- *Corresponding Author:
- W. Jiang
Department of Medicine, Nankai University, Tianjin, Nankai 300071, China
E-mail: jiangwentao_tjfch@126.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “282-287” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this study was to explore the role of miR-423-5p in the pathogenesis of liver cancer. Hepatocellular carcinoma cells were transfected with miR-423-5p mimics or miR-423-5p inhibitors, respectively. In this paper, the effect of miR-423-5p on the proliferation and migration of hepatocellular carcinoma cells was identified. Detection of miR-423-5p and genes associated with retinoid-interferon-induced mortality-19 messenger ribonucleic acid by Western blot and quantitative reverse transcription polymerase chain reaction. We verified the relationship of miR-423-5p with genes associated with retinoid-interferon-induced mortality-19 using double luciferase assay. The outcomes indicated that the expression of miR-423- 5p was up-regulated in human hepatoma tissues and cells. Moreover, reducing miR-423-5p can inhibit the proliferation and migration ability of hepatoma cells. Mechanistically, the experimental data proves that genes associated with retinoid-interferon-induced mortality-19 are the direct target of miR-423-5p in hepatoma cells. At the same time, rescue experiments verified that over-expression of genes associated with retinoid-interferon-induced mortality-19 may counteract the ability of miR-423-5p to enhance the proliferation and metastasis of hepatoma. Further, the outcomes showed an abnormal down-regulation of the cell cycle inhibitor p21 in hepatocellular carcinoma cells, which was reversed by the reduction of miR-423-5p. It is suggested that miR-423-5p may enhance the proliferation and metastasis of hepatoma cells by targeting genes associated with retinoid-interferon-induced mortality-19.
Keywords
Vascular dementia, Xuanfu theory, Tong Qiao Yizhi granules, mitogen-activated protein kinase, synaptic plasticity
Liver is an extremely important organ of the human body, which undertakes many important functions such as hematopoiesis, immunity, digestion, detoxification and material metabolism. Worldwide, Southeast Asia and Africa denote high incidence areas of liver cancer, while Northern Europe, United Kingdom and United States of America have low incidence. China has high incidence of liver cancer, and incidence rate in Southeast coastal areas is higher than that in inland areas[1]. According to latest statistics from Ministry of Health, since 1990s, incidence of liver cancer has risen from 3rd place to 2nd place among malignant tumors, 2nd for lung cancer in urban areas, and 2nd for gastric cancer in rural areas. There are 310 000 patients in world every year, and 130 000 patients in China die of liver cancer, accounting for 42 % of total number of liver cancer deaths in world. Because liver cancer is not sensitive to chemotherapy and radiotherapy, surgical resection is main clinical treatment at present, but its recurrence rate is high, and 5 y survival rate is only 40 %-50 %, or even lower. Therefore, liver cancer seriously threatens life and health of the people. Therefore, in-depth study of mechanism of liver cancer is an important topic in current liver cancer research, which is of great significance for improving treatment level of liver cancer[2].
First micro Ribonucleic Acid (miRNA) gene lin- 4 was discovered during study of developmental defects in nematodes[3]. This did not attract attention at time and researchers thought it was just a special case of nematodes[4]. A second miRNA gene, Lethal (Let) 7 was found in Caenorhabditis elegans (C. elegans), which regulates developmental transition from Larval (L4) stage to adult stage in C. elegans and highly conserved in biological evolution. miRNAs are transcribed into primary products of hundreds to thousands of nucleotides primary-miRNA (primiRNA) under action of RNA polymerase II, primiRNA plays a role in Ribonuclease (RNase) III Drosha and its cofactor Pasha (also known as DiGeorge Critical Region 8 (DGCR8). It is cleaved into 60-80 nucleotides of hairpin-like precursors (Precursor miRNA and pre-miRNA), and transporter exportin-5 exports pre-miRNA to cytoplasm with help of Ras-related Nuclear Guanosine Triphosphate Protein (Ran-GTP10)[5]. In cytoplasm, pre-miRNA is recognized by dicer and cleaved to produce a miRNA/miRNA* dimer with a structure similar to small interfering (si) RNA. Both strands of dimer have 2 free nucleotides at 3” end and the two strands are not completely paired. There is a 5” end of miRNA chain that is not paired with corresponding position of miRNA* strand small protrusions which reduces the stability of 5” end of miRNA strand. Since production of mature miRNAs always tend to select more unstable sequences in duplex, this allows the miRNA strand to bind to miRNA-induced silencing complex after unwinding of duplex. In Ribonucleic Acid (RNA)-Induced Silencing Complex (RISC), complete or incomplete pairing with target mRNA can degrade target mRNA or repress its post-transcriptional translation; while miRNA* is rapidly degraded[6]. Studies have found that a large number of miRNAs are located in fragile sites of chromosomes or tumor-related genomic regions, which suggests that miRNAs not only play the role of proto-oncogenes or tumor suppressor genes, but also participate in regulation of tumor cells through various molecular mechanisms, such as growth, differentiation, proliferation and metastasis. Abnormal miRNA has been detected in most malignant tumors among which miR-221 seems to be abnormal in 83 % of liver cancer[7]; Let- 7a-1 and miR-122a was down-regulated in 70 % of liver cancers. Human Let-7 was down-regulated in lung cancer tissues and its level could also reflect prognosis of lung cancer patients. With improvement and application of detection technology such as gene chip, detection of miRNA level will be combined with clinical practice, which opens up a new way of thinking for gene diagnosis and prognosis judgment of tumors[8].
In addition, miRNAs may also become effective drugs for tumor gene therapy. For miRNAs that are abnormally expressed in tumors, antisense oligonucleotides modified with 2’-O-methylation or Locked Nucleic Acid (LNA) could be used specifically to inactivate them[9]. Targeted inhibition of miR-122 using the above methods could completely degrade miR-122 in mice, with long duration and low toxicity. These miRNAs may be overexpressed by viral vectors or liposomes when abnormal downregulation of miRNAs in tumor tissues occurs. This method has been widely used in in vitro study of miRNAs. By overexpressing or inhibiting miRNA, occurrence, development and metastasis of tumors can be intervened, which is a new strategy for tumor gene therapy.
Now world’s 1st miRNA drug-SPC3649, i.e., miravirsen which is a LNA-antimiRTM-122 has entered stage of human clinical research. Uncontrolled cell growth is one of basic characteristics of tumor cells. This process involves activation of a large number of signaling pathways and abnormal gene expression where miRNAs are also involved[10]. Transfection of miR-132 and miR-212 in Pancreatic cancer cell line (Panc-1) could promote cell proliferation, by regulating Retinoblastoma-like (Rbl)[11]. In bladder cancer, while miR-1 and miR- 133a can down-regulate Transgelin 2 (TAGLN2), which can promote cell proliferation; in primary liver cancer cell lines, miR-517a can promote cell proliferation. Although the mechanisms that miRNAs play in the proliferation of liver cancer cells have not been fully understood, miRNAs associated with the proliferation of liver cancer have received increasing attention. In previous experiments of our laboratory, it was found that among chromosomal fragile loci associated with liver cancer, 17qll is a frequent region of Deoxyribonucleic Acid (DNA) copy number amplification associated with liver cancer where miR-423 is located in this region and is highly expressed in liver cancer tissue. Wang et al.[12] studied about miR-423 in Human Leukemia (HL-60) cell line and its level was up-regulated after induction by 12-0-Tetradecanoyl Phorbol Acetate (TPA).
Materials and Methods
Sample source:
This study collected liver cancer tissue samples from 75 patients who underwent total liver tumor resection at Tianjin First Center Hospital from June 2020 to August 2021.
Reagents and instruments:
Eagle medium and liposome 2000 was purchased from Invitrogen Life Technology, Co., Ltd., while fetal bovine serum was purchased from American HycloneTM company. Similarly, Genes associated with Retinoid Interferon Mortality (GRIM)-19 plasmid was purchased from Shanghai Jikai Biotechnology, Co., Ltd., and the dual-luciferase reporter system was purchased from Shanghai Jima Biopharmaceuticals, Co., Ltd.
Luciferase assay:
This study used GRIM-19 3’Untranslated Region (3’UTR) mutants generated by QuikChange sitedirected mutagenesis kit. Promoter (p) GL3-Gene Associated with Retinoid-Interferon-Induced Mortality 19 (GRIM-19) wild type (wt) plasmid and pGL3-GRIM-19-mut plasmid was constructed using PGL3 luciferase reporter vector.
Results and Discussion
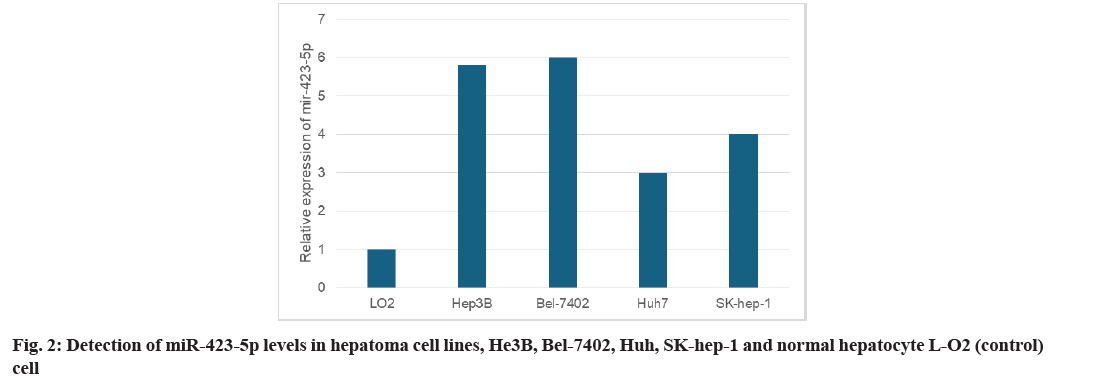
Primarily, quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) was used to detect the levels of miR-423-5p in tumor and adjacent tissues from 75 Hepatocellular Carcinoma (HCC) patients. As shown in fig. 1, the expression of miR- 423-5p in tumor tissues of HCC was higher than that of the corresponding adjacent tissues. Furthermore, miR-423-5p was detected in human Hepatoma cell lines He3B, Bel-7402, Huh7 and SK-hep-1 as well as normal hepatocyte L-O2. The expression of miR- 423-5p He3B, Bel-7402, Huh7 and SK-hep-1 cells increased in different degrees compared with normal hepatocyte L-O2 (fig. 2). These outcomes suggest that miR-423-5p may contribute to the development of HCC.
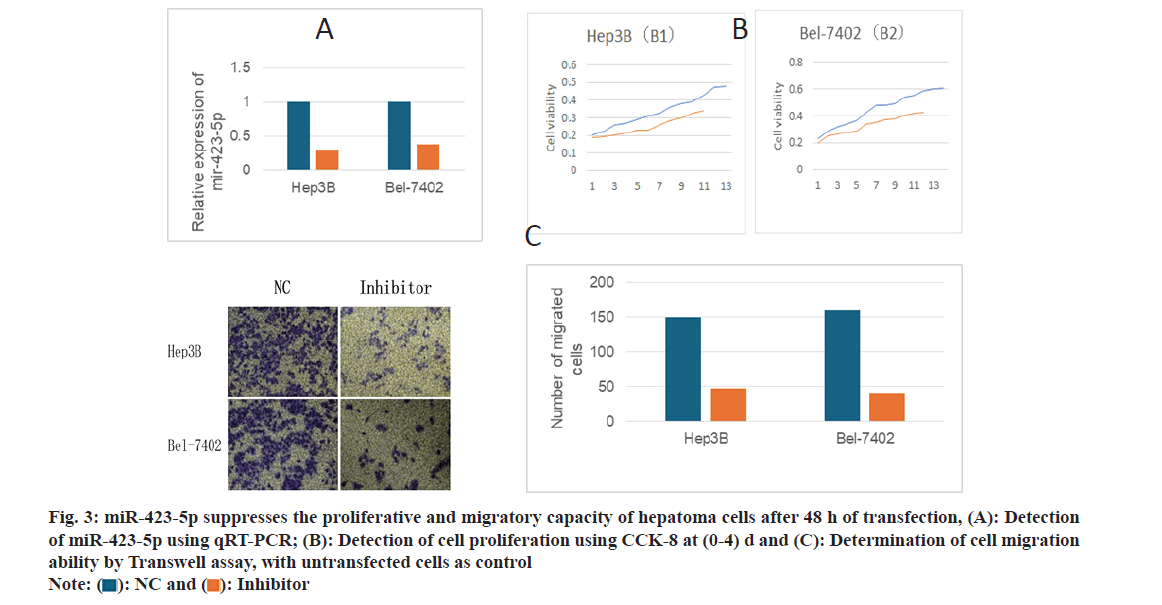
To investigate the biological function of miR-423-5p in liver cancer cells, He3B and Bel-7402 cells were transfected with anti-miR-423-5p and qRT-PCT were used to detect the transfection efficiency; 5p was downregulated (fig. 3A). By 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, the proliferation of He3B and Bel-7402 cells were inhibited following the knockout of miR-423-5p (fig. 3B). The migration ability of He3B and Bel-7402 cells was decreased (fig. 3C) after miR-423-5p knock out. The outcomes indicate that miR-423-5p may enhance the proliferation and migration ability of HCC cells.
Fig. 3:miR-423-5p suppresses the proliferative and migratory capacity of hepatoma cells after 48 h of transfection, (A): Detection of miR-423-5p using qRT-PCR; (B): Detection of cell proliferation using CCK-8 at (0-4) d and (C): Determination of cell migration ability by Transwell assay, with untransfected cells as control
Note: ( ): NC and (
): NC and (  ): Inhibitor
): Inhibitor
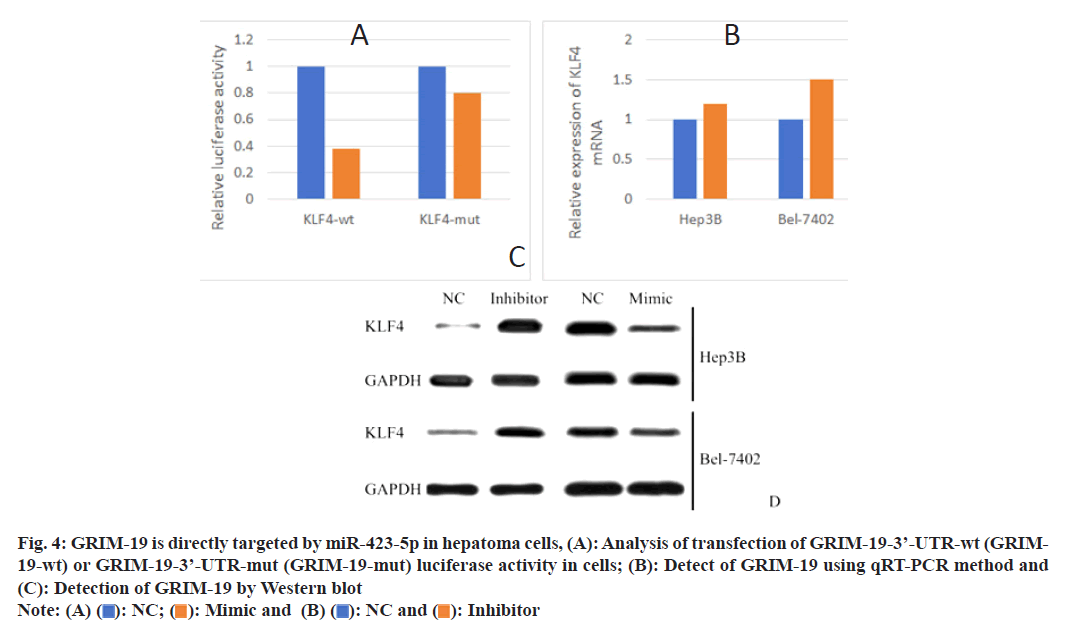
Utilizing TargetScan version 7.2 online software, GRIM-19 might be the target of miR-423-5p. Luciferase reporter assay showed that overexpression of miR-423-5p inhibited luciferase activity of the GRIM-19-3’-UTR-wt reporter gene from He3B and Bel-7402 cells, while the activity of GRIM-19- 3’-UTR-mut reporter gene was not affected (fig. 4A). Using qRT-PCR technology, the miR-423-5p inhibitors did not affect the mRNA of GRIM-19 (fig. 4B). In addition, the outcomes verified that the enhancement of miR-423-5p in He3B and Bel-7402 cells decreased the level of GRIM-19 protein while the inhibition of miR-423-5p increased the GRIM-19 protein level (fig. 4C).
Fig. 4: GRIM-19 is directly targeted by miR-423-5p in hepatoma cells, (A): Analysis of transfection of GRIM-19-3’-UTR-wt (GRIM- 19-wt) or GRIM-19-3’-UTR-mut (GRIM-19-mut) luciferase activity in cells; (B): Detect of GRIM-19 using qRT-PCR method and (C): Detection of GRIM-19 by Western blot
Note: (A) ( ): NC; (
): NC; ( ): Mimic and (B) (
): Mimic and (B) (  ): NC and (
): NC and (  ): Inhibitor
): Inhibitor
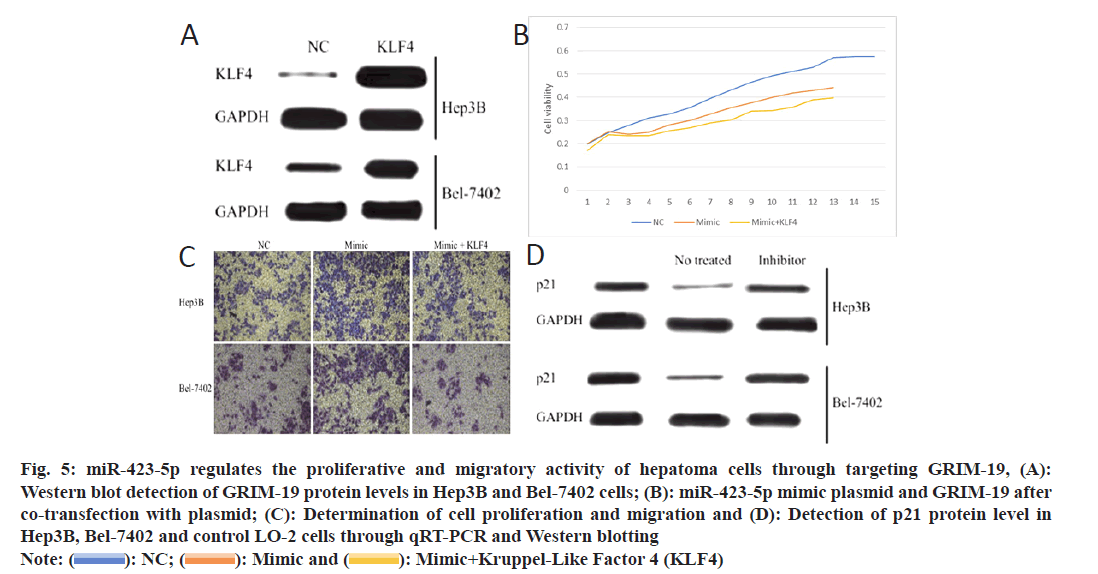
In order to investigate whether GRIM-19 might be the downstream target gene of miR-423-5p for HCC cell motility, He3B and Bel-7402 cells were transfected to over-expression GRIM-19 plasmid (fig. 5A). The effects of miR-423-5p on the proliferation of He3B and Bel-7402 cells were attenuated by up-regulation of GRIM-19 (fig. 5B). At the same time, the ability of miR-423-5p to enhance the cell migration was also inhibited by the overexpression of GRIM-19 (fig. 5C). At the same time, Western blotting verified the down-regulated expression of p21 (GRIM-19 downstream molecule) in He3B and Bel-7402 cells as compared to normal hepatocyte L-O2 which was reversed by miR-423-5p inhibitor. (fig. 5D). The outcomes suggest that miR-423-5p can enhance the proliferation and migration of hepatoma cells through targeting GRIM-19.
Fig. 5: miR-423-5p regulates the proliferative and migratory activity of hepatoma cells through targeting GRIM-19, (A):
Western blot detection of GRIM-19 protein levels in Hep3B and Bel-7402 cells; (B): miR-423-5p mimic plasmid and GRIM-19 after
co-transfection with plasmid; (C): Determination of cell proliferation and migration and (D): Detection of p21 protein level in
Hep3B, Bel-7402 and control LO-2 cells through qRT-PCR and Western blotting
Note: ( ): NC; (
): NC; ( ): Mimic and (
): Mimic and (  ): Mimic+Kruppel-Like Factor 4 (KLF4)
): Mimic+Kruppel-Like Factor 4 (KLF4)
miRNAs play a post-transcriptional negative regulatory role mainly by promoting degradation of target mRNAs or inhibiting their translation[13] and are widely involved in cell proliferation, apoptosis, metabolism and differentiation; miRNAs can be used as a new type of molecular targets in diagnosis and treatment of tumors and have broad application prospects[14]. Furthermore, many miRNAs are located at chromosomal sites or genomic regions associated with cancer[15], often undergoing chromosome replacement, deletion, amplification or abnormal integration[16]. In liver cancer, there are also many chromosome fragility loci[17]. Although abnormal miRNAs in HCC have a close relationship with hepatocarcinogenesis[18], the mechanisms by which the miRNAs regulate the progression of HCC still require further investigation[19]. In this research, miR- 423-5p showed abnormally elevated the expression of miR-423-5p in HCC through inhibition of GRIM-19 to enhance the proliferation and metastasis of hepatoma cells, indicating that miR-423-5p might play a positive role in the regulation of HCC progression[20]. The proliferation and invasion of malignant cells were the main reasons for human tumorigenesis. Our outcomes indicate that miR-423-5p can enhance the proliferation and migration of hepatoma cells while reducing miR-423-5p significantly inhibits the proliferation and migration of hepatoma cells[21].
The innovation of this study is that, for the 1st time, its molecular biological mechanism was explored, depicting a new target of 423-5p. Our study provides new clues to reveal molecular mechanism of liver cancer progression and metastasis. However, this study also has certain limitations[22]. There remains lack of molecular mechanisms for abnormally high miR-423-5p expression in cancer tissues. We speculate that miR-423-5p may be regulated by certain transcription factors associated with tumorrelated transcription factors and thus mediated its abnormally high expression in tumor tissue of liver cancer[23]. Next, we will explore the molecular mechanism behind the abnormally high miR-423-5p expression in tumor tissue of liver cancer[24,25].
In summary, the present research offers a new insight into the pathogenesis of HCC. Up-regulated miR-423- 5p may enhance the proliferation and migration of hepatic cancer cells by targeting downstream GRIM- 19 and p21, which may provide a new therapeutic target for liver cancer.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lin H, Lin T, Lin J, Yang M, Shen Z, Liu H, et al. Inhibition of miR-423-5p suppressed prostate cancer through targeting GRIM-19. Gene 2019;688:93-7.
[Crossref] [Google Scholar] [PubMed]
- Shang Y, Wang L, Zhu Z, Gao W, Li D, Zhou Z, et al. Downregulation of miR-423-5p contributes to the radioresistance in colorectal cancer cells. Front Oncol 2021;10:1-15.
[Crossref] [Google Scholar] [PubMed]
- Dai T, Zhao X, Li Y, Yu L, Li Y, Zhou X, et al. miR-423 promotes breast cancer invasion by activating NF-κB signaling. Onco Targets Ther 2020;13:5467-78.
[Crossref] [Google Scholar] [PubMed]
- Shan G, Gu J, Zhou D, Li L, Cheng W, Wang Y, et al. Cancer-associated fibroblast-secreted exosomal miR-423-5p promotes chemotherapy resistance in prostate cancer by targeting GREM2 through the TGF-β signaling pathway. Exp Mol Med 2020;52(11):1809-22.
[Crossref] [Google Scholar] [PubMed]
- Tao HF, Shen JX, Hou ZW, Chen SY, Su YZ, Fang JL. LncRNA FOXP4‑AS1 predicts poor prognosis and accelerates the progression of mantle cell lymphoma through the miR‑423‑5p/NACC1 pathway. Oncol Rep 2021;45(2):469-80.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Guan R, Gong K, Xie H, Shi L. Circ_FURIN knockdown assuages testosterone-induced human ovarian granulosa-like tumor cell disorders by sponging miR-423-5p to reduce MTM1 expression in polycystic ovary syndrome. Reprod Biol Endocrinol 2022;20(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Zhang P, Zhao F, Jia K, Liu X. The LOXL1 Antisense RNA 1 (LOXL1-AS1)/microRNA-423-5p (miR-423-5p)/Ectodermal-Neural Cortex 1 (ENC1) axis promotes cervical cancer through the Mitogen-Activated protein Kinase (MEK)/Extracellular signal-Regulated Kinase (ERK) pathway. Bioengineered 2022;13(2):2567-84.
[Crossref] [Google Scholar] [PubMed]
- Sun X, Huang T, Zhang C, Zhang S, Wang Y, Zhang Q, et al. Long non-coding RNA LINC00968 reduces cell proliferation and migration and angiogenesis in breast cancer through up-regulation of PROX1 by reducing hsa-miR-423-5p. Cell Cycle 2019;18(16):1908-24.
[Crossref] [Google Scholar] [PubMed]
- Morales-Pison S, Jara L, Carrasco V, Gutiérrez-Vera C, Reyes JM, Gonzalez-Hormazabal P, et al. Genetic variation in microRNA-423 promotes proliferation, migration, invasion, and chemoresistance in breast cancer cells. Int J Mol Sci 2021;23(1):1-13.
[Crossref] [Google Scholar] [PubMed]
- Ke R, Lv L, Zhang S, Zhang F, Jiang Y. Functional mechanism and clinical implications of microRNA-423 in human cancers. Cancer Med 2020;9(23):9036-51.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Wei X, Chen X, Wang Q, Zhang J, Kalvakolanu DV, et al. GRIM-19 inhibits proliferation and induces apoptosis in a p53-dependent manner in colorectal cancer cells through the SIRT7/PCAF/MDM2 axis. Exp Cell Res 2021;407(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Wang B, Yang Y, Deng X, Ban Y, Chao L. Interaction of M2 macrophages and endometrial cells induces downregulation of GRIM-19 in endometria of adenomyosis. Reprod Biomed Online 2020;41(5):790-800.
[Crossref] [Google Scholar] [PubMed]
- Hirschfeld M, Rücker G, Weib D, Berner K, Ritter A, Jäger M, et al. Urinary exosomal microRNAs as potential non-invasive biomarkers in breast cancer detection. Mol Diagn Ther 2020;24(2):215-32.
[Crossref] [Google Scholar] [PubMed]
- Rui X, Shao S, Wang L, Leng J. Identification of recurrence marker associated with immune infiltration in prostate cancer with radical resection and build prognostic nomogram. BMC Cancer 2019;19(1):1-13.
[Crossref] [Google Scholar] [PubMed]
- Ghafouri-Fard S, Shoorei H, Taheri M. Role of microRNAs in the development, prognosis and therapeutic response of patients with prostate cancer. Gene 2020;759:1-14.
[Crossref] [Google Scholar] [PubMed]
- Kushwaha PP, Gupta S, Singh AK, Prajapati KS, Shuaib M, Kumar S. MicroRNA targeting nicotinamide adenine dinucleotide phosphate oxidases in cancer. Antioxid Redox Signal 2020;32(5):267-84.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Yan Y, Yang M, Yang Z. Expressions and clinical significances of STAT3 and GRIM19 in epithelial ovarian cancer. 3 Biotech 2020;10(6):1-10.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Gu J, Ding X, Ge G, Zang X, Ji R, et al. LINC00978 promotes the progression of hepatocellular carcinoma by regulating EZH2-mediated silencing of p21 and E-cadherin expression. Cell Death Dis 2019;10(10):1-15.
[Crossref] [Google Scholar] [PubMed]
- Chen T, Huang H, Zhou Y, Geng L, Shen T, Yin S, et al. HJURP promotes hepatocellular carcinoma proliferation by destabilizing p21 via the MAPK/ERK1/2 and AKT/GSK3β signaling pathways. J Exp Clin Cancer Res 2018;37(1):1-14.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Chen J, Ning D, Liu Q, Wang C, Zhang Z, et al. FBXO22 promotes the development of hepatocellular carcinoma by regulating the ubiquitination and degradation of p21. J Exp Clin Cancer Res 2019;38(1):1-16.
[Crossref] [Google Scholar] [PubMed]
- Wei C, Wang H, Xu F, Liu Z, Jiang R. LncRNA SOX21-AS1 is associated with progression of hepatocellular carcinoma and predicts prognosis through epigenetically silencing p21. Biomed Pharmacother 2018;104:137-44.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Cheng X, Chen C, Huijuan W, Zhao H, Liu W, et al. Apigenin, a flavonoid constituent derived from P. villosa, inhibits hepatocellular carcinoma cell growth by cyclinD1/CDK4 regulation via p38 MAPK-p21 signaling. Pathol Res Pract 2020;216(1):1-15.
[Crossref] [Google Scholar] [PubMed]
- Hui L, Zheng F, Bo Y, Lin SM, Aijun L, Weiping Z, et al. MicroRNA let-7b inhibits cell proliferation via upregulation of p21 in hepatocellular carcinoma. Cell Biosci 2020;10(1):1-12.
[Crossref] [Google Scholar] [PubMed]
- Huang S, Zhang C, Sun C, Hou Y, Zhang Y, Tam NL, et al. Obg-Like ATPase 1 (OLA1) overexpression predicts poor prognosis and promotes tumor progression by regulating P21/CDK2 in hepatocellular carcinoma. Aging 2020;12(3):1-17.
[Crossref] [Google Scholar] [PubMed]
- Baek SY, Hwang UW, Suk HY, Kim YW. Hemistepsin A inhibits cell proliferation and induces G0/G1-phase arrest, cellular senescence and apoptosis via the AMPK and p53/p21 signals in human hepatocellular carcinoma. Biomolecules 2020;10(5):1-14.
[Crossref] [Google Scholar] [PubMed]
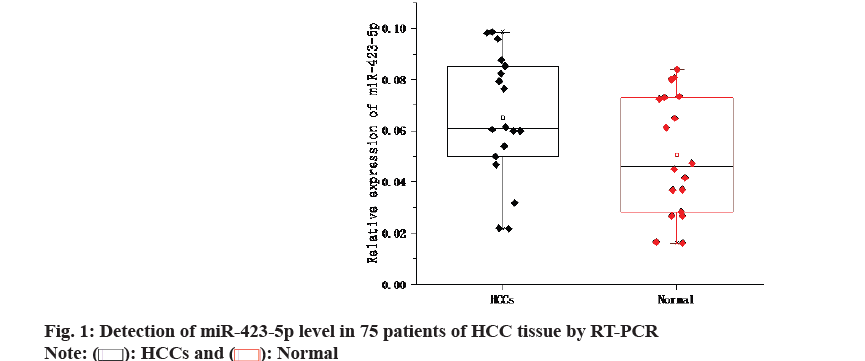
 ): HCCs and (
): HCCs and (  ): Normal
): Normal