- *Corresponding Author:
- A. M. Elazzazy
Department of Biology,
College of Science,
University of Jeddah,
Jeddah 21589,
Saudi Arabia
E-mail: amelazzazy@uj.edu.sa
| This article was originally published in a special issue,“New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “59-65” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Therapeutic outcomes for high grade soft tissue and bone sarcomas still remains poor, which is largely due to severe chemoresistance of this aggressive tumor type. Therefore, alternative approaches are needed addressing novel targets that are different from current multimodal chemotherapy. Natural killer cells are a major component of the innate immune system due to their ability to exert direct cytotoxicity against malignant cells or otherwise stressed cells. Interest in natural killer cell-based immunotherapy has increased since new protocols for the purification and expansion of large numbers of highly cytotoxic cells have become available. Peripheral blood mononuclear cells from patients with different thalassemia subtypes were co-cultured with irradiated, genetically engineered K562-mb15-41BBL cells in the presence of interleukin-2 for 14 d. Expansion efficiency, natural killer cell receptor repertoire and the expression of natural killer cell ligands on cell lines were investigated by flow cytometry. Cytolytic activity of natural killer cells was tested using the europium target detection assay based on time-resolved fluorometry. Ex vivo expansion of peripheral blood mononuclear cells from thalassemia patients allowed the generation of large numbers of natural killer cells exhibiting enhanced activation characteristics and high cytolytic activity against tumor-cell lines, yet retaining tolerance towards normal body cells. Moreover, this method has been adapted to clinical-grade conditions, producing a sufficient quantity of highly cytotoxic natural killer cells for immunotherapy. Based on these data, adoptive transfer of ex vivo expanded and activated natural killer cells deserve further clinical evaluation as a possible new treatment option for tumor and other blood diseases patients.
Keywords
Thalassemia, autologous immunotherapy, natural killer cell expansion, peripheral blood mononuclear cells
Cellular immunotherapy is now being explored for a number of diseases not responsive to standard chemotherapy. Natural Killer (NK) cells are a diverse and complex subset of lymphocytes that constitute a critical component of the innate immune system. In murine models, it has been shown that NK cells can control both local tumor growth and metastasis due to their ability to exert direct cellular cytotoxicity without prior antigen priming for activation [1]. Furthermore, NK cells secrete immunostimulatory cytokines like Interferon- gamma (IFN-γ) participating in cancer elimination by inhibiting cellular proliferation, promoting apoptosis and stimulating the adaptive immune system. Early recognition of the Interleukin-2 (IL-2) activation of NK cells increased their cytotoxicity towards resistant target cells [2,3] led to clinical trials of ex vivo activated autologous or allogeneic NK cells [4-6]. Adoptive transfer of NK cells has emerged as a safe and potentially efficacious immunotherapy for cancer, but a major hurdle in the development of this therapeutic approach has been the production of large numbers of NK cells with high purity and potency. Recently, a novel clinical- scale method of ex vivo NK cell expansion was reported using the Major Histocompatibility Complex (MHC) class I deficient chronic myelogenous leukemia cell line (K562) genetically modified to express a membrane- bound form of IL-15 fused to the T cell receptor Cluster of Differentiation (CD) 8 alpha (α) and the Tumor necrosis factor ligand superfamily member 9 (4-1BB) (CD137) ligand which particularly activate NK cells and promote their proliferation and survival [7,8]. Such activated allogeneic NK cells demonstrated powerful in vitro and in vivo cytotoxicity against pediatric solid tumors, including sarcomas [9]. NK cell function depends on a complex balance between activating and inhibitory signals from a variety of different surface receptors. The interaction of NK cell inhibitory Killer cell Immunoglobulin-like Receptors (KIR) and self- MHC class I molecules mediates NK tolerance to self while conferring functional competence. Recognition of MHC class I proteins by NK cell inhibitory receptors prevents autoreactive behaviour but also permits cytotoxicity against target cells that have downregulated MHC class I antigen expression, where the lack of class I engagement by inhibitory receptors allows NK activation (missing self-recognition) [10,11]. In addition to sensing a loss of MHC class I in target cells, full effector function of NK cells requires triggering of their activating receptors via stress- induced or virus-encoded ligands on target cells [12-14]. NK cells can be activated through a broad array of activating receptors, such as the Natural Cytotoxicity Receptors (NCR) NKp30, NKp44, NKp46 and NKp80, Natural Killer Group 2D (NKG2D), DNAX Accessory Molecule-1 (DNAM-1) and activating receptors of the KIR complex. These receptors endow NK cells with the capacity to distinguish and respond to autologous cells that differentially express their respective ligands in response to malignant transformation, viral infection or other types of stress (induced self-recognition). However, the effective application of autologous NK cell immunotherapy has been limited by the small number of NK cells in peripheral blood, difficulties with large-scale production of clinical-grade cytotoxic NK cells, autologous inhibitory receptor-ligand interactions and decreased functionality of NK cells in cancer patients. To induce autologous cytotoxicity, inhibitory signals must be overcome, either by increased expression of activating NK cell ligands or downregulation of inhibitory ligands on the tumor cells, enhanced expression of activating receptors on NK cells or a combination of the factors mentioned.
Materials and Methods
Ex vivo expansion of NK cells:
Peripheral blood was obtained from patients and healthy donors after informed written consent. Peripheral Blood Mononuclear Cells (PBMC) were isolated by Ficoll- Hypaque density gradient centrifugation. Cells were incubated with irradiated (100 Gy) artificial antigen expressing K562 modified to express a membrane- bound form of IL-15 and 4-1BB Ligand (K562-mb15- 41BBL) cells at a ratio of 1:1.5 (PBMC:irradiated feeder cells) in Roswell Park Memorial Institute (RPMI) 1640 medium (Life Technologies, Darmstadt, Germany) supplemented with 10 % human AB serum (Department of Transfusion Medicine, University Hospital Tuebingen, Germany), L-glutamine (Life Technologies) and 100 IU/ml IL-2 (Proleukin) (Novartis, Basel, Suisse). The K526-mb15-41BBL cell line which was genetically modified to express membrane-bound IL-15 and 4-1BB (CD137) ligand was kindly provided by Dario Campana, Department of Paediatrics, Center for Translational Medicine, National University of Singapore, Singapore. Half of the medium changes were done every 2-3 d with fresh IL-2 containing medium. Cell culture was maintained under the above described conditions for 14 d. Expanded NK cells were purified by CD56 positive cell selection with antibody-conjugated immunomagnetic microbeads (Milteny Biotec, Bergisch Gladbach, Germany) followed by CD3 positive cell depletion using Dynabeads (Life Technologies, Darmstadt, Germany).
Flow cytometry:
Phenotyping of PBMC and ex vivo expanded NK cells was performed using directly conjugated monoclonal antibodies or appropriate isotype controls against: CD16 (clone 3G8) Alexa Fluor 700, CD25 (clone M-A251) Fluorescein Isothiocyanate (FITC), CD56 (clone B159) Phycoerythrin-Cyanine7 (PE-Cy7), CD69 (clone L78) Allophycocyanin (APC), CD158a (clone HP-3E4) FITC, CD161 (clone DX12) PE, CD244 (clone DX26) FITC (Becton Dickinson, Heidelberg, Germany), CD3 (clone BW264/56) VioBlue, CD158b (clone DX27) APC, CD158e (DX9) Peridinin-Chlorophyll- Protein (PerCP), CD158e/k (clone 5.133) PE, CD314 (clone BAT221) APC, NKp30 (clone AF29-4D12) APC, NKp44 (clone 2.29) PE, NKp46 (clone 9E2) PE (Miltenyi Biotec, Bergisch Gladbach, Germany), CD62L (clone DREG-56) APC, CD85j (clone GHJ/75) PE, CD94 (clone DX22) FITC, CD226 (clone TX25) FITC, T-Cell Receptor gamma delta (TCRγδ) (clone B1) FITC (BioLegend, London, Great Britain) and CD159a (clone Z199) PE (Beckman Coulter, Krefeld, Germany).
To evaluate the surface expression of NK cell ligands on sarcoma cell lines, a panel of monoclonal antibodies or matched isotype controls was used: CD48 (clone BJ40) FITC, CD50 (clone CBR-IC3/1) PE, CD112 (clone TX31) PE, Human Leukocyte Antigen-G (HLA-G) (clone 87G) APC, Major histocompatibility complex class I-related Chain A and B (MICA/B) (clone 6D4) APC (BioLegend, London, Great Britain), CD54 (clone 6.5B5) FITC, HLA-ABC (clone W6/32) PE (Dako Denmark A/S, Glostrup, Denmark), CD58 (clone TS2/9) APC, CD 102 (clone CBRIC2/2) FITC, CD155 (clone 2H7CD155) PE (eBioscience, Frankfurt, Germany), Human Leukocyte Antigen E (HLA-E) (clone 3D12) APC (Miltenyi Biotec, Bergisch Gladbach, Germany), UL-16 Binding Protein (ULBP) 1 (clone Z9), ULBP2 (clone F16), ULBP3 (clone 2F9) and ULBP4 (clone 6E6) all unconjugated (Santa Cruz Biotechnology, Heidelberg, Germany). A goat anti- mouse secondary antibody (clone Poly4053) APC from BioLegend was utilized for the detection of ULBP1- 4 expression. Cells were analyzed on a LSRII flow cytometer (Becton-Dickinson, Heidelberg, Germany) using FACSDiva (Becton Dickinson) and FCS Express software (de novo Software, Los Angeles, United States of America (USA)). Mean Fluorescence Intensity (MFI) ratios and percent positive cells were calculated for each cell surface antigen.
Cytotoxicity assay:
Cytolytic activity of PBMC and expanded NK cells against autologous sarcoma cell lines was measured in a 2 h nonradioactive Dissociation-Enhanced Lanthanide Fluorescence Immunoassay (DELFIA®) Europium Target Detection Assay (EuTDA) cytotoxicity assay (PerkinElmer, Rodgau, Germany) based on time- resolved fluorometry. K562 cells were included as target cells in all assays to assess overall cytotoxicity performance. Four different effectors to target cell ratios were tested (10:1, 5:1, 2.5:1) and specific lysis was calculated as follows: Percent (%) specific lysis=(experimental release-spontaneous release)/ (maximum release-spontaneous release)×100.
Statistical analysis:
Statistical analysis was performed using GraphPad Prism version 5 (GraphPad Software Inc, LaJolla, USA). Data were expressed either as median and range or mean plus/minus Standard Error of the Mean (SEM). Significance levels were determined by two- tailed Student’s t-test and p values of 0.05 or less were considered statistically significant.
Results and Discussion
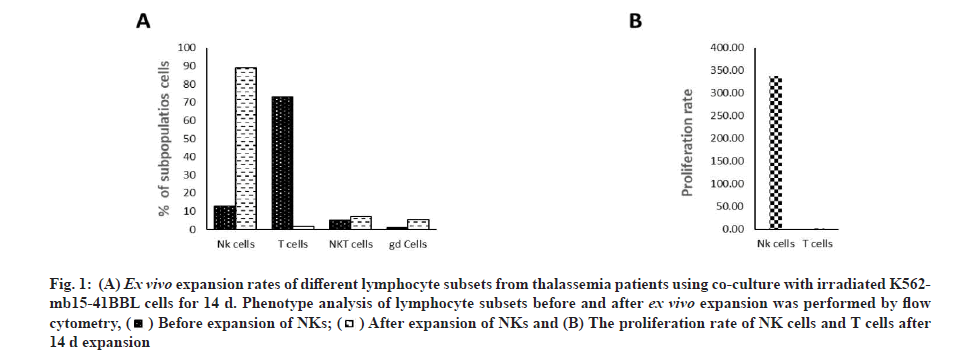
To achieve clinical-grade expansion of NK cells, PBMC were co-cultured with irradiated K562 cells expressing membrane-bound IL-15 and 4-1BB ligand (K652-mb15-41BBL) in culture medium containing 10 % human AB serum and 100 IU/ml IL-2 for 14 d. Cell products became significantly enriched in NK cells (d 0 with median 15.2 %, range 5.2 %-19.0 % vs. d 14 with median 89.1 %, range 77.6 %-93.2 %) (fig. 1). Ex vivo expansion resulted in a considerable increase in NK cells with comparable expansion (median 391.1-fold increase, range 137.4-1773.4-fold) (fig. 2). Patients showed an even higher expansion rate of Natural Killer T (NKT) cells (CD56+CD3+ as defined by flow cytometry) with a median increase of 441.0-fold, range 140.0-903.9-fold, whereas no significant enrichment of NKT cells after ex vivo expansion was observed when data from thalassemia patients. On the contrary, the percentage and number of T cells was decreased, for the expansion of T cells with a median 11.6-fold (range 1.3-3.2-fold). The percentage of T cells declined from a median of 67.4 % (range 54.3 %-73.7 %) on d 0 to 4.0% (range 3.2 %-4.4 %) on d 14. These data suggest that NK cells are efficiently expanded from healthy donors or thalassemia.
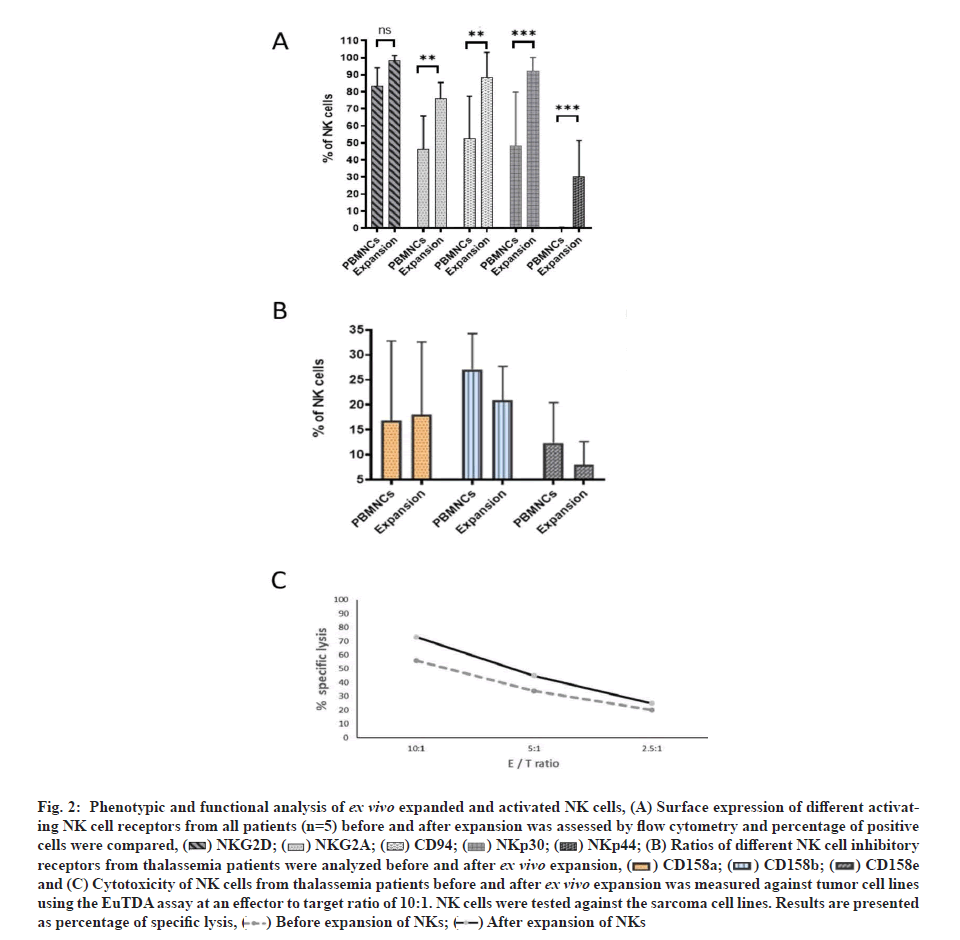
To characterize NK cells in the final expansion product compared with NK cells in the starting material a detailed flow cytometric analysis was carried out. For each receptor or activation marker analysed, percentage of positive cells were determined and compared between after ex vivo expansion and before expansion, and used as an indicator of the change in NK cell receptor expression after ex vivo expansion (fig. 2A and fig. 2B). Ex vivo expanded NK cells from thalassemia patients showed an increase in the percentage of positive cells for different activating receptors like NKp30, NKp44 and NKG2D. After ex vivo NK cell expansion, expressions of activating receptors NKG2D, NKp30 and CD94 increased significantly but NKp44 was not significant. Inhibitory receptors Killer Cell Immunoglobulin-like Receptor 2DL1 (KIR2DL1) (CD158e) and KIR2DL2/ DL3 (CD158b) showed an insignificant upregulation of expressions. No essential changes in expressions of inhibitory receptors KIR3DL1 (CD158e) or the natural cytotoxicity receptor NKp46 could be demonstrated. Expression of inhibitory NKG2A was increased significantly.
The activated NK cell phenotype was accompanied by a considerable increase in cytotoxic activity against K562 cells with comparable values of specific lysis at an effector to target ratio of 10:1 in mean 78.6 %±6.0 % (fig. 2C).
To confirm the cytotoxicity of NK cells after ex vivo expansion against human leukaemia cells, we test this toxicity in vivo using humanized NSG mice. 24 h after injection of leukaemia cells, we started the treatment with ex vivo expanded NK cells alone or with gamma delta (γδ) cells and CD20 Immunoglobulin G 1 (IgG1) antibody, we analyzed the effect of treatment on the survival of mice.
The survival curves are shown in fig. 2C. The cumulative percentage survival was significantly higher after treatment with NK cells, γδ cells and CD20 IgG1 antibody in comparison with the untreated mice (67.1 %). In general, treatment with NK cells increased survival rate of mice.
The results of this study indicate that NK cells can be efficiently ex vivo expanded and activated despite functional impairment. Co-culture with irradiated K562 cells genetically modified to express membrane- bound IL-15 and 4-1BB ligand shifted the NK cell receptor balance towards activation, resulting in enhanced cytotoxicity against tumor cell lines. A major hurdle in cancer immunotherapy has been the various mechanisms by which tumors induce dysfunction or tolerance of immune cells [15]. To use NK cells as therapeutic approach for the treatment of cancer, a reversal of phenotypic and functional defects is of paramount importance. Before ex vivo expansion NK cells from patients showed reduced expression of activating receptors and no cytotoxic activity against tumor cell lines. This tumor-associated NK cell phenotype is a common phenomenon in patients with different kind of malignancies, including Ewing’s sarcoma [16,17].
Besidesthe re-establishment of functionality, the number, purity and state of activation of NK cells are the key factors to be considered for adoptive immunotherapy. Co-culture of irradiated K562-mb15-4-1BBL feeder cells with PBMC, resulted in large quantities of highly activated and enriched NK cells. Similar results have been obtained by several investigators using similar NK cell expansion protocols with PBMC from patients with leukemia [8], various solid tumors including gastric, lung, colon and hepatocellular carcinoma [18] and myeloma [19]. It should be highlighted that the absolute level of NK cells needed to induce substantial tumor cytoreduction which is unknown. The magnitude of NK cell cytotoxicity is directly proportional to the ratio between the number of NK cells and target cells. Thus, the infusion of large numbers of preactivated NK cells is predicted to achieve a maximum anticancer effect. In addition, a critical prerequisite for efficient NK cell- based immunotherapies seems to be the reduction of the tumor mass by surgical removal, chemotherapy or radiation treatment to give the effector cells a numerical advantage. Adoptively transferred ex vivo expanded and activated NK cells have been evaluated for cancer immunotherapy and were found to improve clinical responses without any obvious side effects in patients with various malignancies.
Several strategies to improve NK cell function have been suggested, including blocking of inhibitory receptors with anti-KIR antibodies [20] or small interfering Ribonucleic Acids (RNAs) [21] and genetic manipulation of NK cells to overexpress activating receptors.
Immunomodulatory drugs such as lenalidomide influence NK cell function, either directly or via dendritic cell or T cell stimulation, leading to activation and expansion of NK cells [22,23]. Recent reports have shown that chemotherapy (5-Fluorouracil (5-FU), Cytosine Arabinoside (Ara-C), cisplatin) and radiation treatment targeting the Deoxyribonucleic Acid (DNA) damage pathway can increase the expression of NKG2D ligands on tumor cells, thus inducing enhanced NK cell cytotoxic activity [24]. Pre-treatment of subapoptotic concentrations of doxorubicin sensitized tumor cell lines to both NK and T cell-mediated lysis. This effect was primarily dependent on Tumor Necrosis Factor- Related Apoptosis-Inducing Ligand (TRAIL)/TRAIL receptor signaling, due to an increased expression of TRAIL receptor 2 on doxorubicin treated tumor cells and by downregulation of cellular FLICE inhibitory protein [25]. Bortezomib, a proteasome inhibitor is associated with the upregulation of Death Receptors (DR) Fas and DR5, both of which may be triggered by NK cells to initiate the apoptotic cascade [26]. Several studies have demonstrated that epigenetic drugs, such as Histone Deacetylase inhibitors (HDACi) or DNA Methyltransferase inhibitors (DNMTi) induce the expression of NKG2D ligands on various tumor cell lines, contributing to enhanced NK cell-mediated cytotoxicity [27-29].
Chemotherapy and radiation treatment have proven to be highly effective in eliminating rapidly proliferating tumor cells. However, recent evidence suggests that these therapeutic strategies are less effective at eliminating Cancer Stem Cells (CSCs), also referred to as tumor-initiating cells. CSCs were reported to have low proliferation rates, active DNA repair mechanisms and increased drug efflux capacity, all of which may contribute to their resistance to these treatment modalities [30,31]. Since CSCs possess self- renewal capability, tumorigenic capacity and the ability to form metastases [32], these cells represent an optimal therapeutic target to achieve complete tumor eradication. Recent reports have shown that CSCs are highly susceptible to NK cell cytotoxicity, suggesting that NK cells may be capable of targeting CSCs and non- CSCs populations alike [33-35]. Further studies are needed to analyze the cytotoxic effects of ex vivo expanded and activated NK cells against allogeneic and autologous CSCs, in order to completely evaluate the therapeutic benefits of NK cell-based immunotherapies.
Based on the data presented, ex vivo expansion and activation of NK cells deserves further clinical evaluation as a possible new treatment approach for patients with soft tissue or leukaemia. An ideal therapeutic concept for high-risk sarcoma patients might combine cellular immunotherapy protocols such as adoptive transfer of ex vivo expanded NK cells with immunomodulatory substances and/or tumor sensitizing agents to achieve maximum cytotoxic activity.
Author’s contributions:
All authors listed have made a substantial, direct and intellectual contribution to the work and approved it for publication.
Acknowledgements:
This work was funded by the University of Jeddah, Saudi Arabia, under grant No. UJ-08-18-ICP. The authors, therefore, acknowledges and thanks the University for technical and financial support.
Conflict of interests:
The authors declare that there is no conflict of interest.
References
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007;7(5):329-39.
[Crossref] [Google Scholar] [PubMed]
- Phillips JH, Lanier LL. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med 1986;164(3):814-25.
[Crossref] [Google Scholar] [PubMed]
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg S. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 1982;155(6):1823-41.
[Crossref] [Google Scholar] [PubMed]
- Benyunes MC, Massumoto C, York A, Higuchi CM, Buckner CD, Thompson JA, et al. Interleukin-2 with or without lymphokine-activated killer cells as consolidative immunotherapy after autologous bone marrow transplantation for acute myelogenous leukemia. Bone Marrow Transplant 1993;12(2):159-63.
[Google Scholar] [PubMed]
- Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2005;11(3):181-7.
[Crossref] [Google Scholar] [PubMed]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105(8):3051-7.
[Crossref] [Google Scholar] [PubMed]
- Cho D, Campana D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med 2009;29(2):89-96.
[Crossref] [Google Scholar] [PubMed]
- Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009;69(9):4010-7.
[Crossref] [Google Scholar] [PubMed]
- Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res 2010;16(15):3901-9.
[Crossref] [Google Scholar] [PubMed]
- Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H–2-deficient lymphoma variants suggests alternative immune defense strategy. Nature 1986;319(6055):675-8.
[Crossref] [Google Scholar] [PubMed]
- Ljunggren HG, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 1990;11:237-44.
[Crossref] [Google Scholar] [PubMed]
- Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol 2001;1(1):41-9.
[Crossref] [Google Scholar] [PubMed]
- Diefenbach A, Raulet DH. Strategies for target cell recognition by natural killer cells. Immunol Rev 2001;181(1):170-84.
[Crossref] [Google Scholar] [PubMed]
- Arase H, Lanier LL. Virus-driven evolution of natural killer cell receptors. Microbes Infect 2002;4(15):1505-12.
[Crossref] [Google Scholar] [PubMed]
- Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjöberg J, Pisa P, et al. Tumor-induced immune dysfunction. Cancer Immunol Immunother 1999;48(7):353-62.
[Crossref] [Google Scholar] [PubMed]
- Sutlu T, Alici E. Natural killer cell?based immunotherapy in cancer: Current insights and future prospects. J Intern Med 2009;266(2):154-81.
[Crossref] [Google Scholar] [PubMed]
- Verhoeven DH, de Hooge AS, Mooiman EC, Santos SJ, Monique M, Gelderblom H, et al. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Mol Immunol 2008;45(15):3917-25.
[Crossref] [Google Scholar] [PubMed]
- Voskens CJ, Watanabe R, Rollins S, Campana D, Hasumi K, Mann DL. Ex vivo expanded human NK cells express activating receptors that mediate cytotoxicity of allogeneic and autologous cancer cell lines by direct recognition and antibody directed cellular cytotoxicity. J Exp Clin Cancer Res 2010;29(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Garg TK, Szmania SM, Khan JA, Hoering A, Malbrough PA, Moreno-Bost A, et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica 2012;97(9):1348-56.
[Crossref] [Google Scholar] [PubMed]
- Romagné F, André P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti–KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 2009;114(13):2667-77.
[Crossref] [Google Scholar] [PubMed]
- Figueiredo C, Seltsam A, Blasczyk R. Class, gene and group-specific HLA silencing by lentiviral shRNA delivery. J Mol Med 2006;84(5):425-37.
[Crossref] [Google Scholar] [PubMed]
- Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 2001;98(1):210-6.
[Crossref] [Google Scholar] [PubMed]
- Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: Clinical application. Br J Haematol 2005;128(2):192-203.
[Crossref] [Google Scholar] [PubMed]
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005;436(7054):1186-90.
[Crossref] [Google Scholar] [PubMed]
- Wennerberg E, Sarhan D, Carlsten M, Kaminskyy VO, D'Arcy P, Zhivotovsky B, et al. Doxorubicin sensitizes human tumor cells to NK cell?and T?cell?mediated killing by augmented TRAIL receptor signaling. Int J Cancer 2013;133(7):1643-52.
[Crossref] [Google Scholar] [PubMed]
- Hallett WH, Ames E, Motarjemi M, Barao I, Shanker A, Tamang DL, et al. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol 2008;180(1):163-70.
[Crossref] [Google Scholar] [PubMed]
- Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood 2008;111(3):1428-36.
[Crossref] [Google Scholar] [PubMed]
- Chávez-Blanco A, la Cruz-Hernández D, Dominguez GI, Rodriguez-Cortez O, Alatorre B, Perez-Cardenas E, et al. Upregulation of NKG2D ligands and enhanced natural killer cell cytotoxicity by hydralazine and valproate. Int J Oncol 2011;39(6):1491-9.
[Crossref] [Google Scholar] [PubMed]
- Schmiedel BJ, Arélin V, Gruenebach F, Krusch M, Schmidt SM, Salih HR. Azacytidine impairs NK cell reactivity while decitabine augments NK cell responsiveness toward stimulation. Int J Cancer 2011;128(12):2911-22.
[Crossref] [Google Scholar] [PubMed]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5(4):275-84.
[Crossref] [Google Scholar] [PubMed]
- Diehn M, Clarke MF. Cancer stem cells and radiotherapy: New insights into tumor radioresistance. J Natl Cancer Inst 2006;98(24):1755-7.
[Crossref] [Google Scholar] [PubMed]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer and cancer stem cells. Nature 2001;414(6859):105-11.
[Crossref] [Google Scholar] [PubMed]
- Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol 2009;182(6):3530-9.
[Crossref] [Google Scholar] [PubMed]
- Pietra G, Manzini C, Vitale M, Balsamo M, Ognio E, Boitano M, et al. Natural killer cells kill human melanoma cells with characteristics of cancer stem cells. Int immunol 2009;21(7):793-801.
[Crossref] [Google Scholar] [PubMed]
- Tseng HC, Arasteh A, Paranjpe A, Teruel A, Yang W, Behel A, et al. Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells. PLoS One 2010;5(7):e11590.
[Crossref] [Google Scholar] [PubMed]
 14 d expansion
14 d expansion