- *Corresponding Author:
- Rajeswari Hari
Department of Biotechnology, Dr. M. G. R. Educational and Research Institute, Maduravoyal, Chennai, Tamil Nadu 600077, India
E-mail: rajihar@gmail.com
| Date of Received | 19 August 2021 |
| Date of Revision | 25 November 2022 |
| Date of Acceptance | 06 February 2023 |
| Indian J Pharm Sci 2023;85(1):107-118 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study aimed to investigate the comparative in vitro and in silico antioxidant and anti-proliferative activity of ethanolic root extract of Aconitum heterophyllum and Aconitum heterophyllum roots loaded phytoniosomes. Initially the ethanolic root extract of Aconitum heterophyllum and Aconitum heterophyllum roots loaded phytoniosomes were prepared and their antioxidant potential were studied in terms of 2,2-diphenyl-1-picrylhydrazyl, super oxide, hydroxyl radical scavenging by employing in vitro method. Assessment of anti-proliferative activity of MCF-7 human breast cancer cell lines was performed using 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide assay and trypan blue exclusion assay. In silico docking was performed using Auto Dock Schrodinger Glide to identify the suitable antagonistic ligand which can inhibit the proteins breast cancer gene 1, breast cancer gene 2 and human epidermal growth factor receptor 2 to exert the cytotoxic effect. In the present study a concentration dependent cytotoxic activity was observed for both the extract. However, the enhanced cytotoxic activity observed for Aconitum heterophyllum roots loaded phytoniosomes extract may be due to the increased bioavailability of phytochemicals in the form of phytoniosomes. IC50 values were for the Aconitum heterophyllum roots loaded phytoniosomes calculated and it was found to be 43.81 μg/ml, 50.46 μg/ml respectively for 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide and trypan blue assay. Among the 21 phyto components identified in the gas chromatography-mass spectrometry analysis molecular docking analysis revealed the antagonistic nature of phytoligands namely α-D-Glucopyranoside,β-D-Fructofuranosyl and pyrrlidine,2α-1-pyrrolidinoformyl has the ability to inhibit the three tumour progressive proteins namely breast cancer gene 1, breast cancer gene 2 and human epidermal growth factor receptor 2 and their amino acid interactions were comparable to the positive drug Doxorubicin. Based on these studies the Aconitum heterophyllum loaded phytoniosomes can be a novel drug delivery tool in treating breast cancer.
Keywords
Aconitum heterophyllum, phytoniosomes, cytotoxic activity, ligands
Globally, breast cancer continues to be a most frequently diagnosed cancer in women. The incidence of breast cancer varies worldwide; high incidence was reported in more developed countries than developing ones[1]. One in eight women afflicts breast cancer during their lifetime[2]. The projected number of cases for breast cancer in India, in the year 2020 is 179 790 people were affected and this will constitute about 10 % of all the cancers[3]. And recent research status says that exposure to pesticides is also shown to have a positive correlation to incidence of breast cancer[4]. Developing breast cancer is associated with non-modifiable risk factors such as gender (female), age, reproductive factors such as early menarche, late menopause, nulliparous etc. Modifiable risk factors include high fat and calorie diet, obesity, sedentary lifestyle, use of tobacco, consumption of alcohol, contraceptive pills, endocrine disruptors and work shift[5].
Phytochemicals and derivatives present in plants are promising options to improve treatment efficiency in cancer patients and decrease adverse reactions. A number of these phytochemicals are naturally occurring biologically active compounds with significant anti-tumor potential. The development of effective and side-effects free phytochemical based anticancer therapy begins with the testing of natural extracts[6]. Aconitum heterophyllum, a member of the Ranunculaceae family, has long been used to treat a various human disease as anti-inflammatory and hepatoprotective agent. It is a good source of diterpene alkaloids, flavonoids[7]. Novel anti-cancer agents have been developed in recent decades with the goal of increasing drug pharmacodynamics and bioavailability, as well as delivering targeted drug concentrations to cancer cells with minimal toxicity to healthy cells. Phytosomes represents advanced herbal drug technology that offers defined bioavailability of plant drugs over the herbal extract[8]. Phytosomes has demarcated the undefined bioavailability of lipid insoluble secondary metabolites[9]. The term ‘Phyto’ means plant while ‘Some’ means cell-like. Phytosome is vesicular drug delivery system in which phytoconstituents of herb extract surround and bound by lipid (one phyto-constituent molecule linked with at least one phospholipid molecule). Phytosome protect valuable component of herbal extract from destruction by digestive secretion and gut bacteria and because of which they show better absorption which produces better bioavailability and improved pharmacological and pharmacokinetic parameters than conventional herbal extract[10]. Phytosomes are formulated by the processes in which the standardized extracts of active ingredients of herb are bound to phospholipid like Phosphatidylcholine (PC), phosphatidyl ethanolamine or phosphatidyl serine through a polar end[11].
Molecular Docking is the process by which two molecules fit together in 3D space; it is a key tool in structural biology and computer-aided drug design[12]. Women with defects in either the Breast Cancer gene 1 (BRCA1) or Breast Cancer gene 2 (BRCA2) have a greater than 80 % chance of developing breast cancer. The majority of breast cancer cases are due to BRCA1, BRCA2 and Human Epidermal growth factor Receptor 2 (HER 2) gene mutations[13]. With the above scenario the present investigation is aimed to study, the antioxidant and anti-proliferative effect of the Ethanolic root Extract Aconitum heterophyllum (EEAH) and phytoniosomes loaded with Aconitum heterophylum root extract (nEEAH) on human breast cancer (MCF-7) cell lines and in silico studies to identify the suitable antagonistic ligand for the protein 5JIK, 4IJK and 1N0W.
Materials and Methods
Preparation of EEAH:
Aconitum heterophyllum roots (1 kg) were dried and grinded into a coarse powder at room temperature. The powder was sieved through a 40-mesh sieve and extracted in a soxhlet unit at 60° with ethanol. After ethanol extraction, the filtrate was evaporated under decreased pressure using a rotatory evaporator until all the solvent was removed and the EEAH was then kept refrigerated for further studies.
Preparation of nEEAH:
nEEAH were synthesized by film hydration method. Initially, 10 mM of Tween 60 and cholesterol were dissolved in chloroform with 1:1 molar ratio and 1.0 mg/ml of Aconitum heterophyllum roots extract was mixed in a round bottom flask. The excess chloroform was removed at 55° using a rotary evaporator to obtain a thin film of nEEAH on the surface of the flask which was hydrated with phosphate-buffered saline by agitation in a water bath at 55° for 2 h. The resulting solution was then subjected to bath sonication for 20 min to obtain finer vesicles. Phytoniosomes are separated from the untrapped materials by performing dialysis against distilled water for overnight.
In vitro antioxidant activity:
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity: DPPH radical scavenging ability was examined by the method of Cuendet et al.[14]. In varying concentration range of EEAH and nEEAH extracts (10 to 50 μg/ml) were added to 3.0 ml of methanolic solution of DPPH (0.1 mM). In control, the root extract was replaced for methanol. The reaction mixture was incubated for 30 min in the darkroom at 37° and absorbance was read at 517 nm by Ultravioletvisible spectrophotometer. The percentage of inhibition was determined by the following equation:
Percentage inhibition=A0-A1×100/A0
Where A0 and A1 represents the absorbance of the control and test samples respectively. Ascorbic acid was used as a standard.
Super oxide scavenging activity: Superoxide anion scavenging activity was measured based on the described method by Robak et al.[15]. Superoxide radicals were generated in Phenazine Methosulphate- Nicotineamide Adenine Dinucleotide, (PMS-NADH) system by the oxidation of NADH and assayed by the reduction of Nitro Blue Tetrazolium (NBT). The assay mixture contains different concentrations of EEAH and nEEAH extracts in 1 ml of PMS solution which was incubated at 25° for 5 min and the absorbance was measured at 590 nm against the corresponding blank solution. L-Ascorbic acid was used as the positive control. The decrease of NBT reduction measured by the absorbance of the reaction mixture correlates with the superoxide radical scavenging activity of the study extracts.
Inhibition percentage of superoxide radical=[(A0–A1/ A0)×100]
Where A0 is the absorbance of the control and A1 is the absorbance of sample.
Hydroxyl radical scavenging activity: The hydroxyl radical scavenging activity was determined according to the method of Halliwell et al.[16]. The hydroxyl radical scavenging activity of EEAH and nEEAH extracts were measured by the competition between deoxyribose and the extracts for the hydroxyl radicals generated from the Fe3+/ascorbate/EDTA/H2O2 system (non-site specific assay).
Briefly, the reaction mixture containing 2.8 mM deoxyribose, 0.1 mM FeCl3, 0.1 mM ascorbic acid, 0.1 mM EDTA and 1 mM H2O2 were mixed with various concentrations of plant extracts in 1 ml of final volume made with KH2PO4-KOH buffer (20 mM pH 7.4), and was incubated in a water bath at 37° for 1 h. The extent of deoxyribose degradation was measured by Thiobarbituric Acid (TBA) method. 1 ml of 1 % (w/v) TBA and 1 ml, 2 % (w/v) Trichloro Acetic acid (TCA) were added to the mixture and heated at 100° for 20 min. After cooling to room temperature the absorbance was measured at 532 nm against the reagent blank. Mannitol, a classical hydroxyl radical scavenger was used as positive control. The inhibition percentage of hydroxyl radical was calculated.
Anti-proliferative activity:
Maintenance of MCF-7 cell lines: The MCF 7 cell lines were obtained from National Centre for Cell Sciences, Pune, India were cultured and maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with L glutamine, sodium bicarbonate, 10 % FBS, streptomycin (100 μg/ml), and penicillin (100 U/ml), non-essential amino acids and Hank’s salt at appropriate conditions. A confluent monolayer cells were harvested by 0.25 % trypsin and cultured in 10 % growth medium.
3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay: MTT method was used to assess the cytotoxicity and anti-cancer potential of nEEAH and EEAH on MCF-7 cell according to Horiuchi et al.[17]. A 100 μl cell suspension (5×104 cells /well) was seeded in 96-well tissue culture plates with 0.2 ml of medium/ well. To this different concentration of nEEAH and EEAH extracts were added and incubated at 37° with 5 % CO2 for 24 h, 48 h and 72 h in a humidified incubator to study the anti proliferative activity. At the end of incubation of 24 h, 48 h and 72 h approximately 30 μl of MTT solution was added and further incubated for 4 h with same incubation conditions. MTT solubilization solution in Dimethyl Sulfoxide (DMSO) 100 μl was added to the resultant supernatant in each well and mixed with a micropipette for 45 sec to solubilize the formazan crystals. The suspension was transferred to the cuvette of a spectrophotometer and the optical density values were read at 595 nm by using DMSO as a blank. Doxorubicin was used as a positive control. The percentage of cell viability was calculated as follows:
Cell viability=Optical density of test drugs/optical density of control×100
Trypan blue dye exclusion assay: A trypan blue staining assay was performed to further investigate the nEEAH cytotoxic effect in MCF-7 cells. In this method, viable cells with intact membranes do not absorb the trypan blue dye, whereas dead (non-viable) cells with intact membranes will absorb. MCF-7 cells were seeded in a 96-well plate and the plates were incubated in a 5 % CO2 incubator for 24 h at 37°. The medium was then replaced with new medium containing different concentrations of the Aconitum heterophyllum roots loaded Phytoniosomes (nEEAH) for MCF-7 (10, 20, 40, 60, 80, 100 μg/ml-1) in DMSO and then incubated for 24 h at 37° with 5 % CO2. Cells without any treatment were considered as negative control. The cells were placed on the Neubauer counting chamber at the end of the incubation period, stained with trypan blue dye and the number of viable and nonviable cells counted under the microscope at 100x magnification[18]. The percentage of viable cells compared to the control was used to calculate cell viability.
Gas Chromatography-Mass Spectrometry (GCMS) Analysis:
A Shimadzu GC-2010 Plus gas chromatograph was armed with a straight deactivated 2 mm direct injector liner and a 15 m Alltech EC-5 column (250 μ internal diameter, 0.25 μ film thickness). A split injection was useful for sample administration and the split ratio was set to 10:1. The oven temperature program was programmed to start at 35°, hold for 2 min, then ramp at 20° per min to 450° and hold for 5 min. The helium carrier gas was fixed to 2 ml/min flow rate. A Direct connection with capillary column metal quadrupole mass filter pre-rod mass spectrometer operating in electron ionization mode with software GC-MS solution ver. 2.6 was used for all analyses. Low-resolution mass spectra were acquired at a resolving power of 1000 (20 % height definition) and scanning from 25 m/z to 1000 m/z at 0.3 sec per scan with a 0.2 sec inter-scan delay. High-resolution mass spectra were acquired at a resolving power of 5000 (20 % height definition) and scanning the magnet from 65 m/z to 1000 m/z at 1 sec per scan.
In silico anti-cancer activities:
Preparation of receptor protein: The X-ray crystal structures of the breast cancer targeting protein HER2, BRCA1 and BRCA2 with PDB Code: 5JIK, 4IJK and 1N0W respectively were downloaded from the Research Collaboratory for Structural Bioinformatics databank. The preparation of the crystal structures was performed by correcting the missing residues and missing hydrogens using the protein preparation wizard of Schrodinger Glide. The bond orders were assigned. The non-essential water molecules were deleted and polar hydrogens were added. The crystal structures were optimized using the optimized potentials for liquid simulations force field.
Preparation of the ligand: The structures were drawn using ACD labs Chemsketch software and converted into MDL mol format, downloaded and imported. The LigPrep module in Glide was used to geometrically refine the chemical structures. The ligands were possibly set to a pH of 7.0. Desalting and generation of tautomers with conformer generation were performed and at most 32 conformers per ligand were generated.
Receptor grid generation and molecular docking: The receptor grid indicates the area of interaction between the ligand and protein. The grid indicates an area of active site that is given coordinates of x, y and z. The center of the active site residues is highlighted and the grid parameters were set to enable docking between the ligand and the prepared protein. The prepared and optimized protein structures were docked into ligands that were prepared using Lig Prep. The docking analysis was performed using the Glide tool on Maestro 11.5. The target proteins 5JIK, 4IJK and 1N0W were determined with their active site and the docking analysis was performed with the prepared ligands. The Glide Standard Precision (SP) algorithm was selected and flexible docking was performed. The docked results were viewed to determine the ligand interaction by viewing the interaction diagram at the residues of the active site of the protein.
Statistical analysis:
All data was analyzed statistically with Statistica/ Macsoftware (Prism, USA). The experimental results were mean±SD of three parallel measurements. Effective Concentrations (EC50) value was calculated by regression analysis. Mean differences were analyzed statistically by running one-way Analysis of Variance test (ANOVA). p<0.05 was considered statistically significant when compared to relevant controls.
Results and Discussion
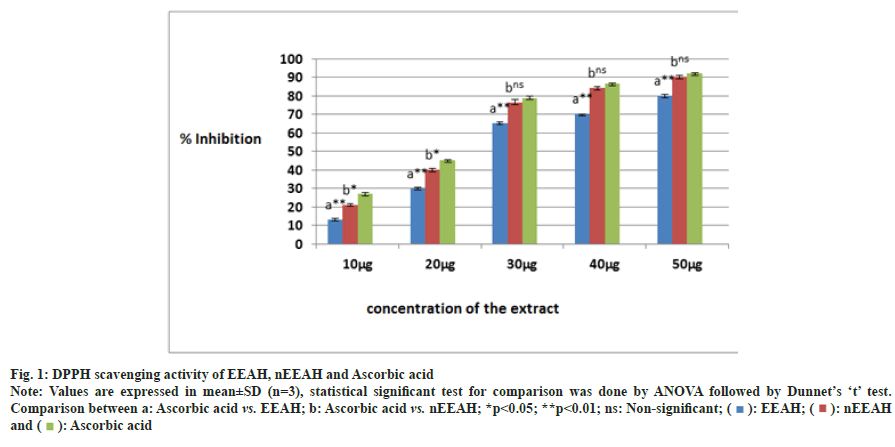
The DPPH scavenging activity of EEAH and nEEAH extracts were shown in the fig. 1. A concentration dependent DPPH scavenging activity was observed for both the extract in the present investigation. But at higher concentrations starting from 30 μg/ml, the phytoniosome extract of Aconitum heterophyllum exhibited similar inhibitory activity as that of positive control ascorbic acid.
Fig. 1: DPPH scavenging activity of EEAH, nEEAH and Ascorbic acid.
Note: Values are expressed in mean±SD (n=3), statistical significant test for comparison was done by ANOVA followed by Dunnet’s ‘t’ test.
Comparison between a: Ascorbic acid vs. EEAH; b: Ascorbic acid vs. nEEAH; *p<0.05; **p<0.01; ns: Non-significant; ( ): EEAH; (
): EEAH; ( ): nEEAH
and (
): nEEAH
and ( ): Ascorbic acid
): Ascorbic acid
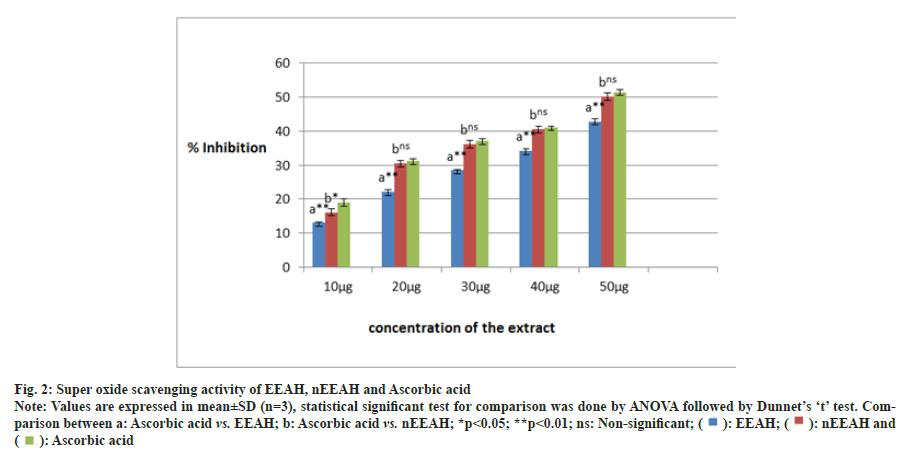
The potential of EEAH and nEEAH extracts in neutralizing the super oxide radicals were shown in fig. 2. Both the extracts neutralized the generated super oxide radicals effectively. However, the phytoniosomes form of Aconitum heterophyllum neutralized the super oxide radicals effectively when comparable to the conventional ethanolic extract.
Fig. 2: Super oxide scavenging activity of EEAH, nEEAH and Ascorbic acid.
Note: Values are expressed in mean±SD (n=3), statistical significant test for comparison was done by ANOVA followed by Dunnet’s ‘t’ test. Comparison
between a: Ascorbic acid vs. EEAH; b: Ascorbic acid vs. nEEAH; *p<0.05; **p<0.01; ns: Non-significant; ( ): EEAH; (
): EEAH; ( ): nEEAH and
(
): nEEAH and
( ): Ascorbic acid
): Ascorbic acid
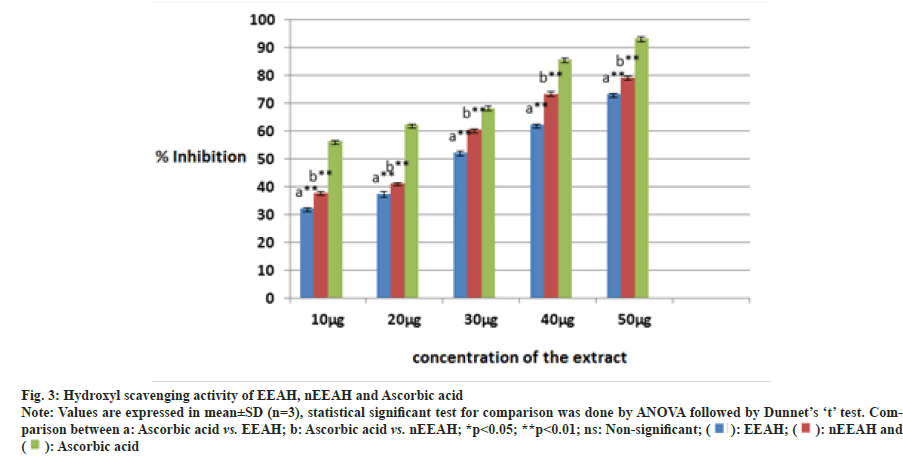
Fig. 3 depicts the hydroxyl radical scavenging activity of the three extracts namely EEAH, nEEAH and Ascorbic acid. Though all the three extracts scavenged the hydroxyl radicals in a dose dependent manner, we could not observe any increase in the scavenging potential nEEAH extracts. In the present investigation ascorbic acid exhibits maximum scavenging potential compared with the two extracts.
Fig. 3: Hydroxyl scavenging activity of EEAH, nEEAH and Ascorbic acid.
Note: Values are expressed in mean±SD (n=3), statistical significant test for comparison was done by ANOVA followed by Dunnet’s ‘t’ test. Comparison
between a: Ascorbic acid vs. EEAH; b: Ascorbic acid vs. nEEAH; *p<0.05; **p<0.01; ns: Non-significant; ( ): EEAH; (
): EEAH; ( ): nEEAH and
(
): nEEAH and
( ): Ascorbic acid
): Ascorbic acid
MTT assay determines the cell viability due to the plant extract treatment. In the present investigation decrease in the cell viability is observed due to the treatment of EEAH and nEEAH (Table 1). The effect was found to be concentration dependent the cell viability of nEEAH treated cells showed a deep decrease which was comparable with the doxorubicin the positive drug used in the present study. IC50 values were calculated and it was found to be 43.81 μg/ml for nEEAH extract.
| Concentration of extract (µg/ ml) | % cell viability by EEAH extract | % cell viability by nEEAH extract | % cell viability by Doxorubicin |
|---|---|---|---|
| 10 | 93.21±2.98a** | 81.60±1.65b* | 80.27±1.99 |
| 20 | 85.21±2.01a** | 72.19±0.95b* | 71.93±2.50 |
| 40 | 76.31±1.95a** | 66.44±1.48b* | 62.86±1.57 |
| 80 | 67.27±1.97a** | 61.46±1.52b* | 56.51±2.51 |
| 100 | 57.49±2.14a** | 56.81±1.50b* | 44.59±2.60 |
Note: Values are expressed in mean±SD (n=3), statistical significant test for comparison was done by ANOVA followed by Dunnet’s ‘t’ test. Comparison between a: Ascorbic acid vs. EEAH; b: Ascorbic acid vs. nEEAH; *p<0.05; **p<0.01 and ns: Non-significant
Table 1: Cell Viability of Mcf-7 Cell Lines after the Treatment of Different Extracts
The trypan blue assay is used to find out the antiproliferative activity of the plant extracts which measures the percentage of cell death due to the drug treatment. The ability of the EEAH and nEEAH extracts to induce the cell death in MCF-7 cells is analysed by trypan blue assay method and shown in Table 2. The nEEAH extract significantly inhibited the growth of MCF-7 cells and starting from the lower concentrations the anti-proliferative activity was quite similar to doxorubicin the positive drug with the IC50 concentration of 50.46 μg/ml.
| Concentration of extract ( µg/ ml) | % cell death by EEAH extract | % cell death by nEEAH extract | % cell death by Doxorubicin |
|---|---|---|---|
| 10 | 6.81±0.18a** | 18.70±2.25b* | 19.97±0.59 |
| 20 | 14.62±0.66a** | 26.89±1.66b* | 29.13±1.70 |
| 40 | 22.45±0.79a** | 33.42±0.88b* | 36.16±0.37 |
| 80 | 34.07±1.27a** | 37.76±2.22b* | 40.01±1.41 |
| 100 | 40.99±3.04a** | 42.66±2.70b* | 45.39±1.80 |
Table 2: Cell Death of Mcf-7 Cell Lines after the Treatment of Different Extracts
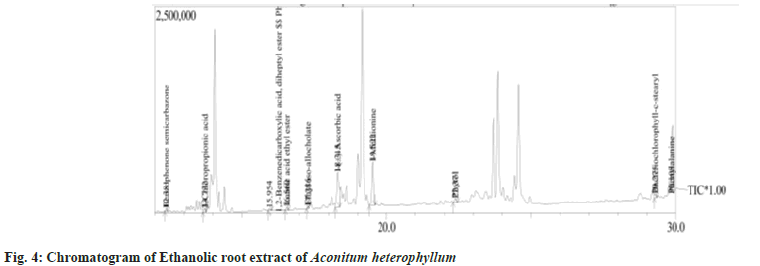
In the present investigation GC-MS analysis was performed to find out the components responsible for the antioxidant and anti-proliferative activity. Fig. 4 and Table 3 depict the chromatogram and list of phytochemicals with their molecular weight and formula. Totally 24 phyto constituents were identified in the ethanolic root extract of Aconitum heterophyllum. These compounds were further subjected to in silico docking studies to find suitable antagonistic ligand for the tumor progressing proteins.
| Peak# | R.Time | Name of compound | Molecular Formula | Molecular Wt | Area % |
|---|---|---|---|---|---|
| 1 | 5.139 | L-Gala--ido-octose | C8H16O8 | 240 | 25.06 |
| 2 | 5.585 | Butane, 2,3-dichloro-2-methyl- | C5H10Cl2 | 140 | 0.87 |
| 3 | 5.803 | Aziridine, 2-methylene-1-(1-methylethyl) | C6H11N | 97 | 1.12 |
| 4 | 6.094 | 4,5-Diamino-6-methyl-2-thiopyrimidine | C5H8N4S | 156 | 0.6 |
| 5 | 6.45 | 1,3-Butanedione, 1-(2-furanyl) | C8H8O3 | 152 | 0.69 |
| 6 | 6.745 | 6-Tridecene, 7-methyl- | C14H28 | 196 | 5.1 |
| 7 | 7.133 | Pyrrolidine, 2 alpha-[1-pyrrolidinoformyl] | C9H16N2O | 168 | 13.8 |
| 8 | 7.317 | alpha-L-Galactopyranoside, methyl 6-deoxy | C7H14O5 | 178 | 1.38 |
| 9 | 7.531 | alpha-D-Glucopyranoside, beta-D-Fructofuranosyl | C12H22O11 | 342 | 2.43 |
| 10 | 7.75 | 2-Pipecoline-1-dithiocarbamate | C7H13NS2 | 175 | 1.32 |
| 11 | 8.704 | 4-Amino-2,3-xylenol | C8H11NO | 137 | 1.83 |
| 12 | 11.14 | Mannitol, 1,1'-O-1,16-hexadecanediylbis- | C28H58O12 | 586 | 2.05 |
| 13 | 12.38 | Benzophenone semicarbazone | C14H13N3O | 239 | 1.24 |
| 14 | 13.73 | 3-Chloropropionic acid,heptadecyl ester | C20H39ClO2 | 346 | 1.38 |
| 15 | 15.95 | 1,2-Benzenedicarboxylic acid, diheptyl ester | C22H34O4 | 362 | 0.72 |
| 16 | 16.56 | Dodecanoic acid, ethyl ester | C14H28O2 | 228 | 1.01 |
| 17 | 17.29 | Ethyl iso-allocholate | C26H44O5 | 436 | 0.99 |
| 18 | 18.32 | I-(+)-Ascorbic acid 2,6-dihexadecanoate | C38H68O8 | 652 | 14.5 |
| 19 | 19.52 | I-Methionine, N- neopentyloxycarbonyl-, ethyl ester | C13H25NO4S | 291 | 18.07 |
| 20 | 22.37 | Phytol | C20H40O | 296 | 1.58 |
| 21 | 29.28 | Bacteriochlorophyll-c stearyl | C52H72MgN4O4 | 840 | 1.61 |
| 22 | 30.11 | Phenylalanine, 4-acetylamino | C11H14N2O3 | 222 | 0.63 |
| 23 | 30.3 | 3-pyrrolidin-2-yl-propionic acid | C7H13NO2 | 143 | 1.68 |
| 24 | 31.58 | alpha-D-Glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic butylboronate | C16H32BNO6Si | 373 | 0.35 |
Table 3: Phyto-Components Identified in Aconitum heterophyllum Ethanolic Root Extract
The in silico docking analysis was performed by molecular docking using “Schrodinger” software to find a suitable antagonistic ligand from the plant compound for the tumor progressive proteins BRCA1, BRCA2 and HER2. All 24 compounds identified from GC-MS analysis were docked against the proteins BRCA1, BRCA2 and HER2 and compounds showing lowest binding energy were selected as a suitable antagonistic ligand and the mode of inhibition is analyzed in terms of their interaction with specific amino acids. Table 4 shows the docking score in terms of binding energies of the selected ligand and the protein.
| S No. | Name of the compound | Binding energy expressed in (k cal mol-1) | ||
|---|---|---|---|---|
| BRCA1 | BRCA2 | HER2 | ||
| 1 | L-Galaido-octose | -3.100 | -0.973 | -2.176 |
| 2 | butane,2,3-dichloro-2-methyl | -3.624 | -6.802 | -2.290 |
| 3 | Aziridine,2-methylene-1(-1methylethyl) | -2.883 | -8.521 | -2.976 |
| 4 | 4,5-diamino-6-methyl-2-furanyl) | -3.070 | -0.800 | -3.497 |
| 5 | 1,3-butanedione,1-(2-furanyl) | -3.050 | -1.890 | -3.392 |
| 6 | 6-tridecene,7-methyl | -0.716 | -1.910 | -1.851 |
| 7 | pyrrolidine,2 alpha-(1-pyrrolidinoformyl) | -5.624 | -3.372 | -3.424 |
| 8 | alpha-L-Galactopyranoside,methyl 6-deoxy | -3.014 | -1.845 | -3.261 |
| 9 | alpha-D-Glucopyranoside,beta-D-Fructofuranosyl | -5.529 | -5.969 | -4.795 |
| 10 | 4-Amino-2,3-xylenol | -4.785 | -1.910 | -3.600 |
| 11 | Mannitol,1,1-O-1,16-Hexadecanediylbis | -5.327 | -1.890 | -3.611 |
| 12 | 3-chloropropionic acid,hepatadecyl ester | -2.836 | -1.786 | -1.709 |
| 13 | 1,2-Benzenedicarboxilic acid,diheptyl ester | -4.587 | -1.657 | -1.540 |
| 14 | dodecanoic acid ethyl ester | -1.156 | -1.811 | -2.411 |
| 15 | ethyl iso allocholate | -2.736 | -1.720 | -4.144 |
| 16 | I-(+)-Ascorbic acid 2,6-dihexadecanoate | -4.233 | -1.633 | -2.402 |
| 17 | I-methionine,N-neopentyloxycarbonyl-ethyl ester | -3.813 | -1.453 | -2.290 |
| 18 | phytol | -4.885 | -1.789 | 0.976 |
| 19 | bacteriochlorophyll-c stearyl | -4.646 | -1.894 | -5.512 |
| 20 | 3-pyrrolidin-2-yl-propionic acid | -4.946 | -1.880 | -4.119 |
| 21 | alpha –D-Glucopyranoside,methyl 2-(acetylamino)2-deoxy-3-O-(trimethylsilyl)-cyclic butylboronate | -4.196 | -1.768 | -2.476 |
| 22 | Doxorubicin | -6.569 | -5.517 | -6.559 |
Table 4: Comparative Analysis on the Docking Results of the Compounds against Different Targets for Breast Cancer
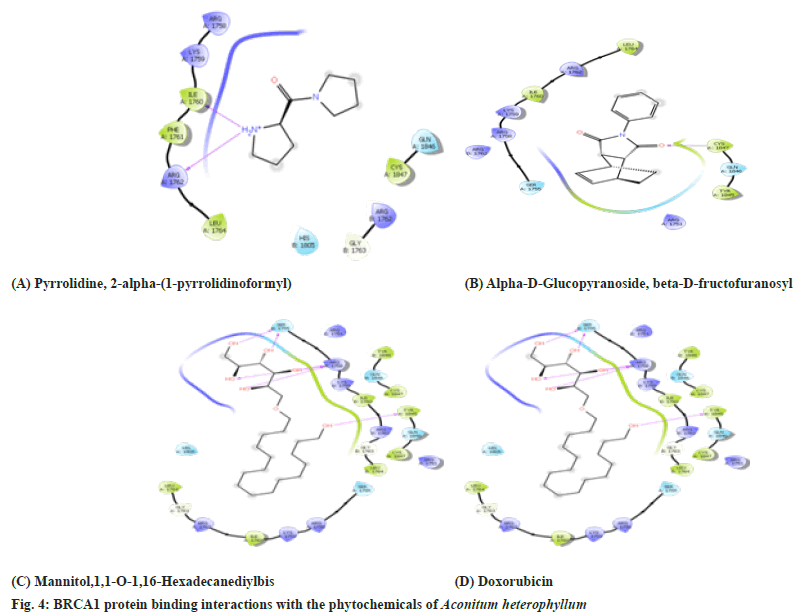
Based on the binding energies three ligands namely pyrrolidine, 2-α-(1-pyrrolidinoformyl) α-D-Glucopyranoside, β-D-Fructofuranosyl and Mannitol,1,1-O-1,16-Hexadecanediylbis showed the lowest binding energy. So these three ligands were further investigated for their inhibition of the BRCA1 protein. The Protein BRCA1 interaction with their phytochemical ligand in terms of amino acid binding is shown in the Table 5 and fig. 5. It is observed the phytoconstituents inhibited certain amino acids like valine and tyrosine which were inhibited by the positive drug Doxorubicin.
| S No. | Compound | BARC1 Protein amino acid binding site | |
|---|---|---|---|
| H bonding sites | Hydrophobic contact sites | ||
| 1 | pyrrolidine,2 alpha-(1-pyrrolidinoformyl) | Ile1760, Arg 1762 | Arg 1758, Lys 1759, Phe 1761, Arg 1762, Gly 1763, Leu1764, His 1805, Gln 1846, Cys 1847 |
| 2 | Alpha-D-Glucopyranoside,beta-D-Fructofuranosyl | Arg 1758, Lys 1759, Leu 1760, Arg 1762 | Phe 1761, Arg 1762, Gly 1763, Leu 1764, His 1805, Cys 1847 |
| 3 | Mannitol,1,1-O-1,16-Hexadecanediylbis | Ser 1755, Arg1758, Tyr 1845 | Arg 1751, Ser 1755, Arg 1758, Lys 1759, Ile 1760, Arg 1762, Gly 1763, Leu 1764, His 1805, Tyr 1845, Gln 1846, Cys 1847,Gln 1846, Cys 1847 |
| 4 | Doxorubicin | Tyr 836, Val 851 | Val850,Trp780,Pro778,Ser774,Ile800,Met772,Lys 802,Ser773,Gln859,Thr856,Ser919,His917, Asn920, Asp933, Ile848,Phe930,Glu849, Met922 |
Table 5: Amino Acid Interaction with the Barc1 Protein Phyto Ligands of EEAH
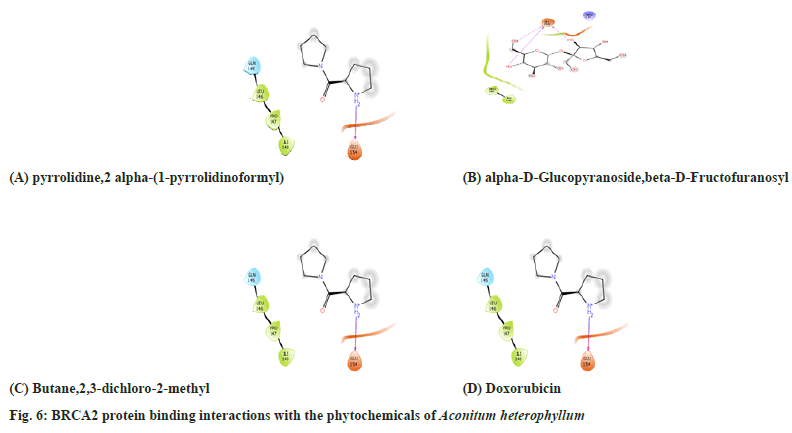
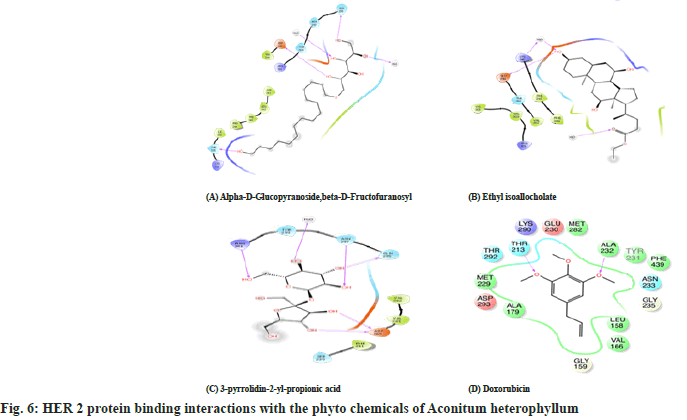
Table 6 and Table 7, fig. 6 and fig. 7 shows the amino acid interactions observed between the protein BRCA 2 protein and the phytoligands namely which were selected based on their binding energies. Here also the same ligands pyrrolidine, 2α-(1-pyrrolidinoformyl) and α-D-Glucopyranoside exhibited lowest binding energy for BRCA 2 also. Additionally, Butane 2,3-dichloro- 2-methyl ligand also showed better binding. So these three ligands were further investigated for their amino acid binding with the protein BRCA 2 to show their antagonistic nature. Here only few amino acid interactions were observed unlike the interactions seen in BRCA 1. The phytoligands such as pyrrolidine, 2α-(1-pyrrolidinoformyl) and α-D-Glucopyranoside and ethyl iso allocholate from Aconitun heterophyllum exhibited antagonistic action in terms of amino acids binding with protein HER2.
| S No. | Compound | BARC2 Protein amino acid binding site | |
|---|---|---|---|
| H bonding sites | Hydrophobic contact sites | ||
| 1 | pyrrolidine,2 alpha-(1-pyrrolidinoformyl) | Glu 154 | Gln145,Leu146,Pro147,Ile147 |
| 2 | alpha-D-Glucopyranoside,beta-D-Fructofuranosyl | Glu 154 | Pro 147, Ile 148, Arg 215 |
| 3 | butane,2,3-dichloro-2-methyl | - | Ile 100, Ile148, Gly 153, Gly 154, Arg 215 |
| 4 | Doxorubicin | His47, Gly29 Phe5 | His27, Cys44, Gly22, Phe98, Tyr21, Ile9, Ala94, Ala17, His6, Ala18, Leu2, Val3, Cys28, Val30, Asp48 |
Table 6: Amino Acid Interactions with the Barc2 Protein Phyto Ligands of EEAH
| S No. | Compound | BARC2 Protein amino acid binding site | |
|---|---|---|---|
| H bonding sites | Hydrophobic contact sites | ||
| 1 | alpha-D-Glucopyranoside,beta-D-Fructofuranosyl | Asp 265, Gln 295, Asn 297, Arg 301 | Ser 239, Phe 241, Val 262, Val 264, Thr 299 |
| 2 | ethyl iso allocholate | Lys 246, Glu 258 | Phe 241, Phe 243, Thr 260, Val262,Lys 290, Arg 301, Val 303, Val 305 |
| 3 | 3-pyrrolidin-2-yl-propionic acid | Asp 265, Gln 295,Thr 335 | Phe 241, Leu 242, Phe 243, Pro 244, Val 262, Asn 297, Thr 299, Arg 301, Lys 334, Ile 336 |
| 4 | Doxorubicin | Ala232,Thr213 | Lys290, Glu230, Met282,Tyr231, Phe439 ,Asn233, Gly235, Leu158, Val166, Gly159, Ala179, Asp293, Met229, Thr292 |
Table 7: Amino Acid Interactions with the HER2 Protein Phyto Ligands of EEAH
Living cells during metabolic reactions produces free radicals like hydroxyl, nitric oxide and super oxide radicals which are collectively called as Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) according to Hari et al.[19]. These are recognized as the major cause of oxidative stress and also are responsible for uncontrolled cell proliferation and various number of disorders. Antioxidants play important role in maintaining balance between the formation of ROS and their removal from the system. Every human cells prevent themselves from free radical by some antioxidant mechanism, sometimes these are not adequate to protect cells from the ROS damage. Several parts of medicinal plants have previously been reported as antioxidants. The plant bioactive compounds due to their antioxidant potential can inhibit the development of cancer by reducing oxidative stress caused by free radicals. Phytosomes are herbal formulations which have a greater bioavailability when compared to ordinary plant extracts. They are obtained by adding conventional plant extracts or phytochemicals with phospholipids, generally PC to produce lipid-compatible molecular structures. PC possesses medicinal properties in addition to acting as a carrier, offering it a dual benefit when it is used in phytosome preparations[20]. In the present investigation we have demonstrated the comparative antioxidant and anti-proliferative activity of ethanolic root extract and phytosomes of Aconitum heterophyllum. The antioxidant potential of the root extracts to scavenge the DPPH free radical by donating hydrogen proton was indicated by stage of discolouration. According to Shyaula, the root contains many phenolic and flavonoids which may contribute towards the DPPH scavenging activity observed in the present study. Superoxide anion radical plays a key role in the production of other ROS such as hydrogen peroxide or singlet oxygen. So deactivation of superoxide by the plant extracts is considered as an important activity since most of the chain reactions initiated by the super oxide radicals can be halted. The role of hydroxyl radicals as mutagenic agent is well documented since it attacks the deoxy ribose sugar present in the DNA molecule leading to its breakdown leading to carcinogenicity and cytotoxicity. Phaniendra et al.[21], Sarkar et al.[22] have done the phytochemical analysis of the roots of Aconitum heterophyllum and reported the presence of carbohydrates, alkaloids, amino acids, proteins, cardiac glycosides, tannins, quinones, steroids, saponins tannins, terpenoids and flavonoids. The presence of diverse group of phytochemicals with high therapeutic nature makes the plant extract in scavenging the free radicals in an efficient manner and contributes towards its antioxidant activity. In the present study the phytoniosomes of Aconitum heterophyllum extract exhibited higher free radical scavenging potential than its conventional ethanolic extract which may attributed to their increased bioavailability in the form of niosomes.
MCF-7 breast cancer cells used as an ideal model for understanding the cancer pathway occurring in the human breast cancer and the MTT assay is a colorimetric assay to assess the cellular metabolic activity. It is a widely used method for screening the cytotoxic activity of crude extracts, isolated compounds and synthetic drugs proposed for anticancer activity in different cell lines[23]. Moreover, the trypan blue assay is usually performed to find out the viability of the tumor cells as a result of drug treatment was estimated by trypan blue dye. In the present investigation significant cytotoxicity was observed for the Aconitum heterophyllum loaded phytoniosomes in the MTT assay and trypan blue assay. Babazadeh et al.[24] have developed food grade nano delivery systems in the form of phyto niosomes using the phytochemicals such as rutin, anthocyanidins, catechins, and silymarin to treat several types of cancer. These lipids based nano carriers facilitate the entry of large molecular sized and poor lipid soluble phyto molecules through the biological membranes to exert its effect[25]. The increased cytotoxic activity observed for the Aconitum heterophyllum loaded phyto niosomes may be due to the increased bio availability of its phytoconstituents to the cancer cells leading to their death.
It is also important to gain a deeper understanding of breast cancer molecular processes in order to find new therapeutic targets. The mutation in the BRCA1, BRCA2, and HER-2 genes plays a significant role in breast cancer. BRCA and HER2 proteins, which play a critical role in developing breast and ovarian cancer in women, are functionally disrupted by mutations in the BRCA1 and BRCA2 genes. So in the present investigation we made an attempt to identify a suitable antagonistic ligand which can bind with the BRCA and HER2 proteins to down regulate the actions of these proteins. Based on the docking studies two phyto ligands α-D-Glucopyranoside,β-D-Fructofuranosyl and pyrrlidine,2 α-(1-pyrrolidinoformyl has the ability to inhibit the three tumour progressive proteins namely BRCA1, BRCA2, and HER-2 among the 21 phytocomponents identified in the GC-MS analysis. Anh et al.[26] have isolated α-D-Glucopyranoside,β-DFructofuranosyl a sucrose derivative from the roots of Canna indica and showed it possess antioxidant activity. Hwa-JinSuh et al.[27] have shown the antioxidant activity of pyrrlidine,2α-(1-pyrrolidinoformyl)
Thakur et al.[28] have studied the anticancer activity of the steroidal derivative Ethyl iso allocholate isolated from the Trigonella foenum-graecum. These compounds may be responsible for the anti-proliferative activity of MCF-7 cell lines observed in the present study.
It can be concluded that phyto niosomes produced from the root extract of Aconitum heterophyllum (nEEAH) exhibited enhanced anti-carcinogenic activity and bioavailability compared to its EEAH. Due to the encapsulation of Aconitum heterophyllum in the form of niosomes the release rate of the phytoconstituents were slow to prolong the therapeutic activity. Moreover, bio active compounds present in Aconitum heterophyllum could act as an antagonistic ligand for the proteins namely BRCA1, BRCA2, and HER-2, thereby used as a potential drug for reducing the cytotoxic condition observed in Breast cancer.
Acknowledgements:
The author would like to thank the Molecular Biology and Bioinformatics Laboratory staffs for their continuous help and support for the completion of this study.
Conflict of Interest:
The authors declared that there is no potential conflict of interest to be disclosed.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127(12):2893-917
[Crossref] [Google Scholar] [PubMed]
- Tirona MT, Sehgal R, Ballester O. Prevention of breast cancer (part I): Epidemiology, risk factors, and risk assessment tools. Cancer Invest 2010;28(7):743-50.
[Crossref] [Google Scholar] [PubMed]
- Mathur P, Sathishkumar K, Chaturvedi M, Das P, Sudarshan KL, Santhappan S, et al. Cancer statistics, 2020: Report from national cancer registry programme, India. JCO Glob Oncol 2020;6:1063-75.
[Crossref] [Google Scholar] [PubMed]
- Bassil KL, Vakil C, Sanborn M, Cole DC, Kaur JS, Kerr KJ. Cancer health effects of pesticides: Systematic review. Can Fam Physician 2007;53(10):1704-1711.
[Google Scholar] [PubMed]
- Weir R, Day P, Ali W. Risk factors for breast cancer in women. New Zealand Health Technology Assessment (NZHTA) Report. 2007;10(2):1-328.
- Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front Pharmacol 2020;10:1614.
[Crossref] [Google Scholar] [PubMed]
- Paramanick D, Sharma N, Parveen N, Patel N, Keshri M. A review article on ayurvedic/herbal plant? aruna. Int J Adv Res 2017;5(2):319-325.
- Kumar S, Baldi A, Sharma DK. Phytosomes: A modernistic approach for novel herbal drug delivery-enhancing bioavailability and revealing endless frontier of Phytopharmaceuticals. J Dev Drugs 2019;9:1-8.
- Kumari P, Singh N, Cheriyan BP. Phytosome: A Noval Approach For Phytomedicine. Int J Inst Pharm Life Sci 2011;1(2):89-100.
- Pawar HA, Bhangale BD. Phytosome as a novel biomedicine: A microencapsulated drug delivery system. J Bioanal Biomed 2015;7(1):6-12.
- Amit PY, Tanwar YS, Rakesh S, Poojan P. Phytosome: Phytolipid drug delivery system for improving bioavailability of herbal drug. J Pharm Sci Biosci Res 2013;3(2):51-57.
- Palleti JD, Chitti S, Ajay Babu P. Virtual screening and molecular docking analysis of Zap-70 Kinase inhibitors. Int J Chem Anal Sci 2011;2(9):1208-1211.
- Thakur S, Phadke SR. Familial breast cancer: Genetics and counseling. Indian J Surg 2005;67:297-301.
- Cuendet M, Hostettmann K, Potterat O, Dyatmiko W. Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helvetica Chimica Acta. 1997;80(4):1144-1152.
- Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol 1988;37(5):837-841.
[Crossref] [Google Scholar] [PubMed]
- Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 1987;165(1):215-219.
[Crossref] [Google Scholar] [PubMed]
- Horiuchi N, Nakagawa K, Sasaki Y, Minato K, Fujiwara Y, Nezu K, et al. In vitro antitumor activity of mitomycin C derivative (RM-49) and new anticancer antibiotics (FK973) against lung cancer cell lines determined by tetrazolium dye (MTT) assay. Cancer Chemother Pharmacol 1988;22:246-250.
[Crossref] [Google Scholar] [PubMed]
- Haldar PK, Kar B, Bala A, Bhattacharya S, Mazumder UK. Antitumor activity of Sansevieria roxburghiana rhizome against Ehrlich ascites carcinoma in mice. Pharm Biol 2010;48(12):1337-1343.
[Crossref] [Google Scholar] [PubMed]
- Reddy PR, Hari R, Durairaj P. Anti-proliferative potentials of Excoecaria agallocha Leaf extract in human breast cancer cell line-an antioxidant enzyme approach. J Appl Pharm Sci 2022;1-5.
- Jain N, Gupta BP, Thakur N, Jain R, Banweer J, Jain DK, et al. Phytosome: A novel drug delivery system for herbal medicine. Int J Pharm Sci Drug Res 2010;2(4):224-228.
- Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 2015;30:11-26.
[Crossref] [Google Scholar] [PubMed]
- Sarkar PK, Prajapati PK, Pillai AP, Chauhan MG. Pharmacognosy of aconite sold under the name Vatsanabha in Indian market. Indian J Trad Knowledge 2012;11(4):685-696.
- Morshed MA, Uddin A, Rahman A, Hasan T, Roy S, Amin AA, et al. In vitro antimicrobial and cytotoxicity screening of Terminalia arjuna ethanol extract. Int J Biosci 2011;1(2):31-38.
- Babazadeh A, Ghanbarzadeh B, Hamishehkar H. Novel nanostructured lipid carriers as a promising food grade delivery system for rutin. J Functional Food 2016;26:167-175.
- Bhattaram VA, Graefe U, Kohlert C, Veit M, Derendorf H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine 2002;9:1-33.
[Crossref] [Google Scholar] [PubMed]
- Anh LT, Hieu N, Trang DT, Huu Tai B, van Kiem P. Two new acylated sucroses from the roots of Canna indica L. and their antioxidant activity. Nat Prod Commun 2021;16(2):1934578X21991720.
- Suh HJ, Kim SR, Lee KS, Park S, Kang SC. Antioxidant activity of various solvent extracts from Allomyrina dichotoma (Arthropoda: Insecta) larvae. J Photochem Photobiol B 2010;99(2):67-73.
[Crossref] [Google Scholar] [PubMed]
- Thakur RS, Ahirwar B. A steroidal derivative from Trigonella foenum graecum L. that induces apoptosis in vitro and in vivo. J Food Drug Anal 2019;27(1):231-239.
[Crossref] [Google Scholar] [PubMed]