- *Corresponding Author:
- Sneh Priya
Department of Pharmaceutics, NGSM Institute of Pharmaceutical Sciences, Nitte (Deemed to be University), Mangalore, Karnataka 575018, India
E-mail: snehpriya123@nitte.edu.in
| Date of Received | 20 April 2023 |
| Date of Revision | 12 Dec 2023 |
| Date of Acceptance | 22 May 2024 |
| Indian J Pharm Sci 2024;86(3):1-9 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Quetiapine fumarate is an atypical antipsychotic drug to treat schizophrenia. It has poor oral bioavailability (9 %) owing to its extensive first-pass metabolism. In the present study, quetiapine nanoemulsion were formulated for brain targeting through the intranasal route. Ultrasonication method was employed for nanoemulsion preparation by employing water, Tween 20 and propylene glycol as the surfactant mixture (surfactant and co-surfactant mixture) and isopropyl myristate as oil. Rat models were used to examine the concentration of quetiapine in the brain and blood (plasma) following oral, intranasal and free medication administration. When compared to the intranasal-free drug and the oral route, intranasal nanoemulsion of quetiapine led to noticeably greater drug levels in the brain. The results showed that intranasal nanoemulsion, as opposed to intranasally or orally delivered free drug had a longer half-life in the brain. Hence, using intranasal quetiapine nanoemulsion to treat schizophrenia and bipolar disorder may be a novel way to handle this illness.
Keywords
Quetiapine fumarate, schizophrenia, nanoemulsion, intranasal, brain drug delivery, pharmacokinetics
Schizophrenia is a heterogeneous syndrome characterized by perturbation of language, social activity, perception, volition and affection. Delusions, conceptual confusion and hallucinations are examples of positive symptoms and loss of function, reduced capacity for emotional expressiveness, anhedonia, trouble concentrating, and reduced social interaction are examples of negative symptoms that schizophrenia patients may have. Antipsychotic drugs (APDs) are the keystones of treatment and management of positive and negative symptoms of schizophrenia[1,2]. Quetiapine fumarate is a short-acting atypical APD to treat schizophrenia, major depressive disorder and bipolar disorder. It is mostly marketed as tablets, which show substantial first-pass metabolism; where around 60 % of the dose is metabolized before entering the systemic circulation[3]. Because the brain is quetiapine's intended site of action, it is required to prevent its first-pass metabolism, thus increasing the Bioavailability (BA) and at the same time improving receptor targeting and bypassing the Blood-Brain Barrier (BBB), hence lowering the drug concentration at non-targeted locations and minimising adverse effects, to attain the necessary drug concentration at the site of action[4,5].
Previous studies[6-9] have shown that the intranasal route of administration offers a non-invasive, practical and rapid brain drug delivery. It is also an advantageous route due to being simple, convenient and cost-effective. It is also a better option for drug targeting owing to the direct transport of drugs bypassing the barriers of the brain. There is some evidence that quetiapine can be absorbed intranasally as indicated by its illicit abuse through this route as reported by morin et al.[10]. The designed formulation should target the olfactory region of the nasal cavity for its quick permeation across the nasal mucosa. Further, the mucoadhesion of the drug aids permeation across the nasal mucosa and by circumventing nasal mucociliary clearance, residence time in the posterior nasal cavity is extended[11]. Nanoemulsion (NE) is widely used as a delivery system to enhance uptake across the nasal mucosa due to their small globule size and lipophilic nature and materials such as polyelectrolyte polymer help in the mucoadhesion of the drug and thus its retention in the nasal mucosa[12].
The objective of this study was to prepare and characterize Quetiapine NE (QNE) and their evaluation on animal models. This NE is assumed to result in rapid nose-to-brain transport of quetiapine and a more significant transport and distribution into and within the brain. This may help lower the price of the therapy in addition to other benefits like reduced adverse effects, dosage, and frequency of administration.
Materials and Methods
Quetiapine fumarate obtained as a generous gift from Orchid Pharmaceuticals Pvt. Ltd., (Chennai, India). Tween 20 (polyoxyethylene sorbitan monolaurate) was obtained from Sigma-Aldrich Chemicals Pvt. Ltd., USA. Propylene glycol was acquired from SD Fine Chemicals (Mumbai, India). Isopropyl myristate, methanol and acetonitrile, High-Performance Liquid Chromatography (HPLC) grade water was purchased from E-Merck (Mumbai, India). All other chemicals and solvents were of analytical grade.
Preparation and characterization of QNEs:
NE was prepared by ultrasonication using a probe sonicator (Table 1)[13]. In this method, the sonication process helps in the reduction of the droplet size of the conventional emulsion. But only small batches of the NE can be prepared. The aqueous and the oil phases were first prepared. The oil phase was prepared by dissolving the drug in oil and Smix mixture at 50 % amplitude, pulse 5 s on and 5 s off for 3 min in the Probe Sonicator. The aqueous phase includes water. With constant stirring at 250 rpm, the oil phase was introduced drop wise to the heated aqueous phase (50°). The obtained emulsion was sonicated for 10 min at 50 % amplitude, pulse 5 s on and 3 s off to give NE.
| Formulation Code | Drug (mg) | Oil (% w/w) | Smix (3:1) (% w/w) | Water (% w/w) |
|---|---|---|---|---|
| QNE1 | 50 | 5 | 30 | 65 |
| QNE2 | 50 | 10 | 30 | 60 |
| QNE3 | 50 | 15 | 30 | 55 |
| QNE4 | 50 | 20 | 30 | 50 |
Table 1: Composition of Quetiapine Fumarate Loaded NE
The transparency of NE formulation was determined by measuring percentage transmittance at 560 nm through an Ultraviolet (UV) spectrophotometer and the percentage drug content of the NE was also estimated using UV-visible spectrophotometry at 254 nm. A pH meter (Systronics, India) was used to determine the pH of the NE at 25°. A C50-1 spindle of the Brookfield R/S plus rheometer (Brookfield Engineering, Middleboro, MA) at 25° was used to measure the viscosity of the NE. A Zetasizer (Nano ZS, Malvern Instruments, UK) was used to measure the globule size, size distribution and zeta potential. The shape and surface morphology of the NE were determined by fluorescent microscope as well as TEM.
In vitro drug release studies:
In vitro drug release from NE was studied with the help of Franz diffusion cell across the dialysis membrane (12 Kda, Hi Mediam)[14]. Before use, the membrane was given a 12 h soak in deionized water. 80 ml of phosphate buffer solution as the dissolution medium (n=3) which was in the pH range in the nasal cavity (pH 6.4), was placed in the receptor compartment and 1 ml (2 mg/ml) of NE was placed in the donor compartment. With the aid of a tiny Teflon-coated magnetic bead, the medium was agitated at 200 rpm while being kept at 37±0.5° using a circulating water bath. Aliquots were withdrawn from the medium at suitable time intervals and were analysed using a UV/Visible (Vis) spectrophotometer (1700, Shimadzu®, Tokyo, Japan) at wavelength 254 nm. To maintain the sink condition, the removed volume was substituted with an equal volume of the new medium.
In vitro drug permeability studies:
The permeation barrier consisted of sheep nasal mucosa that was procured from a nearby slaughterhouse within an hour of the animal's sacrifice and Franz diffusion cells were used for the in vitro drug permeation studies. The mucosa was carefully cut with a scalpel and mounted on the diffusion chamber with mucosal surface facing the donor compartment and serosal surface facing the receptor compartment. The samples were collected and assessed in the same manner as in vitro drug release studies.
Calculation of nasal mucosal permeation parameters:
Plots depicting the cumulative drug permeation per unit area as a function of time were made. The slope of the linear part of the curve was used to determine the flux. The relation derived from Fick’s first law of diffusion was used to determine the permeability coefficient (Kp) of QTP across sheep nasal mucosa and this is shown using the equation, Kp=J/C, where C is the drug concentration in the donor compartment and J is the flux.
Bioanalytical HPLC method:
Reverse phase HPLC method with a mobile phase made up of 10 mm ammonium acetate buffer (pH 3.5) and acetonitrile (60:40 v/v) through a Varian Microsorb-MVC18 Column (250×4.6 mm, 5) at a flow rate of 1 ml/min, was used for the chromatographic separation. The effluents were measured by Photodiode Array (PDA) detection at wavelength 254 nm. Extraction of quetiapine from the plasma and brain sample was carried out by protein precipitation method using methanol. The working stock solution (50 mg/ml) was prepared by combining 25 μl of the internal standard (Triprolidine) and 150 μl of either rat plasma or brain sample. This solution was then vortexed for 60 s. To this, 150 μl of methanol cold acetonitrile (1:1 ratio) was added and vortexed for 60 s. The resulting solution was centrifuged in the cold centrifuge at 4° at 7000 rpm for 10 min. The supernatant was separated and 40 μl of this was injected into the HPLC system.
In vivo studies:
Plasma and brain pharmacokinetic studies were carried out in Albino rats. The preclinical study protocol was approved by the Institutional Animal Ethics Committee of the K. S. Hegde Medical Academy, Mangalore vide Approval no: KSHEMA/IAEC/01/2015. Three groups of rats were formed. Group III had 18 animals, while the other two groups each had 15 animals. Quetiapine pure drug solution was given to group I through the oral route. Group II was given quetiapine pure drug solution intranasally, while group III was given Quantitative Nasal Eosinophilia (QNE) intranasally. The rats were treated orally and intranasally as given in Table 2. To each of the 3 groups of rats, 2.25 mg/kg quetiapine was administered. After administration of the dose at a predetermined time interval, heparinized capillary tubes were used to collect 500 μl blood samples from the retro-orbital vein and place them in centrifuge tubes with dipotassium Ethylene Diamine Tetraacetic Acid (K2 EDTA) added. The animals were immediately euthanized using the cervical necrosis method following the collection of each blood sample and the removed rat brain was washed twice in saline, wiped using soft tissue, weighed and frozen until analysis. Phosphate buffer saline was utilized to homogenise the brain tissue (pH 7.4), centrifuging the collected blood samples at 3000×g (Remi cooling centrifuge, India) for 5 min at 0°. Segmented plasma was kept at a temperature of -20°. HPLC was used to extract and examine samples of both blood and brain tissue.
Pharmacokinetics parameters:
Statistics were assessed by using GraphPad Prism software package and PK solution 2.0 software (non-compartmental modelling) (version 4.03) was used to analyse pharmacokinetic parameters. The pharmacokinetics parameters such as Cmax, Tmax, and Area Under the Curve (AUC), Mean Residence Time (MRT) and elimination half-life (t1/2) were calculated for all groups. p<0.05 was considered significant with 95 % confidence intervals in a one-way Analysis of Variance (ANOVA) to look for any differences. The relative BA of the different quetiapine formulations/administration routes was calculated as follows:
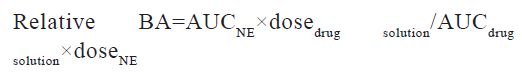
Histopathological studies:
Sheep nasal mucosa was employed to study any damage done to the integrity of tissue. From a local slaughterhouse (Mangalore, India), sheep nasal mucosa was obtained within 1 h of sacrificing the animal and it was cleaned with an isotonic saline solution. Optimized NE formulations were applied on nasal mucosa followed by fixing in 10 % neutral carbonate buffered formalin solution. After embedding in paraffin wax, a thin section was stained with haematoxylin and eosin. Under a light microscope, the smeared sections were inspected. The control was untreated mucosa.
Results and Discussion
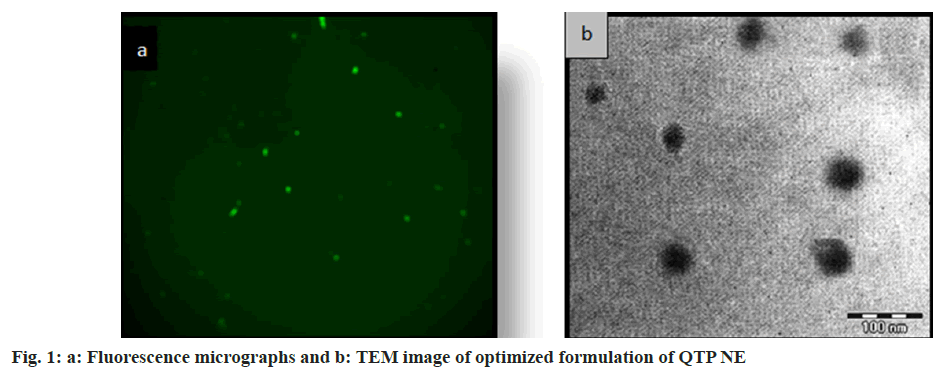
NEs were prepared and evaluated for pH, viscosity, globule size, zeta potential and size distribution and percent transmittances of drug content (Table 2). All the NEs formulations were nearly transparent and their percent transmittances were higher than 98 %. In all the NEs formulations drug content was higher than 98 % and there was no trace of undissolved drug. Each formulation's pH was well within the range for the nasal pH (6.4-6.8), which may help to reduce the nasal mucosal irritation which has developed upon instillation. Every formulation had nanoscale droplets (61-105 nm) with a very uniform size distribution as indicated by their low polydispersity values. The zeta potential of all NEs formulations was in the range of -30 to -35 mv suggesting that the NEs were remarkably stable. Indeed, colloidal nanodispersions have a higher chance of remaining stable at larger zeta potentials because of the repulsion among charged droplets that overcomes their natural tendency to aggregate. The concentrations of the surfactant, water and oil components affect how viscous NEs were. Decreasing the water content and increasing the oil content tends to increase the viscosity, as seen in formulation QNE1-QNE4, where the oil concentration was increased from 5 %-20 %. The selection of oil concentration is very crucial because if higher concentration of oil reached in the lung may cause lipoidal pneumonia. Therefore, the minimum concentration of oil is used in the formulation. Viscosity >30 cps is regarded as sufficient to keep the formulation in the nasal cavity for adequate absorption through the nasal mucosa. All NEs formulations viscosity was >30 cps. Viscosity is also an essential factor required for stability and efficient drug release. The lower viscosity of NEs formulation is anticipated to result in a quicker release of the active ingredients. The droplets in the NE appeared as spherical particles, uniform in size, bright at fluorescence microscopy and dark at transmission electron microscopy shown in fig. 1a and fig. 1b, respectively.
| Form code | % Drug content ±SD | pH | Viscosity (cps) ±SD | Globule size (nm) | PDI | Zeta potential mV |
|---|---|---|---|---|---|---|
| QNE1 | 99.11±2.17 | 6.24±0.41 | 32.6±0.13 | 61.24±1.07 | 0.187 | -30.56±2.1 |
| QNE2 | 98.65±1.45 | 6.31±0.14 | 52.5±0.19 | 81.74±1.07 | 0.211 | -33.49 ±1.11 |
| QNE3 | 99.32±2.51 | 6.43±0.25 | 66.3±0.37 | 95.59±1.11 | 0.210 | -32.13 ±1.51 |
| QNE4 | 98.55±1.06 | 6.16±0.19 | 81.4±0.25 | 105.15±1.07 | 0.211 | -35.76 ±1.87 |
Note: All values are expressed as mean ±standard deviation, (n=3)
Table 2: Characterization of QNE Formulations
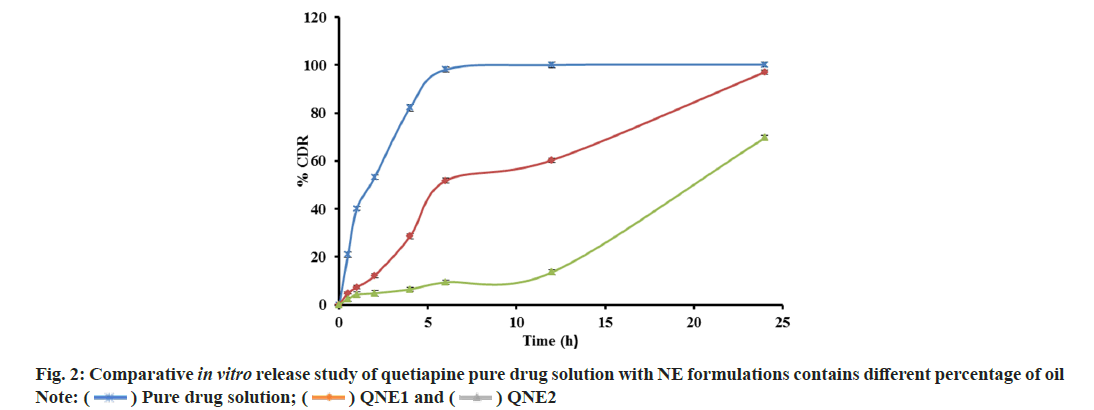
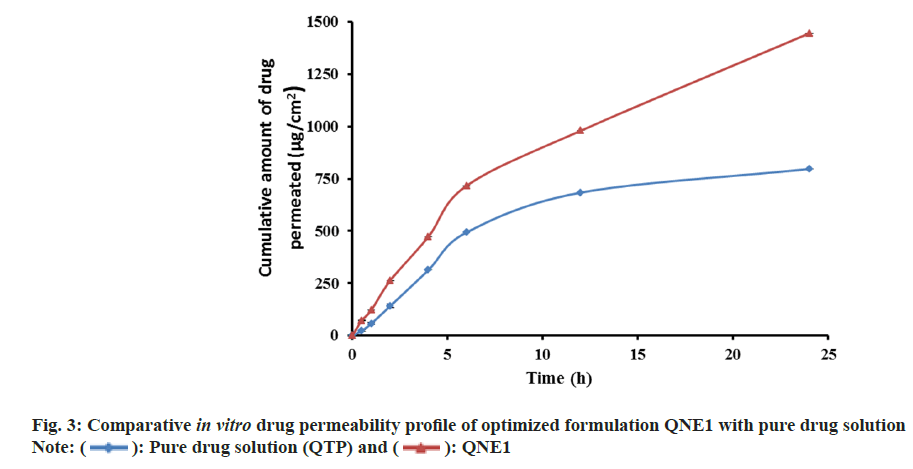
An in vitro drug release study from NE was performed and shown in fig. 2. It was found that drug release from NEs decreased as the concentration of oil increased, possibly because of the increase in the viscosity of the formulations. Based on in vitro drug release profile QNE1 was considered as the optimized formulation. In vitro drug release profiles of optimized formulations (QNE1) were also compared with that of pure drug solution (fig. 3). The percentages of the drug released 6 h from the beginning of the incubation were 100 % and 45.15 %±1.11 % for pure drug solution and for QNE1, respectively. It was only after 24 h incubation that 100 % of the drug was released from QNE1. An in vitro permeation study of QTP through sheep nasal mucosa was conducted for 24 h. Fig. 4, shows the QTP penetration profile and Table 3, shows the QTP permeation parameters through the sheep nasal mucosa that has been excised. The cumulative amount of drug permeated across sheep nasal mucosa after 24 h for purse drug solution (796.64 μg/cm2) was considerably lower when compared with QNE1 NE formulation (1445.68 μg/cm2) indicating that NE can enhance nasal delivery of hydrophilic drugs such as QTP.
| Form code | Permeated amount at 24 h (µg/cm2) | Flux (µg/cm2.h) | Peameability constant (Kp) (cm/h) |
|---|---|---|---|
| Pure drug solution | 796.64±11.21 | 40.26±1.65 | 0.007888 |
| QNE1 | 1445.68±13.03 | 15.75±2.02 | 0.02013 |
Table 3: Permeated Amount of QTP at 24 H, FLUX, and Permeability Coefficient
Results of the permeability coefficient indicated that QNE1 was 2.8 times more permeable than the drug solution.
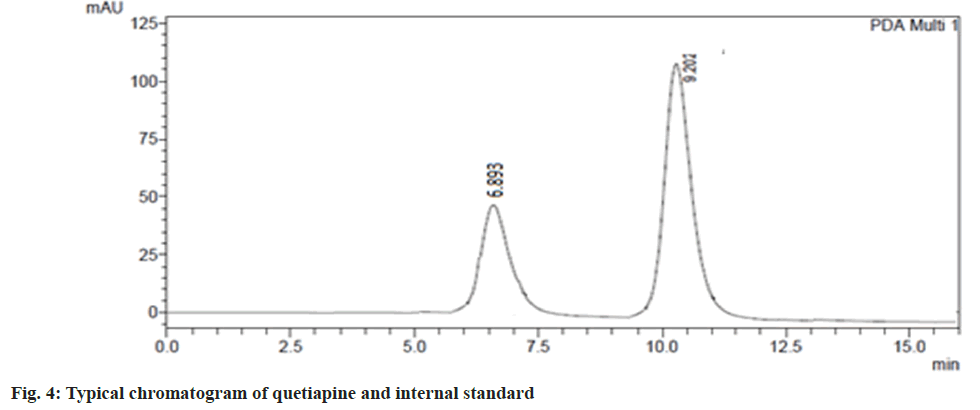
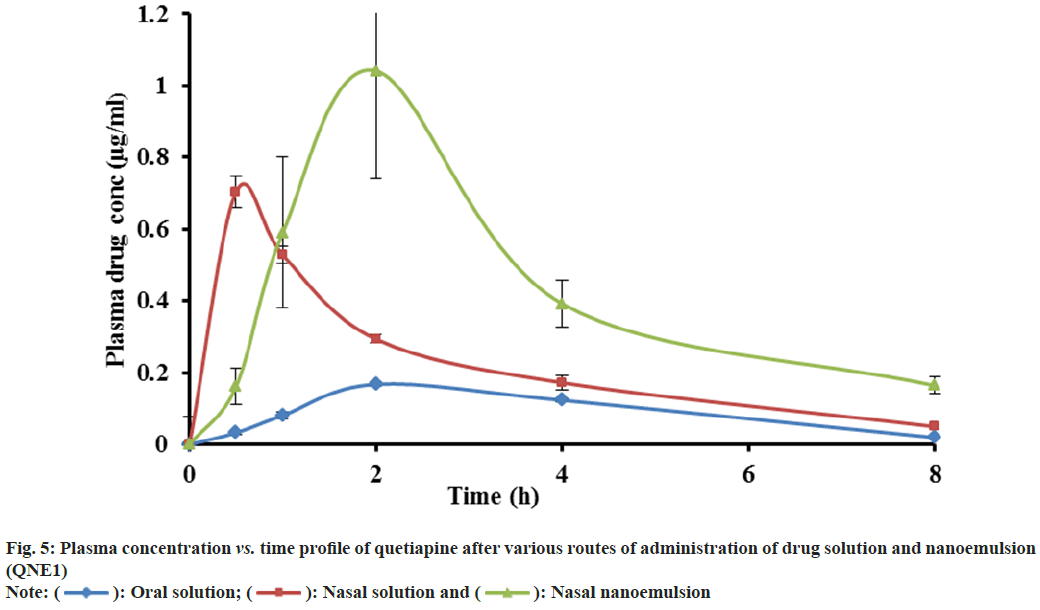
The validated HPLC method was very sensitive to quantifying quetiapine in plasma and brain in very low concentrations (50 ng/ml). This method was analysed, and there was no endogenous compound obstruction to the quetiapine retention time (9.2 min) and internal standard (6.87). Fig. 4, depicts the distinctive chromatogram of quetiapine and the internal standard. The concentration range of the calibration line was 1-50 μg/ml (R2=0.998). The average quetiapine unsheathing recovery from brain tissue homogenate was 96.04±1.6 % and 93.560±2.8 %, respectively. Table 4 and fig. 5, show the plasma concentration of quetiapine by oral and nasal free drug administration followed by intranasal NE administration. Plasma quetiapine AUC after the administration of the intranasal NE (4.42±0.47 μg min ml-1) was about 6 and 2.4 fold higher than after the oral (0.78±0.11 μg min ml-1) and intranasal (1.87±0.22 μg min ml-1) administration of the drug solution. Nasally delivered drug solution peaked in 0.5 h when Cmax and Tmax were compared (Cmax=0.7 μg ml-1) whereas the intranasal NE formulation peaked in 1.67 h (Cmax of 1.08 μg ml-1). When an oral solution group and an intranasal NE group were compared, it was found that the Cmax of the NE formulation was 5.7 times stronger. Intranasal QNE was found to have a greater systemic AUC than free drug. Further the relative BA of the NE formulation QNE1 was found to be 2.4 times that of drug solution through intranasal route. This enhancement might be due to avoiding first-pass metabolism of drugs in NE. The nasal solution hit its peak before the NE drug. The drug solution given through the nasal route reached the systemic circulation quickly, whereas the NE drug may have been released slowly into the circulation due to collection in the nasal mucosa, delaying its Tmax. A similar result was reported by Kumar et al. who studied the absorption of olanzapine after intranasal administration[15].
| Parameter | Oral solution | Nasal solution | Nasal nanoemulsion (QNE1) |
|---|---|---|---|
| AUC(0-∞) (µgh/ml) | 0.78±0.11b | 1.87±0.22b | 4.42±0.47bb |
| Tmax (h) | 2.67±1.15 | 0.50 | 1.67±0.58 |
| Cmax (µg/ml) | 0.19±0.01b | 0.70±0.08c | 1.084±0.23bc |
| KE (1/h) | 0.29±0.15 | 0.32±0.12 | 0.22±0.07 |
| MRT (h) | 3.87±0.25 | 3.43±0.90 | 4.98±0.98 |
Note: a: Mean ±SD, n=3; b: Significant difference between free drug oral vs. intranasal NE, **p<0.01 (99 % CI) and c: Significant difference between free drug intranasal vs. intranasal NE, *p<0.05 (95 % CI)
Table 4: Plasma Pharmacokinetic Parameters of Quetiapine with Various Routes of Administration
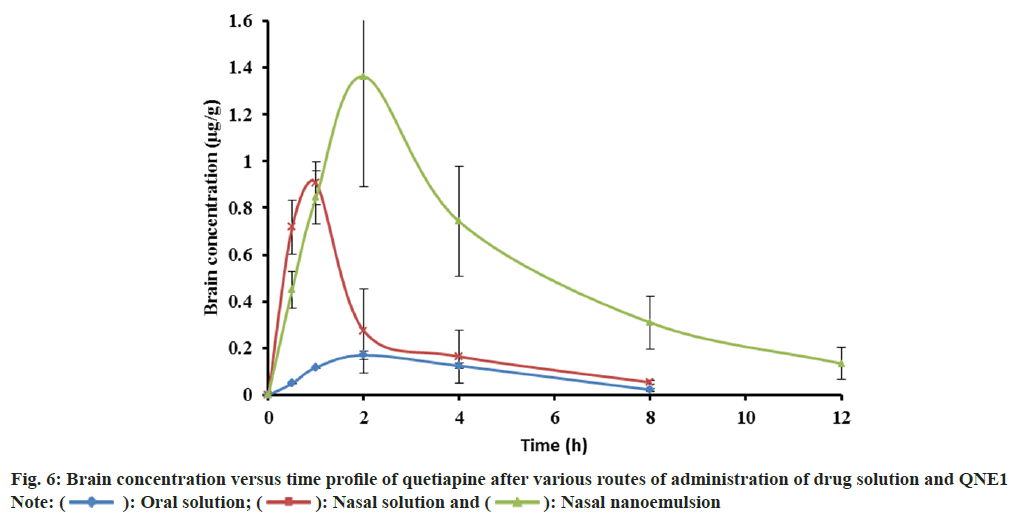
Quetiapine concentration in brain is shown in Table 5 and fig. 6. The AUC of quetiapine in brain after the intranasal administration of the NE (7.33±2.02 μg min ml-1) was about 9 and 3-fold higher than after the oral (0.84±0.02 μg min ml-1) and intranasal (2.44±0.05 μg min ml-1) administration of the drug solution, respectively. When quetiapine concentrations in the brain were examined, nasal drug solution and NE administrations achieved 3 times the amount of quetiapine as compared to the oral route. The intranasal solution had a tmax of 0.83 h, whereas intranasal NE showed a tmax of 1.67 h. Quetiapine MRT was significantly longer after the intranasal administration of the NE formulation (5.09 h) than after the oral administration (3.63 h) and nasal administration (2.91 h) of the drug solution (Table 5). Nasally administered drug solution showed smaller MRT because it gets cleared from the nasal cavity quickly due to mucociliary clearance whereas NE showed longer MRT since it was more viscous than drug solution, which prolonged its contact time with the nasal mucosa, thereby enhancing the rate and extent of drug absorption across the nasal mucosa. NE formulation was found to protect the drug from biological and chemical degradation as well as act as a controlled release system.
| Parameter | Oral solution | Nasal solution | Nasal nanoemulsion (QNE1) |
|---|---|---|---|
| AUC(0-∞) (µgh/g) | 0.84±0.02b | 2.44±0.50b | 7.33±2.02bb |
| Tmax (h) | 2±0.0 | 0.83±0.28 | 1.67±0.58 |
| Cmax (µg/g) | 0.17±0.02b | 0.91±0.08c | 1.412±0.38bc |
| KE (1/h) | 0.32±0.09 | 0.39±0.12 | 0.22±0.04 |
| MRT (h) | 3.63±0.35c | 2.91±0.58b | 5.09±.81b |
Note: a: Mean±SD, n=3; b: Significant difference between free drug oral vs. intranasal NE; **p<0.01 (99 % CI) and c: Significant difference between free drug intranasal vs. intranasal NE, *p<0.05 (95 % CI)
Table 5: Brain Pharmacokinetic Parameters of Quetiapine with Various Routes of Administration
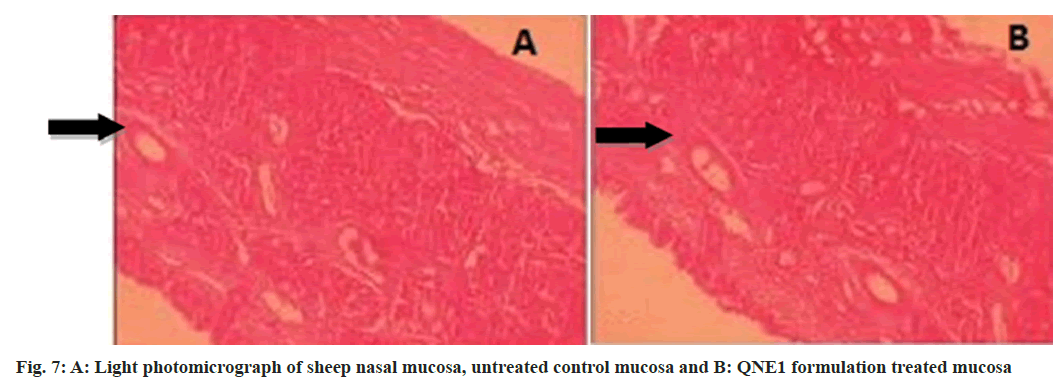
The drug enters the brain primarily through two different pathways from the nasal mucosa. Some of the drugs travel via the systemic circulation and then cross the BBB[16] to reach the brain the systemic pathway and the other is the olfactory pathway where the drug is partly carried from the nasal cavity to CSF and/or brain tissue[17,18]. Many authors have studied the olfactory pathway and have repeatedly demonstrated that intranasal administration is a viable drug delivery route into the CNS system[5,19-21]. A histopathological study was conducted by treating sheep nasal mucosa with optimized formulation then examined by light microscopy and photomicrograph shown in fig. 7. Tissue analysis revealed ciliated respiratory epithelium and normal goblet cells. No serious symptoms of damage to the integrity of the nasal mucosa like epithelial necrosis or desquamation of epithelial cells were identified when treated nasal mucosa was compared to the control[22].
Intranasal NE of quetiapine showed greatly enhanced exposure and high drug concentration in the brain. The sustained release of quetiapine from the NE can be employed to minimise administration frequency and promote patient compliance. The evidence of considerable direct transport of quetiapine into the brain may aid future research in this field as well as investigations of its therapeutic consequences.
Acknowledgements:
The authors are thankful to Orchid Pharmaceuticals Pvt. Ltd., Chennai, India, for providing us with the gift sample of quetiapine. The authors are also thankful to NGSM Institute of Pharmaceutical Sciences, Nitte (Deemed to be University), Mangalore for providing all facilities for carrying out the studies and MCOPS, Manipal for carrying out HPLC studies.
Conflict of interests:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sahni JK, Doggui S, Ali J, Baboota S, Dao L, Ramassamy C. Neurotherapeutic applications of nanoparticles in Alzheimer's disease. J Control Release 2011;152(2):208-31.
[Crossref] [Google Scholar] [PubMed]
- Spuch C, Navarro C. Liposomes for targeted delivery of active agents against neurodegenerative diseases (Alzheimer's disease and Parkinson's disease). J Drug Deliv 2011;2011:469679.
[Crossref] [Google Scholar] [PubMed]
- Mundo E, Cattaneo E, Zanoni S, Altamura AC. The use of atypical antipsychotics beyond psychoses: Efficacy of quetiapine in bipolar disorder. Neuropsychiatr Dis Treat 2006;2(2):139-48.
[Crossref] [Google Scholar] [PubMed]
- Talegaonkar S, Mishra PR. Intranasal delivery: An approach to bypass the blood brain barrier. Indian J Pharmacol 2004;36(3):140-7.
- Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm 2008;358(1-2):285-91.
[Crossref] [Google Scholar] [PubMed]
- Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm 2009;379(1):146-57.
- Li JC, Zhang WJ, Zhu JX, Zhu N, Zhang HM, Wang X, et al. Preparation and brain delivery of nasal solid lipid nanoparticles of quetiapine fumarate in situ gel in rat model of schizophrenia. Int J Clin Exp Med 2015;8(10):17590.
[Google Scholar] [PubMed]
- Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A. Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of sumatriptan. AAPS PharmSciTech 2006;7(8):49-57.
[Crossref] [Google Scholar] [PubMed]
- Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A. Intranasal mucoadhesive microemulsions of clonazepam: Preliminary studies on brain targeting. J Pharm Sci 2006;95(3):570-80.
[Crossref] [Google Scholar] [PubMed]
- Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm 2007;64(7):723-5.
[Crossref] [Google Scholar] [PubMed]
- Chaturvedi M, Kumar M, Pathak K. A review on mucoadhesive polymer used in nasal drug delivery system. J Adv Pharm Technol Res 2011;2(4):215-22.
[Crossref] [Google Scholar] [PubMed]
- Jain AK, Khar RK, Ahmed FJ, Diwan PV. Effective insulin delivery using starch nanoparticles as a potential trans-nasal mucoadhesive carrier. Eur J Pharm Biopharm 2008;69(2):426-35.
[Crossref] [Google Scholar] [PubMed]
- Delmas T, Piraux H, Couffin AC, Texier I, Vinet F, Poulin P, et al. How to prepare and stabilize very small nanoemulsions. Langmuir 2011;27(5):1683-92.
[Crossref] [Google Scholar] [PubMed]
- Seju U, Kumar A, Sawant KK. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: In vitro and in vivo studies. Acta biomaterialia. 2011;7(12):4169-76.
[Crossref] [Google Scholar] [PubMed]
- Kumar M, Misra A, Mishra AK, Mishra P, Pathak K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J Drug Target 2008;16(10):806-14.
[Crossref] [Google Scholar] [PubMed]
- Carreño F, Paese K, Silva CM, Guterres SS, Dalla CT. Pharmacokinetic investigation of quetiapine transport across blood–brain barrier mediated by lipid core nanocapsules using brain microdialysis in rats. Mol Pharm 2016;13(4):1289-97.
[Crossref] [Google Scholar] [PubMed]
- Al-Ghananeem AM, Saeed H, Florence R, Yokel RA, Malkawi AH. Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by AIDS viruses. J Drug Target 2010;18(5):381-8.
[Crossref] [Google Scholar] [PubMed]
- Khan S, Patil K, Bobade N, Yeole P, Gaikwad R. Formulation of intranasal mucoadhesive temperature-mediated in situ gel containing ropinirole and evaluation of brain targeting efficiency in rats. J Drug Target 2010;18(3):223-34.
[Crossref] [Google Scholar] [PubMed]
- Bhanushali RS, Gatne MM, Gaikwad RV, Bajaj AN, Morde MA. Nanoemulsion based intranasal delivery of antimigraine drugs for nose to brain targeting. Indian J Pharm Sci 2009;71(6):707.
- Pailla SR, Talluri S, Rangaraj N, Ramavath R, Challa VS, Doijad N, et al. Intranasal Zotepine Nanosuspension: Intended for improved brain distribution in rats. Daru 2019;27:541-56.
[Crossref] [Google Scholar] [PubMed]
- Arumugam K, Subramanian G, Mallayasamy S, Averineni R, Reddy M, Udupa N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm 2008;58(3):287-97.
[Crossref] [Google Scholar] [PubMed]
- Pardeshi CV, Rajput PV, Belgamwar VS, Tekade AR, Surana SJ. Novel surface modified solid lipid nanoparticles as intranasal carriers for ropinirole hydrochloride: Application of factorial design approach. Drug Deliv 2013;20(1):47-56.
[Crossref] [Google Scholar] [PubMed]