effective in the field of medicine, clinical tests involving animal and human trials should be conducted. If the in vivo findings match the in vitro results, this B. flabellifer seed powder can be considered a natural ingredient for therapeutic purposes.
Acknowledgements:
Authors thank UGC JRF (No. F.15-6 (JAN. 2017)/2017 (NET)) for funding the project, the Managing Director of Whizbang Bioresearch Pvt. Ltd., Thiruverkadu, Chennai, Tamil Nadu for all the laboratory facilities provided and Dr. V. Geetha Baalsubramaniam for statistical assistance.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Tabish SA. Lifestyle diseases: Consequences, characteristics, causes and control. J Cardiol Curr Res 2017;9(3):00326.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394-424.
- Karpuz M, Silindir-Gunay M, Ozer AY. Current and future approaches for effective cancer imaging and treatment. Cancer Biother Radiopharm 2018;33(2):39-51.
[Crossref] [Google Scholar] [PubMed]
- Szablewski L. Diabetes mellitus: Influences on cancer risk. Diabetes Metab Res Rev 2014;30(7):543-53.
[Crossref] [Google Scholar] [PubMed]
- Gandhi GR, Sasikumar P. Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed 2012;2(4):281-6.
[Crossref] [Google Scholar] [PubMed]
- Vishwakarma SL, Rakesh DS, Rajani M, Goyal RK. Evaluation of effect of aqueous extract of Enicostemma littorale Blume in streptozotocin-induced type 1 diabetic rats. Indian J Exp Biol 2010;48(1):26-30.
[Google Scholar] [PubMed]
- Rojas R, Bustamante B, Bauer J, Fernández I, Albán J, Lock O. Antimicrobial activity of selected Peruvian medicinal plants. J Ethnopharmacol 2003;88(2):199-204.
[Crossref] [Google Scholar] [PubMed]
- Forouzanfar MH, Alexander L, Bachman VF, Biryukov S, Brauer M, Casey D, et al. Global, regional and national comparative risk assessment of 79 behavioural, environmental and occupational and metabolic risks or clusters of risks in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386(10010):2287-323.
- Alamelumangai M, Dhanalakshmi J, Mathumitha M, Renganayaki RS, Muthukumaran P, Saraswathy N. In vitro studies on phytochemical evaluation and antimicrobial activity of Borassus flabellifer Linn against some human pathogens. Asian Pac J Trop Med 2014;7:S182-5.
[Crossref] [Google Scholar] [PubMed]
- Yaacob NS, Hamzah N, Nik Mohamed Kamal NN, Zainal Abidin SA, Lai CS, Navaratnam V, et al. Anticancer activity of a sub-fraction of dichloromethane extract of Strobilanthes crispus on human breast and prostate cancer cells in vitro. BMC Complement Alternat Med 2010;10(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Moglad EHO, Abdalla OM, Koko WS, Saadabi AM. In vitro anticancer activity and cytotoxicity of Solarium nigrum on cancers and normal cell lines. Int J Cancer Res 2014;10(2):74-80.
- Manikandaselvi S, Divya R, Thinagarbabu R. In vitro antidiabetic activity of aqueous fruit extract of Solanum torvum Sw. Int Res J Pharm 2018;9(9):145-51.
- Saranya K, Sivakumar G, Gopalasatheeskumar K, Arulkumaran G. An updated overview on phytochemical screening and pharmacological screening of Borassus flabellifer Linn. PharmaTutor 2019;7(5):15-9.
- Sahni C, Shakil NA, Jha V, Gupta RK. Screening of nutritional, phytochemical, antioxidant and antibacterial activity of the roots of Borassus flabellifer (Asian palmyra palm). J Pharmacogn Phytochem 2014;58(34):58-68.
- Bibi Y, Nisa S, Zia M, Waheed A, Ahmed S, Chaudhary MF. In vitro cytotoxic activity of Aesculus indica against breast adenocarcinoma cell line (MCF-7) and phytochemical analysis. Pak J Pharm Sci 2012;25(1):183-7.
[Google Scholar] [PubMed]
- Jamkhande PG, Suryawanshi VA, Kaylankar TM, Patwekar SL. Biological activities of leaves of ethnomedicinal plant, Borassus flabellifer Linn.(Palmyra palm): An antibacterial, antifungal and antioxidant evaluation. Bull Fac Pharm Cairo Univ 2016;54(1):59-66.
- Muthulakshmi V, Shanthi S, Ghosh J. Determination of bioactive compounds from seed coat of Borassus flabellifer linn seed using phytochemical and GC-MS analysis. World J Pharm Res 2018;5(4):2942-5.
- Holder IA, Boyce ST. Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns 1994;20(5):426-9.
- Dantu AS, Shankarguru P, Ramya DD, Vedha HB. Evaluation of in vitro anticancer activity of hydroalcoholic extract of Tabernaemontana divaricata. Asian J Pharm Clin Res 2012;5(3):59-61.
- Telagari M, Hullatti K. In vitro α-amylase and α-glucosidase inhibitory activity of Adiantumcaudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J Pharmacol 2015;47(4):425-9.
[Crossref] [Google Scholar] [PubMed]
- Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of α-glucosidase inhibitors from tochu-cha (Eucommia ulmoides). Biosci Biotechnol Biochem 1997;61(1):177-8.
[Crossref] [Google Scholar] [PubMed]
- Miller SI. Antibiotic resistance and regulation of the gram-negative bacterial outer membrane barrier by host innate immune molecules. MBio 2016;7(5):e01541-16.
[Crossref] [Google Scholar] [PubMed]
- Breijyeh Z, Jubeh B, Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020;25(6):1340.
[Crossref] [Google Scholar] [PubMed]
- Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013;34(1):1-14.
[Crossref] [Google Scholar] [PubMed]
- Vijayakumari B, Vengaiah PC, Kiranmayi P. Qualitative phytochemical screening, GC-MS analysis and antibacterial activity of palmyra fruit pulp (Borassus flabellifer L.). Int J Pharm Bio Sci 2015;6(2):430-5.a
- Gummadi VP, Battu GR, Keerthana Diyya MS, Manda K. A review on palmyra palm (Borassus flabellifer). Int J Curr Pharm Res 2016;8(2):17-20.
- Mohan GK, Yadav M, Rani MS, Shanker K. Antimitotic activity of Borassus flabellifer seed coat. Res J Pharmacogn Phytochem 2016;8(4):223-30.
- Duddukuri GR, Sastry YN, Kaladhar DS, Babu PA, Rao KK, Chaitanya KK. Preliminary studies on in vitro anti-tumor activity of tender seed coat extract of Borassus flabellifer L. on hela cell line. Int J Curr Res 2011;3(7):75-7.
- Sharma R, Arya V. A review on fruits having anti-diabetic potential. J Chem Pharm Res 2011;3(2):204-12.
- Kavatagimath SA, Jalalpure SS, Hiremath RD. Screening of ethanolic extract of Borassus flabellifer flowers for its antidiabetic and antioxidant potential. J Nat Remedies 2016;16(1):22-32.