- *Corresponding Author:
- Shuhe Zhao Department of Neurology,Taiyuan Central Hospital, Taiyuan, Shanxi 030000, China E-mail: 18235198360@163.com
| Date of Received | 28 July 2023 |
| Date of Revision | 17 January 2023 |
| Date of Acceptance | 14 May 2024 |
| Indian J Pharm Sci 2024;86(3):1025-1031 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the protective effect of Cornus officinalis extracts on Alzheimer’s disease cell injury models and its underlying molecular mechanism. Pheochromocytoma cells were treated with 20 μM amyloid beta-peptide 25-35 to establish an Alzheimer’s disease cell injury model in vitro, which was recorded as amyloid beta-peptide 25-35 group. Cells affected by amyloid beta-peptide 25-35 were treated with different-doses of Cornus officinalis extracts and recorded as low-dose Cornus officinalis extracts group, medium-dose Cornus officinalis extracts group and high-dose Cornus officinalis extracts group. Pheochromocytoma cells transfected with si-NC/si-long non-coding RNA Rpph1, treated with 20 μM amyloid beta-peptide 25-35 and 80 mg/ml of Cornus officinalis extracts were recorded as high-dose Cornus officinalis extracts+si-NC group, high-dose Cornus officinalis extracts+si-long non-coding RNA Rpph1 group. Cell viability and apoptosis were examined using cell counting kit-8 assay and flow cytometry. Protein expression was tested by Western blot. Malondialdehyde, superoxide dismutase and catalase levels were assessed by measuring cell oxidative stress. Amyloid beta-peptide, tumor necrosis factor-alpha, interleukin-6 and interferon gamma levels were examined using enzyme-linked immunosorbent assay. Long non-coding RNA Rpph1 expression was detected using quantitative reverse transcriptase polymerase chain reaction. Amyloid beta-peptide 25-35 treatment decreased Pheochromocytoma cell viability, CyclinD1, superoxide dismutase, catalase and long non-coding RNA Rpph1 levels, while increased apoptosis rate, cleaved-caspase-3, malondialdehyde, amyloid beta-peptide, tumor necrosis factor-alpha, interleukin-6 and interferon gamma levels. After treatment with different-doses of Cornus officinalis extracts, cell viability, CyclinD1, superoxide dismutase, catalase and long non-coding RNA Rpph1 levels were enhanced, while apoptosis rate, cleaved-caspase-3, malondialdehyde, Aβ, tumor necrosis factor-alpha, interleukin-6 and interferon gamma levels were reduced in pheochromocytoma cells treated with amyloid beta-peptide 25-35. Downregulation of long non-coding RNA Rpph1 reversed the inhibitory effect of Cornus officinalis extracts on cell injury. Cornus officinalis extracts relieved amyloid beta-peptide 25-35-induced nerve cell injury by upregulating long non-coding RNA Rpph1, suggesting that Cornus officinalis extracts might be used in Alzheimer's disease treatment.
Keywords
Cornus officinalis extracts, Alzheimer’s disease, long non-coding RNA Rpph1, caspase-3, interferon gamma, tumor necrosis factor-alpha
Alzheimer’s Disease (AD), a chronic degenerative neurological disease, is the most common type of dementia in the elderly[1,2]. Clinically, AD is often characterized by the deterioration of cognitive and memory functions, accompanied by mental abnormalities and social life dysfunction[3,4]. AD incidence has increased significantly with the progress of population aging, which brings a huge burden to society[5,6]. Therefore, developing effective therapeutic drugs for AD has become a global research focus.
Traditional Chinese Medicine (TCM) has the effect of preventing and treating AD[7,8]. Cornus officinalis (CO) is a rare TCM in our country, which has antioxidant, anti-inflammatory and neuroprotective effects[9,10]. Loganin, an iridoid glycoside extracted from CO, had been confirmed to improve the cognitive impairment of AD mice models[11]. Besides, iridoid glycosides of CO could inhibit Tau hyperphosphorylation and aggregation by activating protein phosphatase 2A, thus preventing neuron loss[12]. Importantly, cornuside and gallanthin extracted from CO could reduce the activities of ChE and Beta-Amyloid Cleaving Enzyme 1 (BACE1)[13], the important enzymes for AD progression[14,15]. The above studies suggest that CO extracts may have anti-AD effects, but the specific effects and mechanisms remain unclear.
Long non-coding RNA (lncRNA) Rpph1 attenuated Amyloid Beta-peptide (Aβ) 25-35-induced SH-SY5Y cell endoplasmic reticulum stress and apoptosis[16]. Besides, Rpph1 could improve Aβ-induced neuronal apoptosis[17]. Thus, lncRNA Rpph1 may be an important regulator of AD progression. In this, we found that CO could inhibit lncRNA Rpph1 expression. However, whether CO extracts exert its neuroprotective effect by inhibiting lncRNA Rpph1 expression remains unclear. Our study aimed to investigate the effect of CO extracts on Aβ25-35-induced nerve cell injury and whether it was related to lncRNA Rpph1 expression.
Materials and Methods
Cell culture and treatment:
Pheochromocytoma (PC12) cells (Procell, Wuhan, China) were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium plus 10 % Fetal Bovine Serum (FBS) and 1 % penicillin-streptomycin. Cells were treated with 20 μM Aβ25-35 (Sigma-Aldrich, St. Louis, MO, United States of America (USA)) for 24 h to record as Aβ25-35 group. Cells treated with Aβ25-35 and different-doses (20, 40 and 80 mg/ml) of CO extracts (KINGREEN, Xian, China) were recorded as low-dose CO extracts group, medium-dose CO extracts group and high-dose CO extracts group. Cells were transfected with si-NC/si-LncRNA Rpph1, treated with 20 μM Aβ25-35 and 80 mg/ml CO extracts, which were recorded as high-dose CO extracts+si-NC group, high-dose CO extracts+si-LncRNA Rpph1 group.
Cell Counting Kit-8 (CCK-8) assay:
PC12 cells in each group were re-seeded in 96-well plates. Following, cells were incubated with CCK-8 reagent (Dojindo, Kumamoto, Japan) and absorbance (A) value was tested by microplate reader to assess cell viability.
Flow cytometry:
Collected PC12 cells were dyed by Annexin V/Fluorescein Isothiocyanate (FITC) and Propidium Iodide (PI) (Abcam, Cambridge, MA, USA). Cell Apoptosis rate was examined under flow cytometer.
Western blot:
Protein samples isolated from PC12 cells were separated and transferred to Polyvinylidene Difluoride (PVDF) membranes, followed by incubated with anti-CyclinD1 (1:200, ab16663), anti-cleaved-caspase-3 (1:1000, ab2302), anti-β-actin (1:200, ab115777) and secondary antibody (1:2000, ab205718). Protein signals were examined by Enhanced Chemiluminescence (ECL) reagent (Beyotime, Shanghai, China), ChemiDoc XRS+imaging system and Quantity One software.
Measurement of oxidative stress:
Malondialdehyde (MDA), Superoxide Dismutase (SOD) and Catalase (CAT) levels in PC12 cells were tested by MDA Assay Kit (ab118970, Abcam), SOD Activity Assay Kit (ab65354, Abcam) and CAT activity assay kit (ab83464, Abcam) according to kit instructions, respectively.
Enzyme-Linked Immunosorbent Assay (ELISA):
Aβ, Tumor Necrosis Factor-Alpha (TNF-α), Interleukin (IL)-6 and Interferon Gamma (IFN-γ) levels in PC12 cells were analyzed by Aβ ELISA Kit (JL10958-48T, Jianglai, Shanghai, China), TNF-α ELISA Kit (JL13202-48T, Jianglai), IL-6 ELISA Kit (JL20896-48T, Jianglai) and IFN-γ ELISA Kit (JL13241-48T, Jianglai), respectively.
Reverse Transcriptase quantitative Polymerase Chain Reaction (RT-qPCR):
Extracted Ribonucleic Acid (RNAs) from PC12 cells was reverse-transcribed into complementary Deoxyribonucleic Acid (cDNA). SYBR Green was used for PCR with specific primers as below. LncRNA Rpph1, forward: 5'-CGAGCTGAGTGCGTCCTGTC-3', Reserved: 5'- TCGCTGGCCGTGAGTCTGT-3'; Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH), forward: 5'-ACAGCAACAGGGTGGTGGAC-3', Reserved; 5'-TTTGAGGGTGCAGCGAACTT-3'. Relative lncRNA Rpph1 level was analyzed with the 2−ΔΔCt method.
Statistical analysis:
Results were presented as mean±Standard Deviation (SD) by Statistical Package for the Social Sciences (SPSS) 20.0 software. Differences were evaluated by Student’s t-test or Analysis of Variance (ANOVA). p<0.05 was considered statistically significant.
Results and Discussion
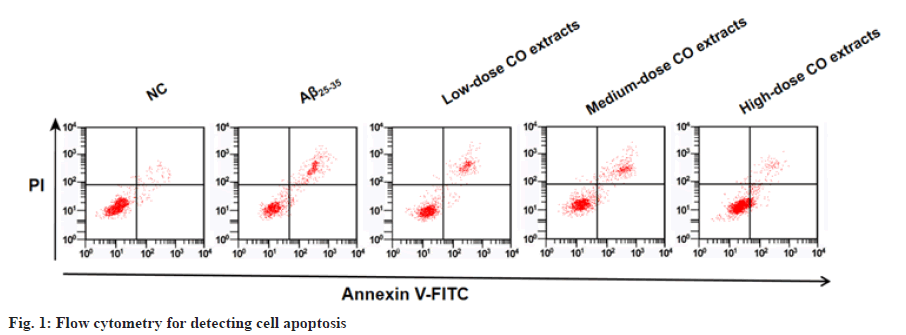
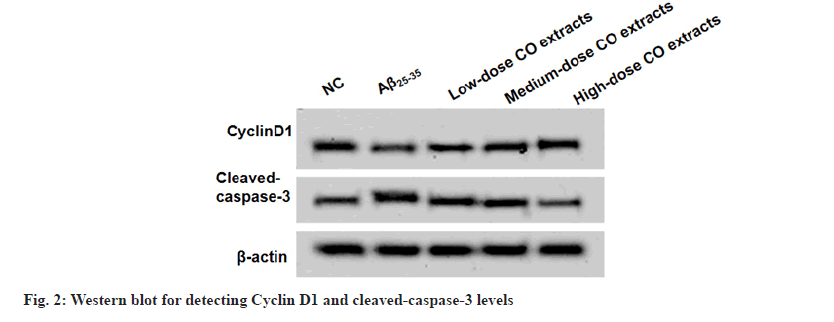
After Aβ25-35 treatment, PC12 cell viability was restrained and apoptosis was enhanced. Under the treatment of CO extracts, PC cell viability was increased and apoptosis was inhibited (fig. 1 and Table 1). Aβ25-35 treatment decreased CyclinD1 level and improved cleaved-caspase-3 level. However, CO extracts markedly enhanced CyclinD1 level and reduced cleaved-caspase-3 level in Aβ25-35-induced PC12 cells (fig. 2 and Table 2).
| Groups | A value | Apoptosis rate % |
|---|---|---|
| NC | 0.902±0.07 | 7.12±0.34 |
| Aβ25-35 | 0.448±0.03* | 25.41±1.52* |
| Low-dose CO extracts | 0.593±0.03# | 21.35±1.25# |
| Medium-dose CO extracts | 0.763±0.05# | 14.81±1.03# |
| High-dose CO extracts | 0.851±0.07# | 9.43±0.52# |
| F | 113.200 | 507.325 |
| p | 0.000 | 0.000 |
Notes: *p<0.05 to NC group and #p<0.05 to Aβ25-35 group
Table 1: Co Extracts Regulated Aβ25-35-Induced PC12 Cell Viability and Apoptosis
| Groups | CyclinD1 | Cleaved-caspase-3 |
|---|---|---|
| NC | 0.83±0.05 | 0.32±0.02 |
| Aβ25-35 | 0.42±0.02* | 0.91±0.07* |
| Low-dose CO extracts | 0.57±0.03# | 0.70±0.05# |
| Medium-dose CO extracts | 0.71±0.05# | 0.52±0.03# |
| High-dose CO extracts | 0.78±0.05# | 0.37±0.02# |
| F | 142.517 | 293.39 |
| p | 0.000 | 0.000 |
Notes: *p<0.05 to NC group and #p<0.05 to Aβ25-35 group
Table 2: Different Doses of Co Extracts Regulated CYCLIND1 and Cleaved-CASPASE-3 Levels
| Groups | MDA (μmol/g) | SOD (U/mg) | CAT (U/mg) |
|---|---|---|---|
| NC | 18.12±1.01 | 12.13±0.86 | 10.56±0.71 |
| Aβ25-35 | 34.25±2.17* | 4.16±0.21* | 4.89±0.27* |
| Low-dose CO extracts | 30.13±1.23# | 7.13±0.46# | 6.07±0.41# |
| Medium-dose CO extracts | 24.81±1.11# | 10.05±0.73# | 8.27±0.61# |
| High-dose CO extracts | 21.06±1.24# | 11.91±0.81# | 9.48±0.53# |
| F | 193.840 | 238.263 | 177.890 |
| p | 0.000 | 0.000 | 0.000 |
Notes: *p<0.05 to NC group and #p<0.05 to Aβ25-35 group
Table 3: CO Extracts Regulated MDA, SOD AND CAT Levels
| Groups | Aβ (ng/l) | TNF-α (ng/l) | IL-6 (ng/l) | IFN-γ (ng/l) |
|---|---|---|---|---|
| NC | 283.16±15.13 | 75.46±4.12 | 35.69±2.16 | 15.67±1.02 |
| Aβ25-35 | 427.32±22.56* | 179.26±11.26* | 80.57±5.14* | 45.97±3.14* |
| Low-dose CO extracts | 383.72±18.49# | 151.02±10.43# | 61.27±4.86# | 38.93±3.07# |
| Medium-dose CO extracts | 327.17±21.43# | 114.06±9.16# | 48.62±3.19# | 27.53±1.55# |
| High-dose CO extracts | 298.52±18.83# | 90.27±4.13# | 39.02±1.76# | 19.71±1.05# |
| F | 86.581 | 234.117 | 222.217 | 307.410 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Table 4: Effects of CO Extracts on Aβ and Inflammation
Aβ25-35 treatment promoted MDA level, while decreased SOD and CAT levels in PC12 cells. However, MDA level was reduced, while SOD and CAT levels were enhanced with the increasing of CO extracts (Table 3). Aβ25-35 treatment enhanced Aβ, TNF-α, IL-6 and IFN-γ levels in PC12 cells, while CO extracts reduced their levels in Aβ25-35-induced PC12 cells (Table 4). Aβ25-35 treatment reduced lncRNA Rpph1 expression in PC12 cells, while CO extracts could promote lncRNA Rpph1 expression in a dose-dependent manner (Table 5).
| Groups | Aβ (ng/l) | TNF-α (ng/l) | IL-6 (ng/l) | IFN-γ (ng/l) |
|---|---|---|---|---|
| NC | 283.16±15.13 | 75.46±4.12 | 35.69±2.16 | 15.67±1.02 |
| Aβ25-35 | 427.32±22.56* | 179.26±11.26* | 80.57±5.14* | 45.97±3.14* |
| Low-dose CO extracts | 383.72±18.49# | 151.02±10.43# | 61.27±4.86# | 38.93±3.07# |
| Medium-dose CO extracts | 327.17±21.43# | 114.06±9.16# | 48.62±3.19# | 27.53±1.55# |
| High-dose CO extracts | 298.52±18.83# | 90.27±4.13# | 39.02±1.76# | 19.71±1.05# |
| F | 86.581 | 234.117 | 222.217 | 307.410 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Notes: *p<0.05 to NC group and #p<0.05 to Aβ25-35 group
Table 4: Effects of CO Extracts on Aβ and Inflammation
| Groups | LncRNA Rpph1 |
|---|---|
| NC | 1.00±0.10 |
| Aβ25-35 | 0.41±0.02* |
| Low-dose CO extracts | 0.61±0.03# |
| Medium-dose CO extracts | 0.79±0.05# |
| High-dose CO extracts | 0.94±0.07# |
| F | 141.618 |
| p | 0.000 |
Notes: *p<0.05 to NC group and #p<0.05 to Aβ25-35 group
Table 5: CO Extracts Enhanced LNCRNA RPPH1 Expression
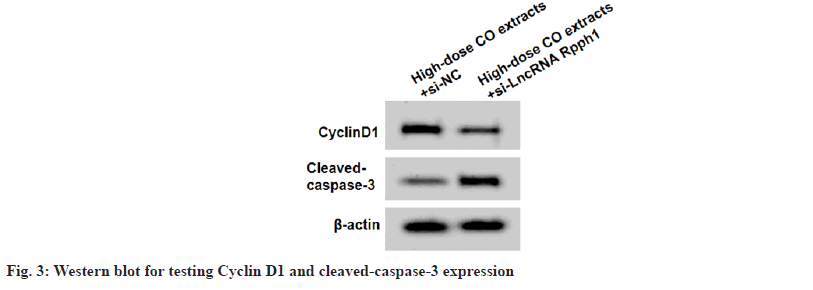
In High-dose CO extracts+si-LncRNA Rpph1 group, lncRNA Rpph1 expression, CyclinD1 level, cell viability, SOD and CAT levels were reduced, while cleaved-caspase-3 level, apoptosis rate, MDA, Aβ, TNF-α, IL-6 and IFN-γ levels were increased (fig. 3 and Table 6).
| Groups | LncRNA Rpph1 | CyclinD1 | Cleaved caspase-3 | A value | Apoptosis rate (%) | MDA (μmol/g) | SOD (U/mg) | CAT (U/mg) | Aβ (ng/l) | TNF-α (ng/l) | IL-6 (ng/l) | IFN-γ (ng/l) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-dose CO extracts+si-NC | 1.00±0.07 | 0.78±0.05 | 0.38±0.02 | 0.857±0.05 | 9.31±0.67 | 21.11±1.32 | 11.88±0.71 | 9.42±0.69 | 291.52±15.41 | 88.37±5.12 | 39.46±2.04 | 19.49±1.15 |
| High-dose CO extracts+si-LncRNA Rpph1 | 0.48±0.02* | 0.40±0.02* | 0.86±0.07* | 0.438±0.03* | 26.81±1.54* | 36.54±2.47* | 5.43±0.35* | 4.20±0.31* | 407.10±28.13* | 184.29±10.44* | 83.25±6.73* | 48.76±2.91* |
| t | 21.428 | 21.169 | 19.780 | 21.557 | 31.261 | 16.529 | 24.445 | 20.702 | 10.811 | 24.747 | 18.681 | 28.063 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Notes: *p<0.05 to NC group and #p<0.05 to Aβ25-35 group
Table 6: LNCRNA RPPH1 Knockdown Reversed the effect of Co Extracts On Cell Injury
With the increasing number of AD patients, more and more attention has been paid to AD pathogenesis. AD development is not completely clear, mainly involving excessive deposition of Aβ, hyper phosphorylation of Tau protein, cholinergic dysfunction, inflammatory response, oxidative stress and apoptosis[18-20]. Studies have found that the AD in vitro cell model can be simulated by inducing PC12 cell injury with Aβ25-35[21,22]. MDA, SOD and CAT are oxidative stress-related factors[23] and TNF-α, IL-6 and IFN-γ are pro-inflammatory-related factors[24]. It had been reported that IL-6, TNF-α, IL-1β, IFN-γ and MDA levels were elevated, while SOD and CAT levels were reduced in AD models[25,26]. In this, PC12 cell viability was decreased, while cell apoptosis, oxidative stress, inflammation and Aβ level were increased under Aβ25-35 treatment. Therefore, AD cell injury model was successfully established in this study.
Ethanol extract of CO protected keratinocytes from oxidative stress caused by particulate matter[27]. Iridoid glycosides of CO had been confirmed to repress the production of pro-inflammatory factors[28]. Cornuside, an iridoid glycoside from CO, could significantly reduce TNF-α, IL-6 and MDA levels in AD mice models[29]. These results indicate that CO extracts have obvious antioxidant and anti-inflammatory effects. To investigate whether CO extracts played a negative role in AD, PC12 cells were treated with Aβ25-35 and CO extracts. The results suggested that MDA, Aβ, TNF-α, IL-6 and IFN-γ levels were suppressed, while SOD and CAT levels were improved by CO extracts, indicating that CO extracts inhibited Aβ25-35-induced oxidative stress and inflammation in PC12 cells. Additionally, iridoid glycosides of CO had neuroprotective effects on traumatic brain injury by inhibiting apoptosis and promoting nerve repair[30]. Our data confirmed that CO extracts promoted PC12 cell viability and reduced apoptosis under Aβ25-35 treatment.
Aberrant expression of lncRNA Rpph1 has been implicated in human disease progression. For example, lncRNA Rpph1 was overexpressed in colorectal cancer, which could enhance cell proliferation and metastasis[31]. Also, high lncRNA Rpph1 was confirmed to promote hepatocellular carcinoma malignancy progression by increasing cell growth and metastasis[32]. LncRNA Rpph1 has been shown to restrain the apoptosis of nerve cells[16,17], indicating that it may participate in regulating AD progression. In this, we observed low lncRNA Rpph1 expression in PC12 cells after induced by Aβ25-35 and confirmed the increasing effect of CO extracts on lncRNA Rpph1 expression. Moreover, the reversal effect of lncRNA Rpph1 knockdown on CO extracts-mediated cell injury inhibition confirmed that CO extracts increased lncRNA Rpph1 expression to suppress Aβ25-35-induced PC12 cell injury.
In conclusion, our study reveals a novel molecular mechanism by which CO extracts inhibited AD progression. This study suggested that CO extracts might relieve Aβ25-35-induced nerve cell injury by up regulating lncRNA Rpph1. These results provide new evidence for the use of CO extracts in AD treatment and show that lncRNA Rpph1 may be a potential therapeutic target for AD.
Conflict of interests:
The authors declared no conflict of interests.
References
- Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer's disease. Lancet 2021;397(10284):1577-90.
- Rostagno AA. Pathogenesis of Alzheimer's disease. Int J Mol Sci 2022;24(1).
- Li YD, Luo YJ, Xie L, Tart DS, Sheehy RN, Zhang L, et al. Activation of hypothalamic-enhanced adult-born neurons restores cognitive and affective function in Alzheimer’s disease. Cell Stem Cell 2023;30(4):415-32.
[Crossref] [Google Scholar] [PubMed]
- Reiss AB, Muhieddine D, Jacob B, Mesbah M, Pinkhasov A, Gomolin IH, et al. Alzheimer’s disease treatment: The search for a breakthrough. Medicina 2023;59(6):1084.
[Crossref] [Google Scholar] [PubMed]
- Tay LX, Ong SC, Tay LJ, Ng T, Parumasivam T. Economic burden of Alzheimer’s disease: A systematic review. Value Health Reg Issues 2024;40:1-2.
[Crossref] [Google Scholar] [PubMed]
- Monteiro AR, Barbosa DJ, Remião F, Silva R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem Pharmacol 2023;211:115522.
[Crossref] [Google Scholar] [PubMed]
- Ding MR, Qu YJ, Hu B, An HM. Signal pathways in the treatment of Alzheimer’s disease with traditional Chinese medicine. Biomed Pharmacother 2022;152:113208.
[Crossref] [Google Scholar] [PubMed]
- Pei H, Ma L, Cao Y, Wang F, Li Z, Liu N, et al. Traditional Chinese medicine for Alzheimer’s disease and other cognitive impairment: A review. Am J Chin Med 2020;48(03):487-511.
[Crossref] [Google Scholar] [PubMed]
- Quah Y, Lee SJ, Lee EB, Birhanu BT, Ali MS, Abbas MA, et al. Cornus officinalis ethanolic extract with potential anti-allergic, anti-inflammatory and antioxidant activities. Nutrients 2020;12(11):3317.
[Crossref] [Google Scholar] [PubMed]
- Tian W, Zhao J, Lee JH, Akanda MR, Cho JH, Kim SK, et al. Neuroprotective effects of Cornus officinalis on stress-induced hippocampal deficits in rats and H2O2-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Antioxidants 2019;9(1):27.
[Crossref] [Google Scholar] [PubMed]
- Nie L, He K, Xie F, Xiao S, Li S, Xu J, et al. Loganin substantially ameliorates molecular deficits, pathologies and cognitive impairment in a mouse model of Alzheimer’s disease. Aging (Albany NY) 2021;13(20):23739-56.
[Crossref] [Google Scholar] [PubMed]
- Ma D, Luo Y, Huang R, Zhao Z, Wang Q, Li L, Zhang L. Cornel iridoid glycoside suppresses tau hyperphosphorylation and aggregation in a mouse model of tauopathy through increasing activity of PP2A. Curr Alzheimer Res 2019;16(14):1316-31.
[Crossref] [Google Scholar] [PubMed]
- Bhakta HK, Park CH, Yokozawa T, Tanaka T, Jung HA, Choi JS. Potential anti-cholinesterase and β-site amyloid precursor protein cleaving enzyme 1 inhibitory activities of cornuside and gallotannins from Cornus officinalis fruits. Arch Pharm Res 2017;40:836-53.
[Crossref] [Google Scholar] [PubMed]
- Adewole KE, Ishola AA. BACE1 and cholinesterase inhibitory activities of compounds from Cajanus cajan and Citrus reticulata: An in silico study. In Silico Pharmacol 2021;9(1):14.
[Crossref] [Google Scholar] [PubMed]
- Saeedi M, Iraji A, Vahedi-Mazdabadi Y, Alizadeh A, Edraki N, Firuzi O, et al. Cinnamomum verum J. Presl. Bark essential oil: In vitro investigation of anti-cholinesterase, anti-BACE1 and neuroprotective activity. BMC Complement Med Ther 2022;22(1):303.
[Crossref] [Google Scholar] [PubMed]
- Gu R, Liu R, Wang L, Tang M, Li SR, Hu X. LncRNA RPPH1 attenuates Aβ25-35-induced endoplasmic reticulum stress and apoptosis in SH-SY5Y cells via miR-326/PKM2. Int J Neurosci 2021;131(5):425-32.
[Crossref] [Google Scholar] [PubMed]
- Gu R, Wang L, Tang M, Li SR, Liu R, Hu X. LncRNA Rpph1 protects amyloid-β induced neuronal injury in SK-N-SH cells via miR-122/Wnt1 axis. Int J Neurosci 2020;130(5):443-53.
[Crossref] [Google Scholar] [PubMed]
- Athar T, Al Balushi K, Khan SA. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol Biol Rep 2021;48(7):5629-45.
[Crossref] [Google Scholar] [PubMed]
- Ozben T, Ozben S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer's disease. Clin Biochem 2019;72:87-9.
[Crossref] [Google Scholar] [PubMed]
- Breijyeh Z, Karaman R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecule 2020;25(24):5789.
- Zhang YY, Bao HL, Dong LX, Liu Y, Zhang GW, An FM. Silenced lncRNA H19 and up-regulated microRNA-129 accelerates viability and restrains apoptosis of PC12 cells induced by Aβ25-35 in a cellular model of Alzheimer’s disease. Cell Cycle 2021;20(1):112-25.
[Crossref] [Google Scholar] [PubMed]
- Liu N, Lyu X, Zhang X, Zhang F, Chen Y, Li G. Astaxanthin attenuates cognitive deficits in Alzheimer’s disease models by reducing oxidative stress via the SIRT1/PGC-1α signaling pathway. Cell Biosci 2023;13(1):173.
[Crossref] [Google Scholar] [PubMed]
- Celik Topkara K, Kilinc E, Cetinkaya A, Saylan A, Demir S. Therapeutic effects of carvacrol on beta‐amyloid‐induced impairments in in vitro and in vivo models of Alzheimer's disease. Eur J Neurosci 2022;56(9):5714-26.
[Crossref] [Google Scholar] [PubMed]
- Tsagkaris C, Bilal M, Aktar I, Aboufandi Y, Tas A, Aborode AT, et al. Cytokine storm and neuropathological alterations in patients with neurological manifestations of COVID-19. Curr Alzheimer Res 2022;19(9):641-57.
[Crossref] [Google Scholar] [PubMed]
- Du Q, Zhu X, Si J. Angelica polysaccharide ameliorates memory impairment in Alzheimer’s disease rat through activating BDNF/TrkB/CREB pathway. Exp Biol Med 2020;245(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Gao D, Hao JP, Li BY, Zheng CC, Miao BB, Zhang L, et al. Tetrahydroxy stilbene glycoside ameliorates neuroinflammation for Alzheimer's disease via cGAS-STING. Eur J Pharmacol 2023;953:175809.
[Crossref] [Google Scholar] [PubMed]
- Fernando PD, Piao MJ, Zhen AX, Ahn MJ, Yi JM, Choi YH, et al. Extract of Cornus officinalis protects keratinocytes from particulate matter-induced oxidative stress. Int J Med Sci 2020;17(1):63-70.
[Crossref] [Google Scholar] [PubMed]
- Yuan J, Cheng W, Zhang G, Ma Q, Li X, Zhang B, et al. Protective effects of iridoid glycosides on acute colitis via inhibition of the inflammatory response mediated by the STAT3/NF-кB pathway. Int Immunopharmacol 2020;81:106240.
[Crossref] [Google Scholar] [PubMed]
- Shi JZ, Zheng XM, Zhou YF, Yun LY, Luo DM, Hao JJ, et al. Cornuside is a potential agent against Alzheimer’s disease via orchestration of reactive astrocytes. Nutrients 2022;14(15):3179.
[Crossref] [Google Scholar] [PubMed]
- Ma D, Wang N, Fan X, Zhang L, Luo Y, Huang R, et al. Protective effects of cornel iridoid glycoside in rats after traumatic brain injury. Neurochem Res 2018;43(4):959-71.
[Crossref] [Google Scholar] [PubMed]
- Liang ZX, Liu HS, Wang FW, Xiong L, Zhou C, Hu T, et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis 2019;10(11):829.
[Crossref] [Google Scholar] [PubMed]
- Zhou J, Shi K, Huang W, Zhang Y, Chen Q, Mou T, et al. LncRNA RPPH1 acts as a molecular sponge for miR-122 to regulate Wnt1/β-catenin signaling in hepatocellular carcinoma. Int J Med Sci 2023;20(1):23.
[Crossref] [Google Scholar] [PubMed]