- *Corresponding Author:
- Shailasree Sekhar
Department of Biochemistry, School of Life Sciences, Mysuru, JSS Academy of Higher Education and Research (Deemed-to-be-University), Mysuru, Karnataka 570015, India
E-mail: shailasree@ioe.uni-mysore.ac.in
| Date of Received | 06 December 2021 |
| Date of Revision | 11 September 2023 |
| Date of Acceptance | 21 May 2024 |
| Indian J Pharm Sci 2024;86(3):904-914 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Premna serratifolia Linn, a medicinally prominent plant is widely used in Ayruvedic, Siddha and Homeopathy medicinal treatments. In this study, out of its leaf fungal endophytes, about 68 Chaetomium sp. exhibited highest percentage similarity to the nearest genera (98.6 %) and thus were evaluated for its therapeutic properties. Chaetomium ethyl acetate extract contained flavonoids and phenolic compounds exhibited promising antioxidant activity against the 2,2-diphenyl-1-picrylhydrazyl radical. Chaetomium ethyl acetate extract induced cytotoxicity to MDA-MB-231, human breast cancer cells. Maximum cytotoxic activity and cell death was obtained with half maximal effective concentration of 96.82±5.23 μg upon exposure for 48 h. Apoptosis with chromatin condensation was visualized with acridine orange and propidium iodide dual staining at these conditions. It could stimulate cancerous MDA-MB-231 cells to undergo apoptosis (60.24 %) as evidenced by flow cytometer analysis mediated through elevated levels of reactive oxygen species (7.5 folds). Such excess reactive oxygen species generation resulted structural injury of the mitochondrial membrane its dissipation of membrane potential as identified by shift from red to green fluorescence of 5,5,6,6'-tetrachloro-1,1',3,3' tetraethylbenzimi-dazoylcarbocyanine iodide dye. One of the bioactive bestowing therapeutic potential was identified as chrysin, a flavone, by high performance liquid chromatography analysis of the extract against the standard chrysin (Rt, 24.68 min). Thus, an effective MDA-MB-231 apoptotic event by the extract is reported.
Keywords
Chaetomium sp., MDA-MB-231 cells, apoptosis, mitochondrial membrane potential, reactive oxygen species, chrysin
Through several centuries, indigenous global cultures have been involved in arriving at strategies to manage a myriad of human health issues from natural resources. They include naturally occurring non-nutrient entities termed secondary metabolites reported by several researchers[1,2]. They begin to target specific exhibiting minimal or no-toxicity to constitutive cellular components have paved way for newer paradigms with wide range therapeutics[3].
As a fascinating group of microbes, the endophytes associated with several plant species have garnered attention for their metabolite diversity. Post discovery of Taxomyces andreanae, a fungal endophyte from the tree Pacific yew (Taxus brevifolia) producing taxol, endophyte documentation with therapeutic attributes has gained priority. With sampling, isolation and identification of endophytic fungi from less addressed flora gaining attention for their potential to produce novel molecules, such studies have provided an edge for manipulating the growth parameters resulting in increase in yield of metabolite in economically and ecofriendly precision. Endophyte extracts are rich sources of bioactives like alkaloids, chinones, flavonoids[4], phenols exhibiting pharmaceutical properties viz., antibacterial and anti-cancer due to their strong antioxidant strength revolutionizing pharmaceutical industry[5].
Premna serratifolia (P. serratifolia) (Arani or Agnimantha) is an important constituent of ten herb formulation in the Ayurvedic medicine in India, known as “Dasamula”, widely used for treating various ailments in Indian system of medicine. A few molecules reported include acetoside, a derivative of glucoside from its roots[6] and a potent cytotoxic diterpene with antioxidant property, 11,12,16-trihydroxy-2-oxo-5-methyl-10- demethyl-abieta-1[10],6,8,11,13-pentene reported from its root bark[7]. However, there were few reports of endophytes living in this host, which prompted us to investigate this therapeutic plant because lack of scientific screening of this medicinal plant.
Leaves of P. serratifolia were harvested and the endophytes residing in them were cultured of leaf fungal endophytes cultured (about 68), Chaetomium sp. exhibited highest percentage similarity to the nearest genera (98.6 %) and was evaluated for its therapeutic properties. In the present study, Chaetomium genus, generally a terrestrial and a marine alga, widely explored for its in situ metabolite contents with broad range of medicinal properties was identified for further evaluation.
Chaetomium sp. endophyte ethyl acetate extract was prepared and was evaluated for the biological activities by the procedure detailed in materials and methods. Briefly, the mycelial mat of the endophyte in the broth was sonicated and the whole mixture was filtered. The bioactives were extracted thrice in ethyl acetate. The extracts were pooled and the solvent was evaporated. The product Chaetomium Ethyl Acetate Extract (CEAE) obtained was solubilized and was used for further evaluation. It was identified as a mixture of flavonoids and phenolic compounds. It exhibited promising antioxidant activity against the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical. CEAE induced cytotoxicity to MDAMB- 231, human breast cancer cells via dysfunction of mitochondria inducing apoptosis. For the phytoconstitutent analysis High-Performance Liquid Chromatography (HPLC) was carried out. This analysis identified presence of chrysin, a flavone, against the standard chrysin (Rt, 24.68 min). Chrysin is reported to have several beneficial pharmacological activities[8]. Recent studies report on Chaetomium sp. to be rich source of bioactives as chrysin[9] and flavipin[10]. Further work is underway in our laboratory to enhance chrysin production from this fungus via optimizing media and growth parameters.
Materials and Methods
Collection of plant samples:
Leaf samples of P. serratifolia were collected from Kigga region of Western Ghats in Chikmagalore District of Karnataka State. Herbarium specimen has been deposited (Premna serrotifolia # IOE LP00019). The samples were stored in plastic bags, processed within 24-48 h of collection and sampled for the isolation of endophytic fungi.
Culturing of leaf endophytes:
The leaves collected were cleaned under tap water (5 times) resulting in clearing of dust and grim particles. Under sterile conditions, the leaves were rinsed with ethanol (70 %, v/v) for a minute, cleaned in sodium hypochlorite (3 min, containing 3 % accessible chlorine, 4.5 % v/v diluted, Himedia, India). Lastly a rinse was given using distilled water (sterile) resulting in removal of any remnants of sterilant from the surface. All leaf bits were then tapped resulting in removal of adhering water droplets. Leaf bits (10-15 bits; 0.5 cm-1 cm) were placed in media (sterile water agar, 13g.l-1) containing chloramphenicol (240 ppm; Sigma- Aldrich, St. Louis, MO, United States of America (USA)) in a sterile petri dish. After incubation (15 d for 15 h, 28°) under alternate dark with light cycles, the fungal endophytes sprouting from the bits of leaves were photographed. Maturation and sporulation was induced on Potato Dextrose Agar (PDA) media (2 %, Himedia, India). The grown fungal endophytes were stored (-80°) on PDA and glycerol stock (13 %, v/v) in cryovials.
Genomic Deoxyribonucleic Acid (DNA) isolation from fungal endophytes, its PCR amplification and molecular typing:
Initially fungal endophytes were identified depending on their colony parameters. Their spores and fruiting bodies were photographed under microsope (research stereo zoom microscope, stereo discovery V20, Carl Zeiss, Germany). Its typing was carried out as reported in standard manuals specifically for fungal identification[11]. An endophytic fungus was cultivated on PDA media and the fresh culture was harvested for further work. DNA from this culture was extracted by Cetyltrimethyl Ammonium Bromide (CTAB) protocol[11,12] and kept in buffer (Tris (tris (hydroxymethyl) aminomethane)- Ethylenediaminetetraacetic Acid (EDTA), 100 μl). Quantification of the genomic DNA and its purity was checked in nanospectrophotometer (Thermo 2000°, Thermo Fisher Scientific, USA). Pure sample with respect to desirability (1.6/1.8) indicating no contamination with Ribonucleic Acid (RNA), protein or contaminating phenol was confirmed by agarose gel electrophoresis and results were documented (Geldoc XRT, BioRad, USA). Universal Internal Transcribed Spacer (ITS) primers for fungi was used to amplify ITS regions of the extracted DNA [ITS1:5`- TCCGTAGGTGAACCTGCG G-3`; ITS4:5`- TCCTCCGCTTATTGATATGC-3`]. The reaction was carried out in Polymerase Chain Reaction (PCR) tubes (0.2 ml) containing reaction mixture (25 μl) including genomic DNA (1 μl; 50 ng. μl-1) in a thermal cycler (Master Cycler gradient, Eppendorf, Germany). The program was initial denaturation (5 min, 95° followed by 3 min, 94°), primer annealing (1 min, 55°) followed by extension (2 min, 72° and 10 min extension) and it was repeated for 35 cycles. The product of this PCR amplification (5 μl) was assessed on agarose gel (1 %) and the results were reported in comparison to commercially available standard molecular ladder. The amplified PCR samples were sequenced at Bangalore (Chromous Biotech). The resulting DNA sequences were analyzed by tool, Basic Local Alignment Search Tool (BLAST) with NCBI.
Preparation of endophyte secondary metabolite extracts:
Agar pieces (0.5 cm2) with actively growing fungal endophytic colony were inoculated onto sterile potato dextrose broth (PDB, 1 l). The flasks were incubated with alternate light (8 h) and dark cycles (16 h) in stationary phase (25±2°) for 15 d. The mycelial mat in the broth was sonicated and the whole mass was filtered. For extraction of bioactives, the filtrate was transferred to a separating funnel containing equal volumes of ethyl acetate and the mixture was strongly agitated. The solvent layer on the top was separated and condensed to 10 mg in Heidolpha evaporator with rotation at 42°. The procedure was carried out thrice. The fraction was dried and termed CEAE. It was stored (4°) in a colored glass container for further experimentation. It was used for all the further experiments.
Estimation of total phenol concentration in endophyte extract:
The phenol amount of endophyte extracts was determined according to calorimetric method[13,14]. The extract (100 μl, 1 mg.ml-1) was mixed with folin-ciocalteu reagent (0.75 ml, 1:10 diluted, HiMedia, India) and incubated (22°, 5 min). Saturated sodium carbonate (60 g.l-1) was added to neutralize it. Further incubation was in the dark (1.5 h, 22°). The absorbance of blue color was measured at 725 nm (Hitachi U-3900 ultraviolet/ visible spectrophotometer). Phenol content was estimated using standard curve of gallic acid (25- 250 μg/ml, Sigma-Aldrich, St. Louis, MO, USA). The concentration of phenol in the extract was reported as Gallic Acid Equivalence (GAE μg.g-1 extract). All the estimations were performed thrice in triplicates.
Quantifying total flavonoid content:
The extract (100 μl) was combined with methanolic solution of aluminum chloride (2 %, 100μl). After incubation (30 min), the absorbance of the reaction was read at 430 nm. Flavonoid content was reported using rutin standard analytical curve (10-100μg.ml-1, Sigma-Aldrich, St. Louis, MO, USA). The flavonoid concentration in the extract was expressed in mg rutin (μg.g-1 of extract). All estimations were performed thrice in triplicates.
DPPH radical scavenging assay:
DPPH obtained from Sigma-Aldrich (St. Louis, MO, USA) assay was carried out in dark (37°, 30 min) for estimation of the antioxidant potential following the reported protocol[13]. The results were measured at 517 nm (spectra max 340 colorimeter, molecular devises, USA). The reaction mixture included extract (5 μl) mixed with DPPH solution (95 μl, 300 μm). Scavenging activity of DPPH radical was estimated in comparison with a methanol (negative control), ascorbic acid and quercetin (25 to 250 μg.ml-1, Sigma-Aldrich, St. Louis, MO, USA) used as positive control. The values (IC50) representing the concentration of endophyte extract required to scavenge DPPH (50 %) radicals was reported.
Evaluation of Chaetomium sp. extract induced cell cytotoxicity
Cell lines, culture conditions and 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay: in vitro experiments were done using human cell lines, MDA-MB-231 (human breast cancer cells) and non-cancerous cell line (HEK 293 T). They were grown in Dulbecco's Modified Eagle Medium (DMEM), supplemented with FBS (10 %) as per reported protocol[15]. The cells (3×104 cells/ cm2), were plated in humidified CO2 (5%; 37°) and experiments were initiated. The culture medium was replaced two times a week. For the experiments, confluent cells were trypsinized and plated in 96-, 6-well plates, or into tissue culture dishes (6 mm).
Cell lines, culture conditions and 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay: in vitro experiments were done using human cell lines, MDA-MB-231 (human breast cancer cells) and non-cancerous cell line (HEK 293 T). They were grown in Dulbecco's Modified Eagle Medium (DMEM), supplemented with FBS (10 %) as per reported protocol[15]. The cells (3×104 cells/ cm2), were plated in humidified CO2 (5%; 37°) and experiments were initiated. The culture medium was replaced two times a week. For the experiments, confluent cells were trypsinized and plated in 96-, 6-well plates, or into tissue culture dishes (6 mm).
The cell viability was tested using MTT at 1 mg/ml dissolved in sterile Phosphate Buffer Saline (PBS; 0.05 M phosphate buffer, pH 7.2, 0.8 % sodium chloride (NaCl)) at room temperature. Different concentrations of CEAE (1-200 μg) were placed into the respectively labeled wells and incubated (48 h). MTT (10 μl) was added to each well in dark and the results were recorded after 4 h.
The supernatants were aspirated from the wells and washed thrice with PBS. Dimethyl sulfoxide (100 μl) and glycine buffer (25 μl, 0.1 m, pH 10.5) were added to each well. The absorbance was measured at 570 nm after 15 min incubation using multimode plate reader (Varioskan flash top, Thermo Fisher Scientific, Germany). Controls consisting of same concentration of cells without CEAE were maintained. Any absorbance due to reaction of the extract with MTT in wells devoid of cells was subtracted from the readings. Triplicate wells were assayed for each condition.
Apoptosis assessed by nuclear morphology:
Apoptotic nuclear morphological changes of MDAMB- 231 cells upon exposure to the extract were observed by dual staining with Acridine Orange/ Propidium Iodide (AO/PI). The cells (5×104 cells/ well) were seeded in 6-well plates on poly-LLysine coated cover slips (0.01 %, 24 mm) treated with CEAE (97 μg) and camptothecin (2 μg) for 48 h. Following incubation, they were washed with phosphate buffered saline twice and stained with AO/PI (1 mg.ml-1) mixture for 2-3 min. Apoptotic nuclear morphological changes were observed by Confocal Laser Scanning Microscopy (CLSM) Laser Scanning Microscopy (LSM) 710 (Carl Zeiss, Germany).
Apoptosis by CEAE via accumulation of cellular Reactive Oxygen Species (ROS):
Fluorescent, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; Sigma-Aldrich, St. Louis, MO, USA) probe was used in this study. The cells (2×105) were plated in culture vessels (6 mm) containing serum-rich DMEM for optimal growth and the extract (97 μg) was added. After incubation, they were washed with warm PBS. They were stained with H2DCF-DA (10 μmol/l) under dark in carbon dioxide (CO2) incubator for labeling the generated intracellular ROS. The controls were cells minus CEAE. The ROS labeled with H2DCFDA was measured (multimode plate reader, varioskan flash top, Thermo Fisher Scientific, Germany). The images were photographed by fluorescence microscope (AxioImager A2, Zeiss Germany).
Apoptosis by CEAE via of loss of mitochondrial membrane potential (Δψm):
The lipophilic cationic probe, 5,5′,6,6′-tetrachloro- 1,1′,3,3′-tetraethylbenzimidazolcarbcyanine iodide (JC-1; molecular probes, eugene, OR, USA) mostly used for measuring mitochondrial de-polarization was used in this study. The cells (2×105) were plated in culture vessels (6 mm) containing serum-rich DMEM for optimal growth. These cells were subjected to CEAE (97 μg). They were washed with warm PBS and stained with the dye (JC-1, 10μg/ml) under dark in CO2 incubator. After incubation (20 min, 37°) the intensity of fluorescence was measured by multimode plate reader as the function of changes in mitochondrial membrane potential[15].
Further, the cells were inoculated to grow on sterile poly-L-lysine slides (Sigma-Aldrich, MO, USA). They were subjected to CEAE (97 μg) for 72 h and then stained with 5,5,6,6'-tetrachloro-1,1',3,3' tetraethylbenzimi-dazoylcarbocyanine iodide dye (JC-1 dye) dye for 20 min. After incubation, cells were visualized and analyzed by fluorescence microscopy (advanced fluorescence research microscope with digital imaging, AxioImager A2, Zeiss, Germany).
Apoptosis analyzed by Annexin V Fluorescein Isothiocyanate (FITC)-PI labeling and flow cytometer analysis:
After exposure to CEAE (97 μg), these cells and control (minus CEAE) were trypsinized and centrifuged (103×g, 3 min). Cells (5×104) were re-suspended in PBS (200 μl) and incubated with Annexin V FITC-PI according to manufacturers’ protocol (Beckman Coulter, USA). Theoretically, cells stained by Annexin V were early apoptotic cells (lower right) and cells stained by PI were considered as necrotic (dead) cells (upper left). viable cells were not stained (lower left). Late apoptotic cells were double stained (upper right). A fluorescent activated flow cytometer (Cell Lab QuantaTM, SC, Beckman Coulter, USA) was used to examine the cells (1×104).
HPLC analysis:
HPLC analysis of the CEAE and standard chrysin solubilized in methanol and filtered (0.45 μm filter) was carried out in Agilent compact 1120 system using a C18 column with mobile phases of methanol (A, 50 %) and mixture of acetonitrile/ water (B, 50 %) at a run rate of 1 ml.min-1. The samples were detected at 310 nm[16].
Statistical analysis:
All experiments and measurements were made in triplicate. The values are expressed as the mean±standard deviation. The results were subjected to analysis of variance followed by Turkey’s test to analyse differences between the CEAE and control samples. Statistically significant differences (p value<0.001) are reported.
Results and Discussion
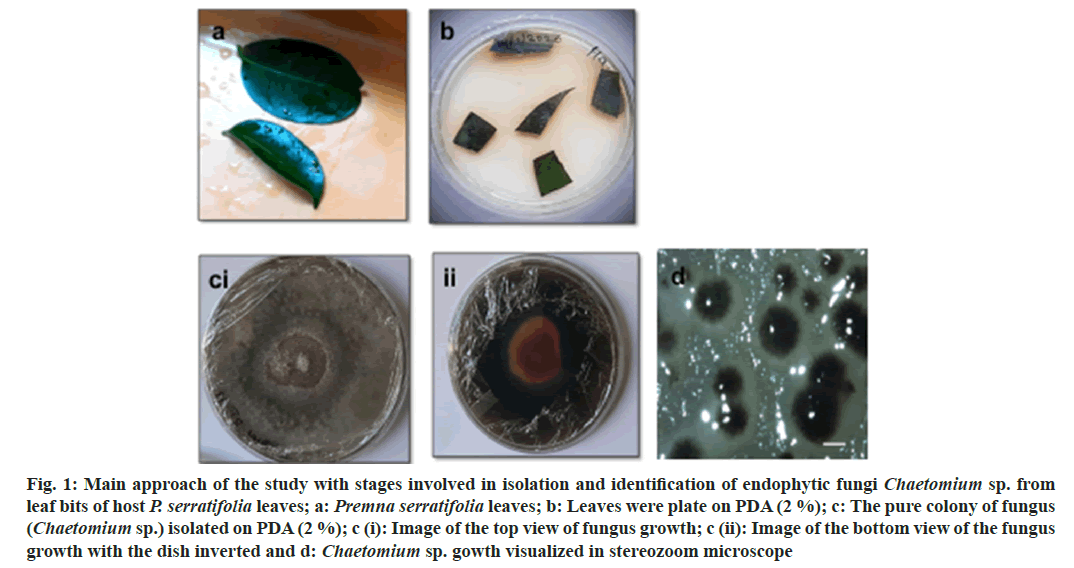
The present study was directed towards isolation and identified the fungal endophyte from the leaves of P. serratifolia Linn. a less discussed medicinal plant, a small tree growing along-side sea coasts. P. serratifolia endophytes identified by molecular typing include Colletotrichum sp., Chaetomium sp., Alternaria sp., Mucor sp., Penicillium sp., Aspergillus sp., and Fusarium sp., Chaetomium sp., was identified by the colony, spore morphology (fig. 1) and ITS analysis (Table 1). The isolated fungus exhibited specific dark green-yellow colony color and such characteristics were reported to be an indicator of its capacity to produce flavonoids and other bioactives. Further, molecular typing by ITS-DNA sequence analysis was carried out. The amplified ITS-DNA presented a clear band of 451 bp. The purified band was sequenced and the results analyzed by National Center for Biotechnology Information Basic Local Alignment Search (NCBI BLAST) identified the isolated endophyte as Chaetomium sp. with ~98.6 % similarity. Further work was carried out using the ethyl acetate extract of this endophyte.
Fig. 1: Main approach of the study with stages involved in isolation and identification of endophytic fungi Chaetomium sp. from leaf bits of host P. serratifolia leaves; a: Premna serratifolia leaves; b: Leaves were plate on PDA (2 %); c: The pure colony of fungus (Chaetomium sp.) isolated on PDA (2 %); c (i): Image of the top view of fungus growth; c (ii): Image of the bottom view of the fungus growth with the dish inverted and d: Chaetomium sp. gowth visualized in stereozoom microscope
| Endophyte key | Endophyte identified | DNA (ng/100mL) | Total DNA | Similarity index (%) | Accession No. w.r.t. GenBank |
|---|---|---|---|---|---|
| PS-6 | Colletotrichum sp. | 136 | 1.73 | Mucor souzae (98 %) | MG980301.1 |
| PS-14 | Chaetomium sp. | 182 | 1.85 | Colletotrichum sp. (98.6 %) | KJ6120621 |
| PS-23 | Alternaria sp. | 163 | 1.76 | Alternaria sp. (91 %) | KP309984.1 |
| PS-33 | Mucor sp. | 155 | 1.71 | Mucor sp. (94 %) | KY992878.1 |
| PS-37 | Penicillium sp. | 172 | 1.82 | Fusarium sp. (90 %) | MG600579.1 |
| PS-42 | Aspergillus sp. | 159 | 1.78 | Aspergillus sp. (94 %) | MT446088.1 |
| PS-51 | Fusarium sp. | 172 | 1.881 | Fusarium sp.(91 %) | MN626707.1 |
Note: PS: P. serratifolia
Table 1: Fungal Endophytes of P. Serratifolia Leaves Identified by its Analysis
Phenol concentration was expressed as Gallic Acid Equivalents (GAE; μg.g-1 of the extract). Flavonoid concentration is reported as rutin equivalents (μg.g-1 of the extract). CEAE contained a total phenol content of 181.28±0.42 (GAE, μg.g-1 of extract). Total flavonoid content of 87.19±0.55 (rutin equivalents, μg.g-1 of extract) was reported in the extract. DPPH radical scavenging property of CEAE were recorded and compared to the reference standards, ascorbic acid and quercetin. CEAE exhibited DPPH scavenging activity with the IC50 value of 51.33±3.21 μg.ml-1.The reference compounds, ascorbic acid and quercetin exhibited IC50 values of 28.14±2.19 and 28.32±1.73 μg.ml-1 respectively.
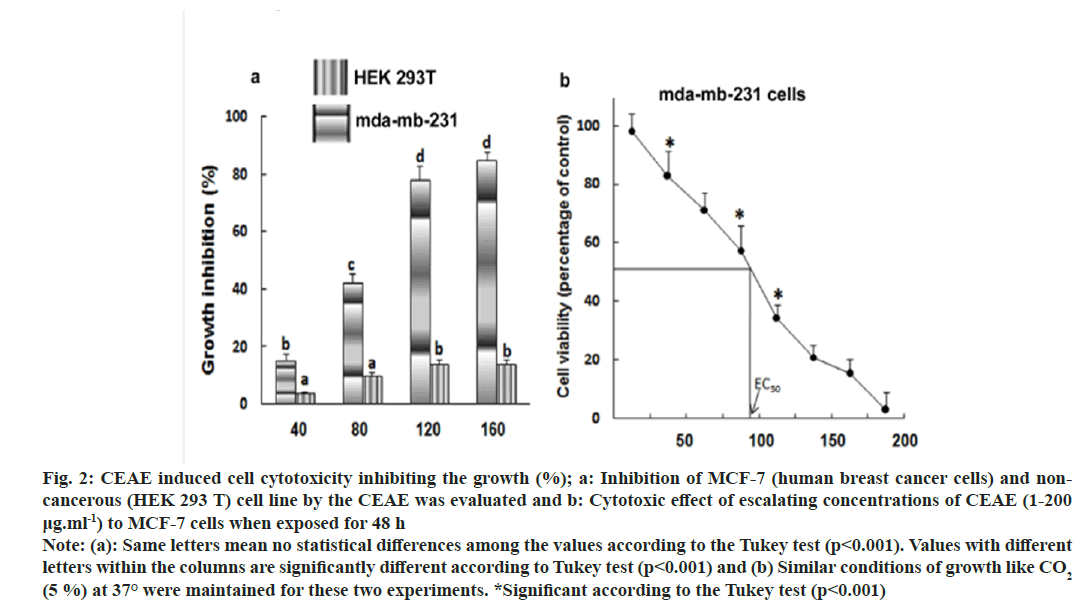
Half maximal Effective Concentration (EC50) is a useful parameter for quantification of drug effect on cell survival. The effect of increasing concentration of extracts on the cell cytotoxicity to MDA-MB-231 (human breast cancer cells) was measured by MTT assay. The minimal cytotoxic activity and no cell death of non-cancerous cell line (HEK 293 T) makes the CEAE extract safe for isolation of anticancer compounds and for use in pharmaceutical industries (fig. 2a). Maximum cytotoxic activity and cell death was obtained with human cervical cancer cell line MDA-MB-231 (EC50, 96.82±5.23 μg) (fig. 2b). The positive control, camptothecin assayed for cytotoxicity under similar conditions exhibited IC50 of 2.75±2.34 μg.
Fig. 2: CEAE induced cell cytotoxicity inhibiting the growth (%); a: Inhibition of MCF-7 (human breast cancer cells) and noncancerous (HEK 293 T) cell line by the CEAE was evaluated and b: Cytotoxic effect of escalating concentrations of CEAE (1-200 μg.ml-1) to MCF-7 cells when exposed for 48 h Note: (a): Same letters mean no statistical differences among the values according to the Tukey test (p<0.001). Values with different letters within the columns are significantly different according to Tukey test (p<0.001) and (b) Similar conditions of growth like CO2 (5 %) at 37° were maintained for these two experiments. *Significant according to the Tukey test (p<0.001)
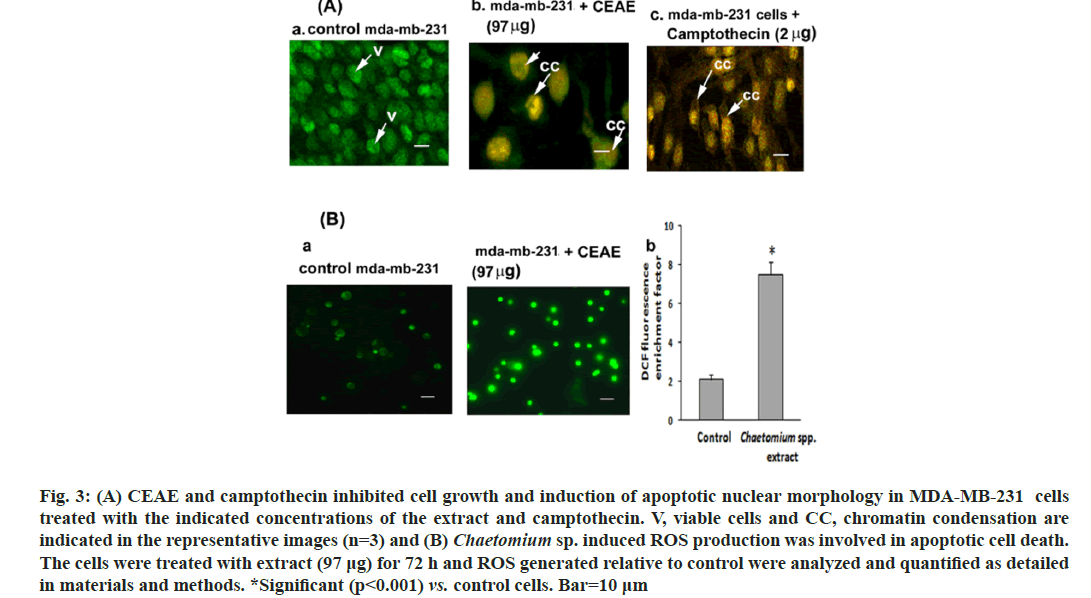
To confirm apoptosis, MDA-MB-231 cells were stained with AO and PI dual stain wherein an emission of green and orange fluorescent wavelengths by AO and PI respectively were captured by CLSM. Control cells did not exhibit significant morphological changes and were green (fig. 3Aa) indicating live cells. However, the cells subjected to treatment with CEAE (97 μg) and camptothecin (2 μg), these groups showed the loss of cell morphology with an uptake of PI. An increase in orange fluorescing nuclear stain indicating apoptosis leading to late apoptotic cells with chromatin condensation for CEAE (fig. 3Ab) and camptothecin (fig. 3Ac) were reported. Chromatin condensation confirms MDA-MB-231 cell cytotoxicity by CEAE to be an apoptotic event.
Fig. 3: (A) CEAE and camptothecin inhibited cell growth and induction of apoptotic nuclear morphology in MDA-MB-231 cells treated with the indicated concentrations of the extract and camptothecin. V, viable cells and CC, chromatin condensation are indicated in the representative images (n=3) and (B) Chaetomium sp. induced ROS production was involved in apoptotic cell death. The cells were treated with extract (97 μg) for 72 h and ROS generated relative to control were analyzed and quantified as detailed in materials and methods. *Significant (p<0.001) vs. control cells. Bar=10 μm
Endophyte extract-induced apoptosis was tested by estimating intracellular ROS after H2DCF-DA fluorescent staining. Endophyte extract-treated MDA-MB-231 cells (fig. 3Ba) exhibited an increase in mean DCF fluorescence in comparison to controls. For example, the DCF fluorescence in MDA-MB-231 cells treated with 97 μg was increased by approximately 7.5-fold, respectively compared to the control group (fig. 3Bb).
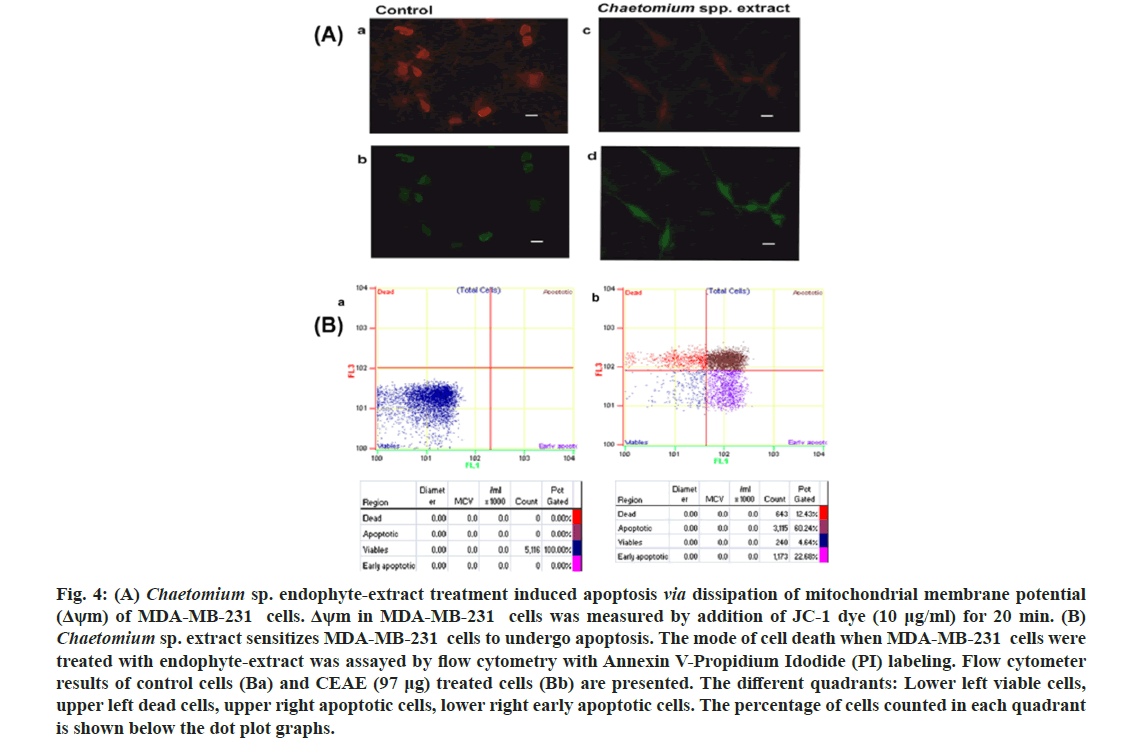
Dissipation of mitochondrial membrane potential as a cellular assay with respect to the effect of CEAE was reported (fig. 4A). The fluorescent probe widely used, JC-1 with the property to accumulate in this organelle was used to stain the cells and mitochondrial membrane potential was reported. It is observed that at a decreased mitochondrial membrane potential a less amount of this molecule resides in this organelle resulting in green fluorescence read as excitation 485/emission 535. In conditions of higher mitochondrial membrane potential, JC-1 dye aggregates resulting in a red-fluorescence with excitation 535/emission 590. Thus, in conditions of loss of mitochondrial membrane potential the shift in red to green fluorescence is reported. Chaetomium sp. extract treatment resulted in fluorescence shift from red (fig. 4A) to green (fig. 4A), indicating loss of mitochondrial membrane potential. It may be suggested that cytotoxicity of MDA-MB-231 cells by endophyte extract (97 μg) treatment via apoptotic pathway may involve targeting the mitochondria in these cells when compared to controls with a negligent shift from red (fig. 4Aa) to green (fig. 4Ab).
Fig. 4: (A) Chaetomium sp. endophyte-extract treatment induced apoptosis via dissipation of mitochondrial membrane potential (Δψm) of MDA-MB-231 cells. Δψm in MDA-MB-231 cells was measured by addition of JC-1 dye (10 μg/ml) for 20 min. (B) Chaetomium sp. extract sensitizes MDA-MB-231 cells to undergo apoptosis. The mode of cell death when MDA-MB-231 cells were treated with endophyte-extract was assayed by flow cytometry with Annexin V-Propidium Idodide (PI) labeling. Flow cytometer results of control cells (Ba) and CEAE (97 μg) treated cells (Bb) are presented. The different quadrants: Lower left viable cells, upper left dead cells, upper right apoptotic cells, lower right early apoptotic cells. The percentage of cells counted in each quadrant is shown below the dot plot graphs.
To gain further insights into the mode of cytotoxicity mediated by endophyte extract, cells were labeled with Annexin V and propidium iodide (fig. 4B). Cells treated with extract (97 μg), (fig. 4Bb) for 72 h presented a decrease in percentage of non labeled alive (lower left) cells (4.64 %) compared to control (100 %) (fig. 4Ba). The percentage of cells labeled only with Annexin V representing early apoptotic stage (lower right) increased upon endophyte-extract treatment (22.68 %) relative to control (0 %). An increase in apoptotic cells to 60.24 % (upper right) in comparison to control (0 %) was presented by flow cytometer analysis.
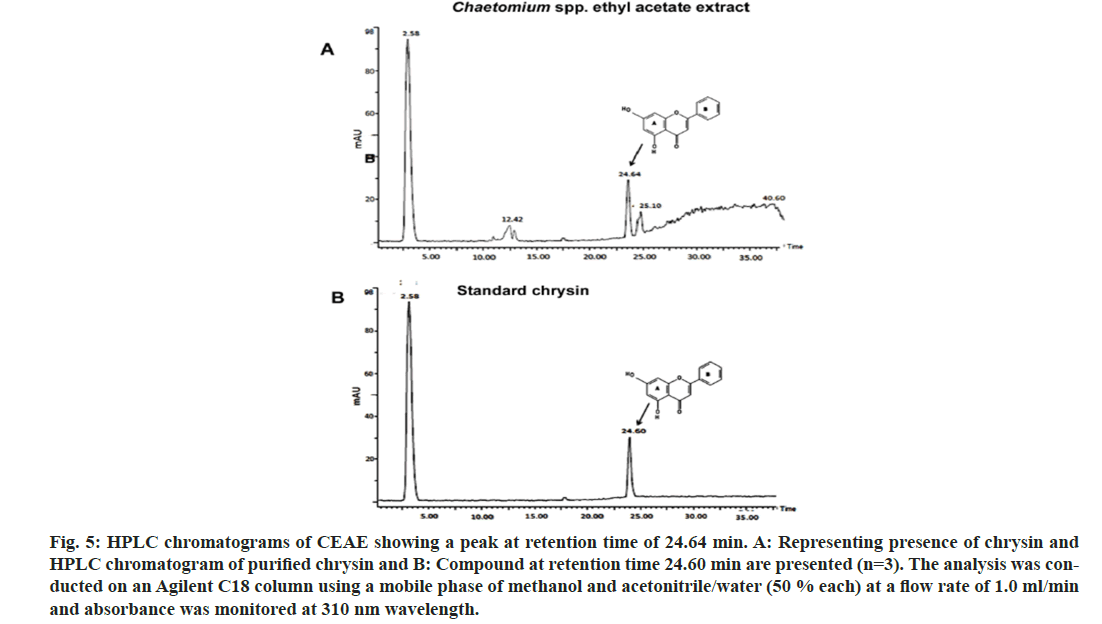
The incubation time interval and solvent extraction has been crucial variable for the extraction of cytotoxic bioactives from the fungal material. Solvent extraction as an imperative step in recovering and isolating secondary metabolites is reported[17]. The solvent extraction of Chaetomium sp. was performed after 15 d of fungal growth, as it has been indicated that the polarity of the solvent provides insight into the chemical nature of metabolites extracted. A maximum extraction of cytotoxic compound (chrysin) was observed in the ethyl acetate extract (fig. 5A). The HPLC results were confirmed with the run using standard chrysin (fig. 5B). Further work is underway in our laboratory to enhance chrysin production from this fungus via optimizing media and growth parameters.
Fig. 5: HPLC chromatograms of CEAE showing a peak at retention time of 24.64 min. A: Representing presence of chrysin and HPLC chromatogram of purified chrysin and B: Compound at retention time 24.60 min are presented (n=3). The analysis was conducted on an Agilent C18 column using a mobile phase of methanol and acetonitrile/water (50 % each) at a flow rate of 1.0 ml/min and absorbance was monitored at 310 nm wavelength.
Research on bioactives from nature has been envisaged an arena wide open for discoveries of therapeutics with broad range of medicinal properties. Fungal endophytes residing as asymptomatic colonies in symbiosis with the host are microbes with either mutualistic or pathogenic characters[18]. However, they do exhibit properties of synthesizing metabolites that are same biologically active molecules as discovered in the hosts flora. Thus, endophytes have offered an alternative avenue for resource for plant based metabolites. Western Ghats medicinal plants is offering an exciting potential relatively underexplored environment with respect to fungal endophytes hot churning factories for novel metabolites due to their special environmental niche with therapeutic properties[19].
In the present study, leaf fungal endophyte extract from a less discussed medicinal plant, a small tree along-side sea coasts, P. serratifolia was chemically finger printed and was evaluated for its cytotoxicity to MDA-MB-231 cells.
Chaetomium sp. was identified by the colony, spore morphology and ITS analysis. The isolated fungus exhibited specific dark green and yellow colony color and earlier it was reported to be an indicator of its capacity to produce terpenoids or flavonoids. This isolate reported here was taken up for further studies. Its ethyl acetate extract was analyzed for secondary metabolite and chrysin was reported in comparison to standard. It was screened for biological activities.
Cancer cell lines as a useful tool in anticancer studies facilitate research on new cytotoxic entities and their structure-activity relationship. It could aid in revealing the potencies of new molecules with therapeutic properties. Documentation of cytotoxicity by potent drugs on cancer cells exhibit blocking the cell cycle program resulting in apoptosis, autophagy and programmed necrosis as their mechanism of action. However, apoptosis as the preferred mode for eliminating cancerous cells inhibiting tumor growth and further arresting cell cycle is prominently preferred[20].
CEAE contained flavonoids and phenolic compounds with promising antioxidant activity. Further, the CEAE was evaluated for its ability to induce cytotoxicity to MDA-MB-231, human breast cancer cells and we report here the possible mechanism of action. Maximum cytotoxicity with EC50, 96.82±5.23 μg.ml-1 for CEAE upon exposure for 72 h was observed. Apoptosis with chromatin condensation was visualized with acridine orange and propidium iodide dual staining at these conditions. It could stimulate cancerous MDAMB- 231 cells to undergo apoptosis, early apoptosis (22.68 %) and apoptotic state (60.24 %) as evidenced by flow cytometer analysis. Apoptosis was mediated through elevated levels of ROS, 7.5 folds over the control. Such excess ROS generation results in structural injury of the mitochondrial membrane resulting in its dissipation of membrane potential as identified by shift from red to green fluorescence of JC-1 dye[15]. One of the bioactive in the extract bestowing therapeutic potential could be was identified as chrysin, a flavones by HPLC analysis of the extract by comparing with the standard chrysin with Rt, 24.68 min. Thus, the CEAE could effectively induce apoptosis to MDAMB- 231 cells in ROS-dependent mitochondrial damage induced cytotoxicity.
Apoptosis in cancer cells, induced particularly through excessive production of free radicals like ROS has been observed in studies relating to chemotherapeutic agents with damage of cellular organelles. The endophyte extract induced excessive ROS production (fluorescently labeled by H2DCF-DA) with a rapid dissipation of mitochondrial membrane potential, mitochondrial membrane potential indicative of mitochondrial dysfunction. Previous studies on chrysin in extracts of prpolis or passion flower and studies on chrysin from honey have reported cytotoxic activity. In studies involving endophytes pioneering work on endophytic fungal extract[21-23] exhibited cytotoxicity to cancer cells in vitro are reported.
Thus, in conclusion, the present study, demonstrated an apoptotic cell death of cancer cell line MDAMB- 231 in a ROS dependent dissipation of mitochondrial membrane event. Thus, the bioactive secondary metabolite productome in the extract of this microbe can be exploited in drug discovery of anti-cancer compounds. It also presents immense scope for the enhanced production of potential anti-cancer compounds in addition to those earlier reported.
Acknowledgements:
Harshitha Chinnadagudihundi Parashiva, Priyanka Shenoy, Sneha Bhaskar, Ramachandra Kukkundoor Kini and Shailasree Sekhar acknowledge the financial support and the experimentation facilities given by the University Grants Commission, New Delhi, Government of India (Grant number UOM/ IOE/RESEARCH/I/2010-11, dt 22.04.2010). SS planned and supervised the work and finalized the manuscript. HCP performed the isolation, identification and standardization of extraction of fungal endophyte. She has analyzed the data. KRK, PS and SB identified fungal strains, conducted ITS experiments and its analysis. Animal cell culture experiments were performed by Shailasree Sekhar. All the authors wrote and edited the manuscript. All the authors approved the final version of the manuscript.
Conflict of interests:
The authors declare no conflict of interest.
References
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 2016;79(3):629-61.
[Crossref] [Google Scholar] [PubMed]
- Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, et al. Flavonoids in cancer and apoptosis. Cancers 2018;11(1):28.
[Crossref] [Google Scholar] [PubMed]
- Lemonnier N, Zhou GB, Prasher B, Mukerji M, Chen Z, Brahmachari SK, et al. Traditional knowledge-based medicine: A review of history, principles, and relevance in the present context of P4 systems medicine. Prog Prev Med 2017;2(7):e0011.
[Crossref] [Google Scholar] [PubMed]
- Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: A versatile source of anticancer drugs. Pharmacogn Rev 2011;5(9):1.
[Crossref] [Google Scholar] [PubMed]
- Ganeshan S, Kim SH, Vujanovic V. Scaling-up production of plant endophytes in bioreactors: Concepts, challenges and perspectives. Bioresour Bioprocess 2021;8:1-6.
[Crossref] [Google Scholar] [PubMed]
- Bose LV, Varghese GK, Habtemariam S. Identification of acteoside as the active antioxidant principle of Premna serratifolia root wood tissues. Phytopharmacology 2013;4(2):228-36.
- Habtemariam S, Varghese GK. A Novel diterpene skeleton: Identification of a highly aromatic, cytotoxic and antioxidant 5‐methyl‐10‐demethyl‐abietane‐type diterpene from Premna serratifolia. Phytother Res 2015;29(1):80-5.
[Crossref] [Google Scholar] [PubMed]
- Kasala ER, Bodduluru LN, Madana RM, Gogoi R, Barua CC. Chemopreventive and therapeutic potential of chrysin in cancer: Mechanistic perspectives. Toxicol Lett 2015;233(2):214-25.
[Crossref] [Google Scholar] [PubMed]
- Kamat S, Kumari M, Sajna KV, Jayabaskaran C. Endophytic fungus, Chaetomium globosum, associated with marine green alga, a new source of Chrysin. Sci Rep 202030;10(1):18726.
[Crossref] [Google Scholar] [PubMed]
- Kumar VS, Kumaresan S, Tamizh MM, Islam MI, Thirugnanasambantham K. Anticancer potential of NF-κB targeting apoptotic molecule “flavipin” isolated from endophytic Chaetomium globosum. Phytomedicine 2019;61:152830.
[Crossref] [Google Scholar] [PubMed]
- Jj D. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 1987;19:11-5.
- Stirling D. DNA extraction from fungi, yeast, and bacteria. Methods Mol Biol 2003:53-4.
[Crossref] [Google Scholar] [PubMed]
- Shailasree S, Ruma K, Prakash HS. Curative properties of Buchanania lanzan: As evaluated by its anti-oxidant, anti-inflammatory and DNA protective properties. J Nat Pharm 2012;3:71.
- Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 1995;28(1):25-30.
- Shailasree S, Venkataramana M, Niranjana SR, Prakash HS. Cytotoxic effect of p-coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Mol Neurobiol 2015;51:119-30.
[Crossref] [Google Scholar] [PubMed]
- Choi JY, Desta KT, Lee SJ, Kim YH, Shin SC, Kim GS, et al. LC‐MS/MS profiling of polyphenol‐enriched leaf, stem and root extracts of Korean Humulus japonicus Siebold and Zucc and determination of their antioxidant effects. Biomed Chromatogr 2018;32(5):e4171.
[Crossref] [Google Scholar] [PubMed]
- Davidson PM, Taylor TM, Schmidt SE. Chemical preservatives and natural antimicrobial compounds. Food Microbiol Fundam Front 2012:765-801.
- Strobel GA. Microbial gifts from rain forests. Can J Plant Pathol 2002;24(1):14-20.
- Sharma H, Rai AK, Dahiya D, Chettri R, Nigam PS. Exploring endophytes forsynthesis of bioactive compounds similar to metabolites produced in vivo by host plants. AIMS Microbiol 2021;7(2):175.
[Crossref] [Google Scholar] [PubMed]
- Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994;73(8):2013-26.
[Crossref] [Google Scholar] [PubMed]
- Seetharaman P, Gnanasekar S, Chandrasekaran R, Chandrakasan G, Kadarkarai M, Sivaperumal S. Isolation and characterization of anticancer flavone chrysin (5, 7-dihydroxy flavone)-producing endophytic fungi from Passiflora incarnata L. leaves. Ann Microbiol 2017;67:321-31.
- Arora D, Sharma N, Singamaneni V, Sharma V, Kushwaha M, Abrol V, et al. Isolation and characterization of bioactive metabolites from Xylaria psidii, an endophytic fungus of the medicinal plant Aegle marmelos and their role in mitochondrial dependent apoptosis against pancreatic cancer cells. Phytomedicine 2016;23(12):1312-20.
[Crossref] [Google Scholar] [PubMed]
- Kamat S, Kumari M, Sajna KV, Singh SK, Kaushalendra, Kumar A, et al. Improved chrysin production by a combination of fermentation factors and elicitation from Chaetomium globosum. Microorganisms 2023;11(4):999.
[Crossref] [Google Scholar] [PubMed]