- *Corresponding Author:
- Veerabhuvaneshwari Veerichetty
Department of Biotechnology, Kumaraguru College of Technology, Coimbatore, Tamil Nadu 641049, India
E-mail: veerabhuvaneshwari.v.bt@kct.ac.in
| Date of Received | 30 June 2023 |
| Date of Revision | 27 February 2024 |
| Date of Acceptance | 03 June 2024 |
| Indian J Pharm Sci 2024;86(3):1032-1041 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Fucoidans are sulphated fucose rich heteropolysaccharides found in marine source seaweed. Fucoidan has gained interest due to its diverse biological activities. Dual inhibition of cyclooxygenase and lipoxygenase prevents shunting of the arachidonic acid metabolism sparing gastric toxicity observed with selective cyclooxygenase-2 inhibitors. The present study investigated dual inhibition potential of fucoidan extracted from Sargassum wightii on cyclooxygenase, lipoxygenase and its anti-inflammatory effect on carrageenan induced paw oedema. Fucoidan and diclofenac demonstrated dual inhibition towards cyclooxygenase and lipoxygenase with half-maximal inhibitory concentration of 44.03±3.74 μg/ml, 34.92±5.14 μg/ml (cyclooxygenase) and 28.26±2.06 μg/ml and 26.54±1.37 μg/ml (lipoxygenase) in lipopolysaccharide stimulated RAW 264.7 macrophage cells respectively. Fucoidan is showing concentration dependant reduction in nitric oxide level of lipopolysaccharide stimulated RAW 264.7 cells with a half-maximal inhibitory concentration of 17.88±2.43 μg/ml and 17.99±3.15 μg/ml for diclofenac. Fucoidan showed suppression of lipopolysaccharide stimulated gene expression of cyclooxygenase-2 and 5-lipoxygenase in RAW 264.7 macrophage cells. Fucoidan showed 99.62±1.69 % cell viability in RAW 264.7 macrophage cells. The acute oral toxicity study of fucoidan showed no abnormal clinical signs. The fucoidan attenuated carrageenan induced increase in paw volume in Wistar rats at the tested doses of 100 mg/kg and 200 mg/kg at 60, 120 and 240 min (p<0.05). Results suggest that the anti-inflammatory activities of fucoidan involve attenuation of eicosanoid production.
Keywords
Cyclooxygenase, lipoxygenase, nitric oxide, paw oedema, RAW 264.7 macrophages, fucoidan, Sargassum wightii
Fucoidan is a fucose rich sulphated polysaccharide present in cell wall fibrillar tissue of brown seaweed. Sulphated polysaccharides are anionic polymers that are present widely in macroalgal community. Utilization of Sargassum wightii (S. wightii) is expected to be a sustainable and easily available source conducive for extensive extraction of fucoidan. Polysaccharide based products from sea weeds were assessed from sustainability and commercial exploitation point of views by Chudasama et al.[1]. Studies suggest that it is multifunctional with antitumor, antidiabetic, cardioprotective, antiviral, neuroprotective and immune-modulating effects[2]. Fucoidan draws great attention due to its bioactivity, biodegradability, hydrophilicity, non-toxic and biocompatible properties. Fucoidan contains a fucose with α-(1-2) or α-(1-3)-linkages. It also has various amounts of other monosaccharides such as galactose, mannose, xylose and uronic acid[3]. Sulfation is the key thing in fucoidan bioactivity[4]. Chronic inflammation is caused by the release of hormone-like substances called Prostaglandins (PGs) and Leukotrienes (LTs). Cyclooxygenase (COX) and Lipoxygenase (LOX) are primary enzymes of arachidonic acid metabolism producing eicosanoids, PGs and LTs respectively[5]. Macrophages play an important role in inflammation. Activation of macrophages by Lipopolysaccharide (LPS) results in induction arachidonic acid metabolism and production of inflammatory mediators like Nitric Oxide (NO), COX-2 and 5-LOX. The present study investigates the anti-inflammatory potential of fucoidan isolated from S. wightii in vitro in the cell lysate of LPS stimulated RAW macrophage cells by measuring total COX, LOX and NO. LPS component of gram-negative bacteria is recognised by Toll like receptor 4 which cleaves membrane bound arachidonic acid by action of cytoplasmic phospholipase 2. Arachidonic acid gets oxygenated via COX and LOX to PGs and LTs respectively. LPS co-induces expression of inducible Nitric Oxide Synthase (iNOS), COX- 2 and 5-LOX in macrophages and fibroblasts accounting for the release of large quantities of NO and eicosanoids at the site of inflammation. NO is considered as a pro-inflammatory mediator that induces inflammation due to over production. The study of carrageenan induced paw oedema is an index of acute inflammation, hence a measure of anti-arthritic activity. It is biphasic with release of histamine and serotonin within 2.5 h in the first phase and release of PGs accompanied with neutrophil infiltration happens after 2-3 h in the second phase. Dual inhibition of COX and LOX prevents oxidative metabolism of arachidonic acid to eicosanoids thereby a potential target for screening anti-inflammatory activity. Dual COX and LOX inhibitors possess the advantage of preventing arachidonic acid shunt and increased leukotriene production observed with selective COX-2 inhibitors[6]. Attenuation of NO production in RAW macrophages and reduction of oedema by fucoidan substantiates it as an anti-inflammatory activity.
Materials and Methods
Cell culture:
RAW 264.7 cell line was purchased from National Centre For Cell Science (NCCS), Pune and was maintained in Dulbecco’s modified eagles' media (Himedia, India) supplemented with 10 % foetal bovine serum (Hi media, India) and grown to confluence at 37° at 5 % CO2 in a CO2 incubator.
Experimental animals:
Female Wistar rats were procured from animal house, JSS College of Pharmacy, Ooty animal ethics committee no JSSCP/OT/IAEC/15/2020- 21 for acute oral toxicity study and carrageenan induced paw oedema model. The animals were housed under the following laboratory conditions, environment temperature of 23.4°-28.8°, relative humidity of 54 %-78 %, with a 12 h light and 12 h dark cycle. The animals were housed individually in standard polypropylene cages with paddy husk bedding and given pelleted food and drinking water in bottle.
Seaweed collection and identification:
Seaweeds were collected from Mandapam coast, Gulf of Mannar, Tamil Nadu, India (latitude: 9°16.811' N, longitude: 79°10.503', Speed Over Ground (SOG): 0.45 knots and Course Over Ground (COG): 6°) and dried before processing for extraction. The collected seaweed (fig. 1) was identified as S. wightii Greville of Sargassaceae family by Botanical Survey of India, Coimbatore. Fresh algae were washed and air dried in the shade at room temperature. Dried samples were pulverized into powder and stored at room temperature.
Extraction of fucoidan from S. wightii:
10 g of powdered seaweed was dissolved in 500 ml of distilled water and boiled at 85° for around 2 h and filtered. The pH of the filtrate was adjusted to 7 to increase the yield of extraction. 4 % (v/v) Trichloroacetic Acid (TCA) was added to the solution and incubated overnight at 4° and centrifuged. 1 % calcium chloride (CaCl2) was added to the supernatant and incubated overnight at 4°. Thrice the volume of absolute ethanol was added to the supernatant and incubated overnight at 4°. The precipitated polysaccharides fraction containing fucoidan was collected through centrifugation. The pellet formed was completely dried. The obtained pellet was dissolved in sterile molecular biology grade water and spray dried. Fucoidan was extracted from S. wightii collected from Mandapam coast using Hot Water Extraction (HWE), method as referred by in literatures [7,8].
Chemical elemental Carbon, Hydrogen, Sulfur and Oxygen (CHSO) analysis:
The presence of elements such as carbon, hydrogen and nitrogen content were measured by CHSO analysis (Laboratory Equipment Corporation (LECO) TruSpec Micro analyser Carbon, Hydrogen, Nitrogen, Sulfur/Oxygen (CHNS/O) analyzer. In this analysis, sulfamethazine, cystine and oxalic acid dihydrate were considered as reference.
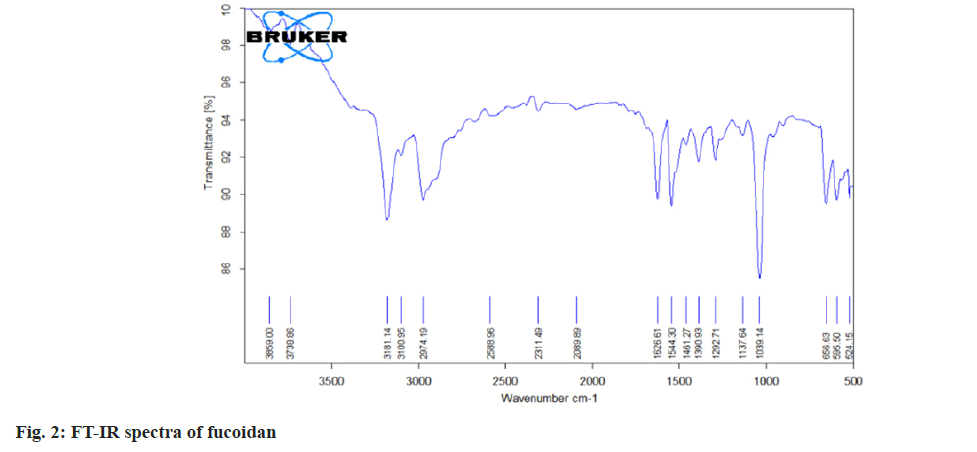
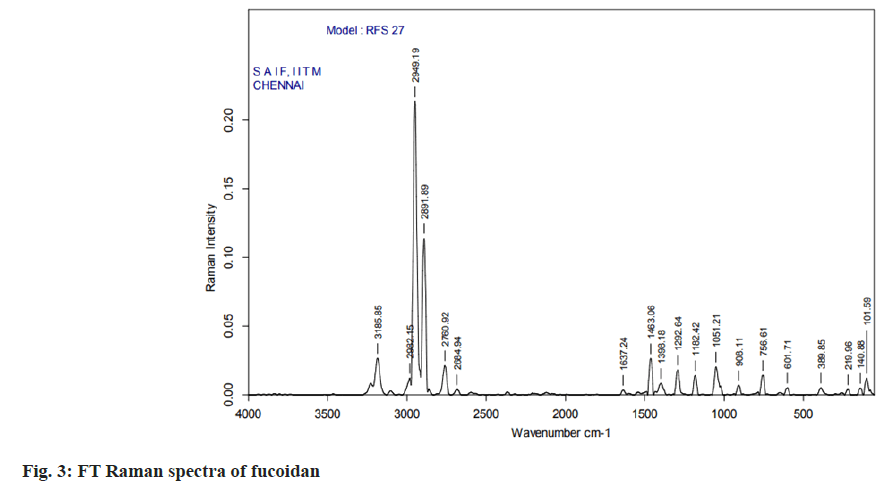
Fourier Transform (FT)-Infrared (IR) and FTRaman spectral characterization of fucoidan:
The nano spray dried fucoidan extract was analyzed using FT-IR spectroscopy and FT Raman spectroscopy. The resulting spectra directly corresponds to the functional groups present in the structures of fucoidan. The structural characterization of fucoidan was determined by FT-IR and FT-Raman. The FT-IR and FT-Raman spectrum was measured using a Bruker model spectrophotometer.
in vitro toxicity assessment in RAW 264.7 macrophage:
3 - ( 4 , 5 - D i m e t h y l t h i a z o l - 2 - y l ) - 2 , 5 - diphenyltetrazolium bromide (MTT) assay and microscopic observation was performed to evaluate the cytotoxic effects of fucoidan on RAW 264.7 macrophage. Initially, 1×104 RAW 264.7 macrophage was seeded in each well and incubated in a CO2 incubator overnight for attachment. Thereafter, cells were exposed to various concentrations of the fucoidan for 24 h. Then, 10 μl of MTT reagent (methyltetrazolium, 1 %) was added in each well and kept in a CO2 incubator for 4 h until purple formazan crystals developed. Followed by the addition of 100 μl of MTT reagent, solubilization buffer, 10 % Sodium Dodecyl Sulfate (SDS) with 0.01 Ammonium Chloride (NHCl) and Dimethyl Sulfoxide (DMSO) the plate was incubated in a CO2 incubator for 12 h after which absorbance was measured at 590 nm[9]. Cells were observed under 10X magnification in inverted phase contrast tissue culture microscope (Labomed TCM-400).
in vivo acute oral toxicity assessment of fucoidan:
The fucoidan was administered once orally as gavage to the fasted Wistar rats at the dose volume of 10 ml/kg body weight, to deliver the dose of 2000 mg/kg body weight. The treated rats were observed five times at 30 min and four times at hourly basis for 14 d. The clinical signs were recorded every day. On 15th d, the rats were euthanized by using diethyl ether anaesthesia and necropsied. The gross necropsy findings were recorded. The body weight of rats was recorded on 1st d, 8th d and 15th d of test. Toxicity assessment was executed as per Organization for Economic Cooperation and Development (OECD) guideline 423, December 2001 [10].
Effect of fucoidan on COX inhibition in RAW 264.7 cells:
LPS stimulated RAW cells were exposed with different concentration of sample and diclofenac sodium standard in varying concentration and incubated for 24 h. After incubation the antiinflammatory assays were performed using the cell lysate. The COX activity was assayed by the method by Walker et al. with slight modifications[11]. The cell lysate in tris-Hydrogen Chloride (HCl) buffer (pH 8) was incubated with glutathione 5 mm/l and haemoglobin 20 μg/l for 1 min at 25°. The reaction was initiated by the addition of arachidonic acid 200 mm/l and terminated after 20 min of incubation at 37°, by the addition of 10 % TCA in 1 N hydrochloric acid. After the centrifugal separation and the addition of 1 % thiobarbiturate, COX activity was determined by reading absorbance at 632 nm. Percentage inhibition of the COX enzyme and half-maximal Inhibitory Concentration (IC50) value (μg/ml) was calculated from the non-linear regression curve using Prism software.
Effect of fucoidan on LOX inhibition in RAW 264.7 cells:
RAW 264.7 cells were grown to 60 % confluence followed by activation with 1 μl LPS (1 μg/ml). LPS stimulated RAW cells were exposed with different concentration of fucoidan sample and diclofenac sodium. LOX activity was determined as per Axelrod et al.[12]. Briefly, the reaction mixture (2 ml final volume) contained tris-HCl buffer (pH 7.4), 50 μl of cell lysate, and 200 μl of sodium linoleate (10 mg/ml). The LOX activity was monitored as difference in absorbance at 234 nm, which reflects the formation of 5-hydroxyeicosatetraenoic acid from linoleate. Percentage inhibition of the LOX enzyme and IC50 value (μg/ml) was calculated from the nonlinear regression curve using Prism software.
Effect of fucoidan on NO production in RAW 264.7 cells:
NO concentration in LPS induced RAW cell culture medium supernatant after treatment with fucoidan and diclofenac was estimated by using Griess reagent following Halonen et al.[13] method. Griess method is an indirect measurement of NO production that involves spectrophotometric determination of nitrite levels. The Griess reagent was prepared by adding 1:1 proportion of 1 % sulphanilic acid in 5 % phosphoric acid and 0.1 % N-(1-naphthyl) ethylenediamine in distilled water. 100 μl of appropriately diluted sample was mixed with 50 μl of Griess reagent and diluted with 1.3 ml of distilled water. The tubes were incubated at room temperature for 30 min and the absorbance was measured at 548 nm in spectrophotometer. The molar concentration of nitrite in the samples was determined from a standard curve generated using known concentrations of sodium nitrite. IC50 value (μg/ml) for inhibition of NO production was calculated from the non-linear regression curve using Prism software.
Gene expression analysis of COX and LOX:
Total Ribonucleic Acid (RNA) was extracted using Trizol from LPS induced RAW macrophage cells treated with fucoidan[14]. The total amount of RNA was measured using nanodrop. 1 μg of the total RNA was reverse transcribed using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT) (Invitrogen Life Technologies). Polymerase Chain Reaction (PCR) was performed in a final volume of 20 μl containing 2 μl of first strand cDNA, 0.25 μl of Taq polymerase 3 U/μl and 10 pmol of primer. PCR cycler was programmed for 35 cycles as follows; 94° for 30 s, followed by annealing at 55°-62° (primer specific, Table 1) for 45 s, and then by extension at 72° for 45 s. Primers were procured from Eurofins. After 35 cycles, the profile was linked on hold at 4° [15,16].
| Oligo name | Sequence |
|---|---|
| GAPDH forward | 5'-cctttcaaggtggggaggga-3' |
| GAPDH reverse | 5'-cgccagaccctgcacttttta-3' |
| COX-2 forward | 5'-gaagtctttggtctggtgcctg-3 |
| COX-2 reverse | 5'-gtctgctggtttggaatagttgc-3' |
| LOX forward | 5'-gcttcgccagtaagatccag-3' |
| LOX reverse | 5'-ttgcgcattttctgtttcag-3' |
Table 1: Primers Sequences for Gene Expression Analysis of Cox and Lox
Assessment acute anti-inflammatory effect in paw oedema model:
Overnight fasted 24 female Wistar rats were divided into 4 groups of 6 each. Group 1 animals received vehicle as control (Carboxymethyl Cellulose (CMC) (0.5 % w/v) 10 ml/kg, post operation (p.o.). Group 2 and 3 received fucoidan at a dose of 100 and 200 mg/kg, p.o respectively. Group 4 received standard diclofenac, 10 mg/kg, p.o. All the groups received the assigned treatments 1 h before sub-plantar administration of aqueous gel of carrageenan (0.1 ml of 0.1 % w/v in saline) to right hind foot produce oedema. The effect of fucoidan on acute phase of inflammation was accessed using plethysmometer. The paw volume was measured at 0, 30, 60, 120, 180 and 240 min. The percentage inhibition at each time interval was calculated[17,18].
Statistical analysis:
In vitro data represented as mean±Standard Error of the Mean (SEM) and IC50 calculated and analysed using Prism software. In vivo data represented as mean±Standard Deviation (SD) and analysed by one-way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison tests using Prism software (version 5). p values≤0.05 were considered significant.
Results and Discussion
The carbon, hydrogen, sulphur and oxygen content in fucoidan S. wightii fucoidan extract were determined by CHNS/O elemental analyser presented in Table 2 and sulphur content varies between 0.04 %-0.2 %[19]. Fucoidan was studied using FT-IR spectroscopy with wavenumber in the range of 4000-500 cm-1. The functional groups present in fucoidan were analysed from literature[20-22] and with obtained spectra. The FTIR Spectra and FT Raman spectra of fucoidan are shown in fig. 2 and fig. 3. Peak at 2974 cm-1 was attributed to C-O and C-C stretching vibrations of pyranose ring and peak at 1626.61 cm-1 the peak was related with the carboxylic vibrations of elongation of the carboxylate anion (COO-) of pyranose rings of polysaccharides. The band at 1039 cm-1 was related to the asymmetric vibration of sulphate ester group (S-O) and overtones near 2000 cm-1 denoted weak sulphur oxygen stretching vibrations[23].
| Chemical elemental analysis | |||||
|---|---|---|---|---|---|
| Standard (fucoidan) | S. code (fucoidan) | % C | % H | % S | % O |
| Sulfamethazine | 1.9220 mg | 4.9575 | 2.6963 | 0.20283 | |
| 1.9300 mg | 5.0304 | 2.1857 | 0.04543 | ||
| Cystine | 2.5030 mg | 4.9206 | 2.7563 | 0.10889 | |
| 2.5520 mg | 5.1763 | 2.5046 | 0.07785 | ||
| Oxalic acid dihydrate | 1.9770 mg | 13.462 | |||
| 2.0250 mg | 12.08 | ||||
Table 2: Chemical Elemental Analysis of Fucoidan
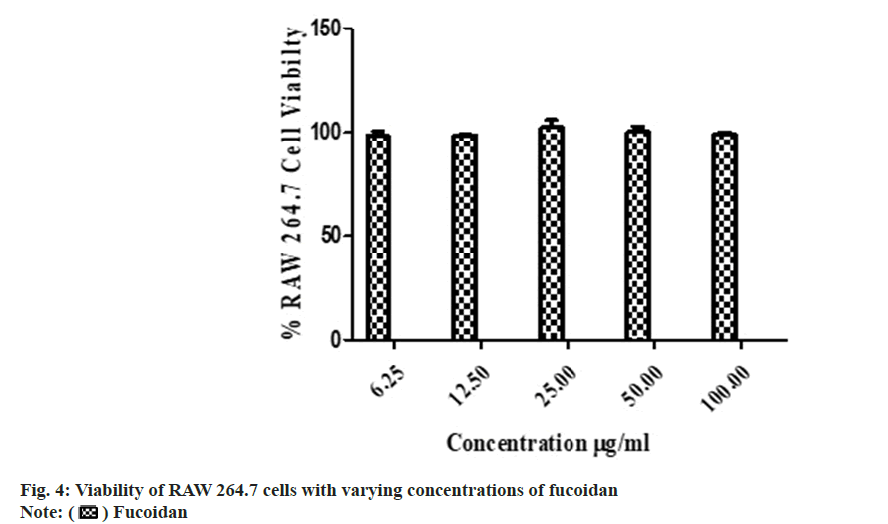
The isolated fucoidan showed no in vitro cytotoxicity with 99.62±1.69 % in MTT cell viability assay (fig. 4). Fucoidan from Turbinaria decurrens showed cell viability up to 400 μg/ ml which suggest minimum toxicity in IC-21 macrophages cells by Manikandan et al.[24]. The acute oral toxicity study of the fucoidan as per Organisation for Economic Co-operation and Development (OECD) guideline No. 423 showed no abnormal clinical signs and was classified into Globally Harmonized System (GHS) category-5 (LD 50>2000 mg/kg, body weight) in Wistar rats. Body weight changes, pre-terminal deaths and gross necropsy findings are presented in Table 3.
| Dose (mg/kg b.wt) | Rat no. | Gender | Body weight (g) | No. of dead/no. of tested | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | 8th d | Weight change (day 8-initial) | 15th d | Weight change (day 15-initial) | ||||
| 2000 | 1 | Female | 141 | 150 | 9 | 162 | 21 | 0/6 |
| 2 | Female | 143 | 150 | 7 | 163 | 20 | ||
| 3 | Female | 159 | 166 | 7 | 172 | 13 | ||
| 4 | Female | 147 | 155 | 8 | 160 | 13 | ||
| 5 | Female | 143 | 156 | 13 | 161 | 18 | ||
| 6 | Female | 142 | 152 | 10 | 161 | 19 | ||
Table 3: Acute Oral Toxicity Study of Fucoidan in Wistar Rats
Diclofenac standard and fucoidan exhibited concentration dependent COX inhibitory activity with an IC50 of 34.92±5.14 μg/ml and 44.03±3.75 μg/ml respectively. Comparative COX percentage inhibition and dose response by fucoidan and diclofenac is shown in fig. 5. Standard nonsteroidal anti-inflammatory compound, diclofenac has showed COX inhibition with an IC50 of 1.155 ug/ml-0.236 ug/ml of COX-1 and COX-2 enzymebased assay[25]. Fucoidan from Fucus vesiculosus possess anti-inflammatory activity and is observed to inhibit the COX-2 enzyme with a greater selectivity over COX-1[26].
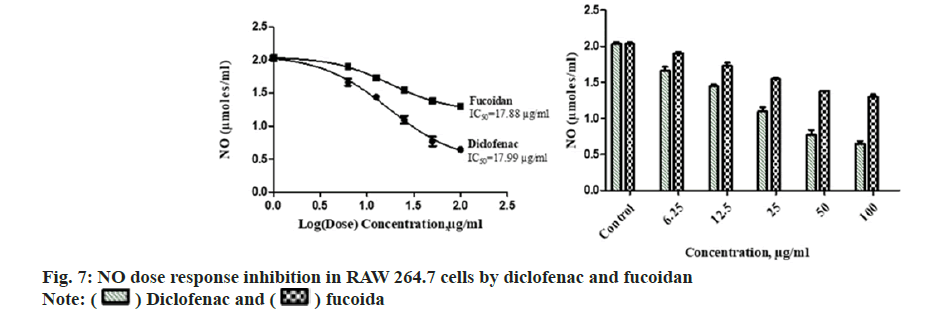
Diclofenac standard and fucoidan exhibited concentration dependent LOX inhibitory activity with an IC50 of 26.54±1.37 μg/ml and 28.26±2.06 μg/ml respectively. Comparative LOX percentage inhibition and dose response by fucoidan and diclofenac is shown in fig. 6. New benzothiophene derivatives shows dual COX and LOX inhibition and LOX inhibitors finds potential use in inflammatory conditions like asthma and arthritis[27]. Dose dependent reduction in NO was observed in RAW macrophage cells with the administration of different concentrations of the extract with an IC50 of 17.88±2.43 μg/ml and 17.99±3.15 μg/ml for standard diclofenac. Fig. 7 illustrates the comparative reduction of NO production, measured in μmol, by fucoidan and diclofenac. Fucoidan isolated from invasive brown Sargassum horneri was also shown to suppress NO production in LPS-activated RAW 264.7 macrophages inflammation via blocking Nuclear Factor kappa B (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) pathways[27,28].
Fucoidan extract from S. wightii treated LPS induced RAW macrophage cells is showed downregulation of COX and LOX gene expression related to inflammation. Fucoidan down regulated LPS-induced COX and LOX gene expression in RAW 264.7 cells and cDNA loaded in agarose gel electrophoresis and relative gene expression of COX and LOX measured using ΔΔCt method is shown in fig. 8. Fucoidan and shikonin derivatives showed downregulation of NF-κB MAPK pathway in inflammation through inhibition of NO in LPS induced RAW macrophages[24,29].
The fucoidan showed a dose dependent antiinflammatory activity at the tested doses of 100 and 200 mg/kg against carrageenan induced paw oedema at 60, 120 and 240 min (p≤0.05) comparable to standard diclofenac (10 mg/kg). The decrease in paw volume after treatment is summarized with representative images of paw edema of treatment group in Table 4 and fig. 9. Manikandan et al.[24] reported anti-inflammatory activity of Turbinaria decurrens derived fucoidan on formalin induced paw oedema in mice. Dong- Seon Kim reported jakyak-gamcho-tang’s antiinflammatory activity in Monosodium Urate (MSU)-induced mice gouty arthritis inflammation model with paw swelling volume as an index of oedema[29]. The dried fucoidan extract mixed with optimized excipients were formulated as oral tablets which followed first order in vitro drug release kinetics[30]. Topical application of fucoidan exerts an anti-inflammatory effect on the skin with well observed pharmacokinetic profile proposed as effective method for the treatment of atopic dermatitis, dermal burns, oral herpes and as an anticoagulant[31]. A comprehensive review highlighting the attractive biopharmaceutical properties of fucoidan with potential as oral, nasal, topical, injectable formulations. Immunomodulatory and anti-inflammatory activity of fucoidan is well studied by acting at different stages of inflammation like blocking lymphocyte invasion, inhibition of inflammatory cascade enzymes and induction of apoptosis[32].
| Treatment groups | Body weight | Paw volume (ml) | Percentage decrease in paw volume | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 120 min | 240 min | 30 min | 60 min | 120 min | 240 min | ||
| Control (0.5 % CMC 10 ml/kg, p.o) | 248±19.6 | 1.03±0.17 | 1.57±0.12 | 1.9±0.17 | 2.41±0.14 | 2.15±0.15 | 38.3±10.81 | 66.95±15.28 | 111.4±12.49 | 88.88±13.15 |
| Fucoidan (100 mg/kg, p.o) | 246.4±13.4 | 1.07±0.1 | 1.51±0.12 | 1.87±0.05 | 2.16±0.12* | 1.80±0.11* | 33.18±10.7 | 64.76±4.62 | 90.05±10.56* | 58.33±10.49* |
| Fucoidan (200 mg/kg, p.o) | 243.1±17.6 | 1.05±0.1 | 1.55±0.1 | 1.81±0.1 | 2.22±0.22 | 1.74±0.07* | 36.4±8.8 | 58.91±9.03 | 95.17±19.83 | 53.36±6.5* |
| Diclofenac (10 mg/ kg, p.o) | 248.7±18.4 | 0.98±0.14 | 1.48±0.1 | 1.7±0.14* | 2.00±0.08* | 1.60±0.09* | 30.55±9.24 | 49.46±12.76* | 76.16±7.87* | 40.78±8.4* |
Table 4: Measurement of Paw Volume Using Plethysmometer at the End of Experimental Period
In conclusion, fucoidan extracted from S. wightii demonstrated dual COX and LOX inhibition in vitro in LPS induced RAW macrophage cells. NO production was attenuated by fucoidan. Fucoidan showed a significant dose dependent anti-inflammatory activity at tested doses and time intervals against carrageenan induced paw oedema in Wistar rats. Fucoidan showed down regulation of COX-2 and 5-LOX gene expression in LPS induced RAW 264.7 cells. The fucoidan obtained showed no cytotoxicity in RAW 264.7 cells and was classified as GHS category-5 in acute oral toxicity in Wistar rats (OECD 423). The FT-IR and FT Raman spectra obtained show the characteristic peaks of fucoidan in addition to peaks signifying the presence of other monosaccharides. This study suggests fucoidan can be used as a potent anti-inflammatory agent for acute inflammation sparing gastric mucosal toxicity and preventing arachidonic acid metabolism shunt.
Conflict of interests:
The authors declare that they have no competing interest.
References
- Chudasama NA, Sequeira RA, Moradiya K, Prasad K. Seaweed polysaccharide based products and materials: An assessment on their production from a sustainability point of view. Molecules 2021;26(9):2608.
[Crossref] [Google Scholar] [PubMed]
- Ramos-de-la-Peña AM, Contreras-Esquivel JC, Aguilar O, González-Valdez J. Structural and bioactive roles of fucoidan in nanogel delivery systems. A review. Carbohydr Polym Technol Appl 2022;4:100235.
- Li B, Lu F, Wei X, Zhao R. Fucoidan: Structure and bioactivity. Molecules 2008;13(8):1671-95.
[Google Scholar] [PubMed]
- Jayawardena TU, Nagahawatta DP, Fernando IP, Kim YT, Kim JS, Kim WS, et al. A review on fucoidan structure, extraction techniques, and its role as an immunomodulatory agent. Mar Drugs 2022;20(12):755.
[Crossref] [Google Scholar] [PubMed]
- Boctor AM, Eickholt M, Pugsley TA. Meclofenamate sodium is an inhibitor of both the 5-lipoxygenase and cyclooxygenase pathways of the arachidonic acid cascade in vitro. Prostaglandins Leukot Med 1986;23(2-3):229-38.
[Crossref] [Google Scholar] [PubMed]
- Manju SL, Ethiraj KR, Elias G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur J Pharm Sci 2018;121:356-81.
[Crossref] [Google Scholar] [PubMed]
- Lu J, Shi KK, Chen S, Wang J, Hassouna A, White LN, et al. Fucoidan extracted from the New Zealand Undaria pinnatifida-physicochemical comparison against five other fucoidans: Unique low molecular weight fraction bioactivity in breast cancer cell lines. Mar Drugs 2018;16(12):461.
[Crossref] [Google Scholar] [PubMed]
- Hanjabam MD, Kumar A, Tejpal CS, Krishnamoorthy E, Kishore P, Kumar KA. Isolation of crude fucoidan from Sargassum wightii using conventional and ultra-sonication extraction methods. Bioact Carbohydr Diet Fibre 2019;20:100200.
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986;89(2):271-7.
[Crossref] [Google Scholar] [PubMed]
- Oecd O. Guidance document on acute oral toxicity testing. Environmental health and safety monograph series on testing and assessment. 2000.
- Walker MC, Gierse JK. in vitro assays for cyclooxygenase activity and inhibitor characterization. Methods Mol Biol 2010:131-44.
[Crossref] [Google Scholar] [PubMed]
- Axelrod B, Cheesebrough TM, Laakso S. Lipoxygenase from soyabean. Meth Enzym 1981;71:441-5.
- Halonen SK, Chiu FC, Weiss LM. Effect of cytokines on growth of Toxoplasma gondii in murine astrocytes. Infect Immun 1998;66(10):4989-93.
[Crossref] [Google Scholar] [PubMed]
- Chomczynski P, Mackey K. Short technical reports. modification of the TRI reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques 1995;19(6):942-5.
[Google Scholar] [PubMed]
- Park EJ, Pezzuto JM, Jang KH, Nam SJ, Bucarey SA, Fenical W. Suppression of nitric oxide synthase by thienodolin in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. Nat Prod Commun 2012;7(6):789-94.
[Crossref] [Google Scholar] [PubMed]
- Cho W, Nam JW, Kang HJ, Windono T, Seo EK, Lee KT. Zedoarondiol isolated from the rhizoma of Curcuma heyneana is involved in the inhibition of iNOS, COX-2 and pro-inflammatory cytokines via the downregulation of NF-κB pathway in LPS-stimulated murine macrophages. Int Immunopharmacol 2009;9(9):1049-57.
[Crossref] [Google Scholar] [PubMed]
- Phyllis E, Whiteley SA. Models of inflammation: Carrageenan-induced paw edema in the rat. Curr Protoc Pharmacol 2001;5(5).
- Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol 2003;225:115-21.
[Crossref] [Google Scholar] [PubMed]
- Zayed A, El-Aasr M, Ibrahim AR, Ulber R. Fucoidan characterization: Determination of purity and physicochemical and chemical properties. Mar drugs 2020;18(11):571.
[Crossref] [Google Scholar] [PubMed]
- Senthil SL, Kumar TV, Geetharamani D, Suja G, Yesudas R, Chacko A. Fucoidan–an α-amylase inhibitor from Sargassum wightii with relevance to NIDDM. Int J Biol Macromol 2015;81:644-7.
[Crossref] [Google Scholar] [PubMed]
- Maneesh A, Chakraborty K. Pharmacological potential of sulfated polygalactopyranosyl-fucopyranan from the brown seaweed Sargassum wightii. J Appl Phycol 2018;30:1971-88.
- Ye J, Chen D, Ye Z, Huang Y, Zhang N, Lui EM, et al. Fucoidan isolated from Saccharina japonica inhibits LPS-induced inflammation in macrophages via blocking NF-κB, MAPK and JAK-STAT pathways. Mar Drugs 2020;18(6):328.
[Crossref] [Google Scholar] [PubMed]
- Vaamonde-García C, Florez-Fernandez N, Torres MD, Lamas-Vazquez MJ, Blanco FJ, Domínguez H, et al. Study of fucoidans as natural biomolecules for therapeutical applications in osteoarthritis. Carbohydr Polym 2021;258:117692.
[Crossref] [Google Scholar] [PubMed]
- Manikandan R, Parimalanandhini D, Mahalakshmi K, Beulaja M, Arumugam M, Janarthanan S, et al. Studies on isolation, characterization of fucoidan from brown algae Turbinaria decurrens and evaluation of it's in vivo and in vitro anti-inflammatory activities. Int J Biol Macromol 2020;160:1263-76.
[Crossref] [Google Scholar] [PubMed]
- El-Miligy MM, Hazzaa AA, El-Messmary H, Nassra RA, El-Hawash SA. New benzothiophene derivatives as dual COX-1/2 and 5-LOX inhibitors: Synthesis, biological evaluation and docking study. Future Med Chem 2017;9(5):443-68.
[Crossref] [Google Scholar] [PubMed]
- Pozharitskaya ON, Obluchinskaya ED, Shikov AN. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents sea. Mar Drugs 2020;18(5):275.
[Crossref] [Google Scholar] [PubMed]
- Sanjeewa KA, Jayawardena TU, Kim SY, Kim HS, Ahn G, Kim J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res 2019;41:101561.
- Cheng YW, Chang CY, Lin KL, Hu CM, Lin CH, Kang JJ. Shikonin derivatives inhibited LPS-induced NOS in RAW 264.7 cells via downregulation of MAPK/NF-κB signaling. J Ethnopharmacol 2008;120(2):264-71.
[Crossref] [Google Scholar] [PubMed]
- Lee YM, Kim DS. The extraction solvent influences the anti-inflammatory effects of Jakyakgamcho-Tang in lipopolysaccharide-stimulated macrophages and mice with gouty arthritis. Int J Mol Sci 2020;21(24):9748.
[Crossref] [Google Scholar] [PubMed]
- Obluchinskaya ED, Pozharitskaya ON, Flisyuk EV, Shikov AN. Optimization of the composition and production technology of fucoidan tablets and their biopharmaceutical evaluation. Pharm Chem J 2020;54:509-13.
- Pozharitskaya ON, Shikov AN, Obluchinskaya ED, Vuorela H. The pharmacokinetics of fucoidan after topical application to rats. Mar Drugs 2019;17(12):687.
[Crossref] [Google Scholar] [PubMed]
- Apostolova E, Lukova P, Baldzhieva A, Katsarov P, Nikolova M, Iliev I, et al. Immunomodulatory and anti-inflammatory effects of fucoidan: A review. Polymers 2020;12(10):2338.
[Crossref] [Google Scholar] [PubMed]

 ) Fucoidan
) Fucoidan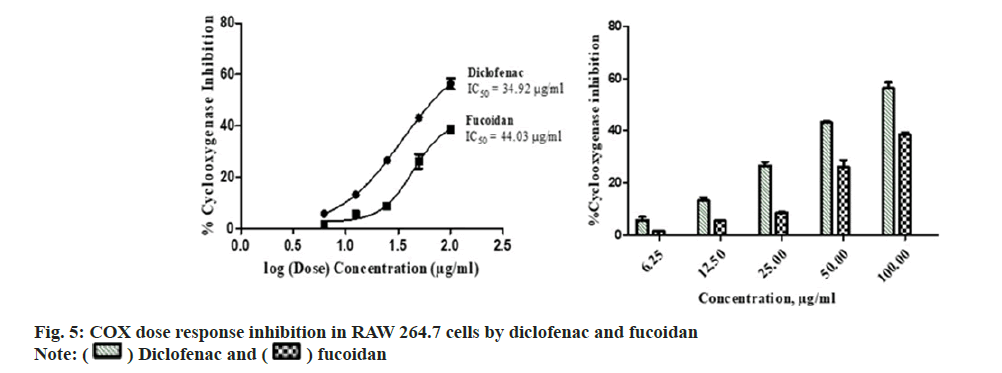
 ) Diclofenac and (
) Diclofenac and ( ) fucoidan
) fucoidan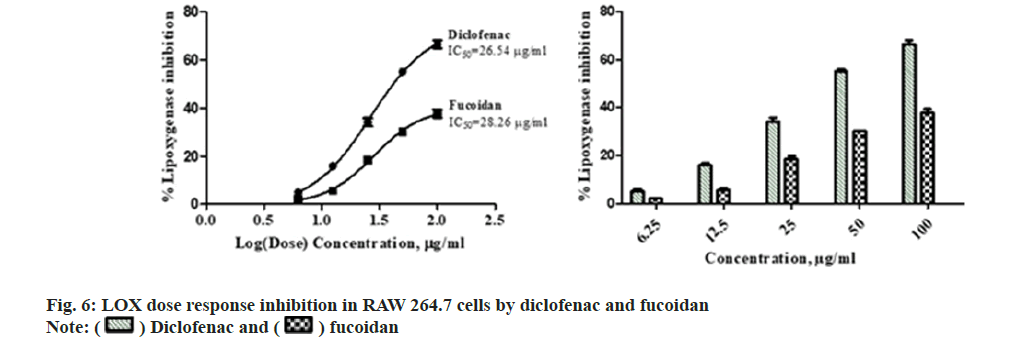
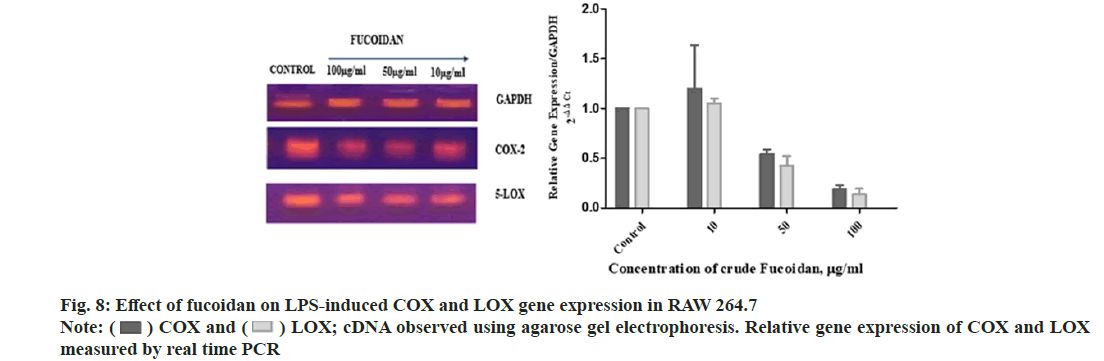
 ) COX and (
) COX and ( ) LOX; cDNA observed using agarose gel electrophoresis. Relative gene expression of COX and LOX
measured by real time PCR
) LOX; cDNA observed using agarose gel electrophoresis. Relative gene expression of COX and LOX
measured by real time PCR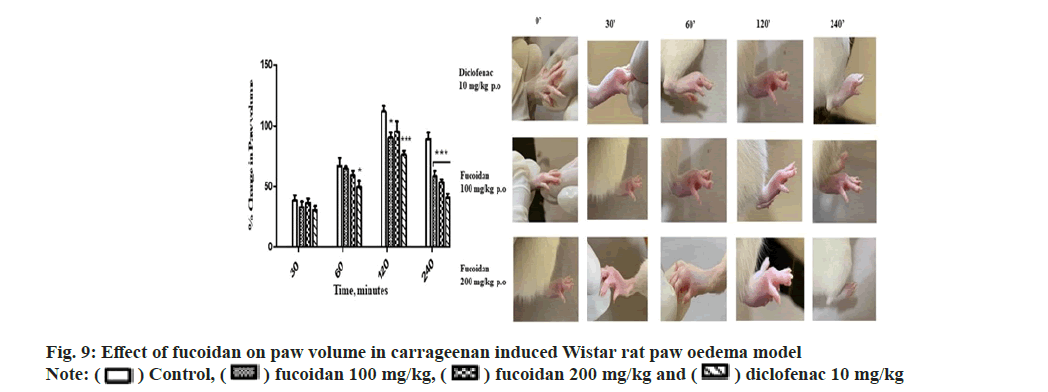
 ) Control, (
) Control, ( ) fucoidan 100 mg/kg, (
) fucoidan 100 mg/kg, ( ) diclofenac 10 mg/kg
) diclofenac 10 mg/kg



