- *Corresponding Author:
- Madhubal Natarajan
Department of Pharmacology, Sree Balaji Medical College and Hospital, Chromepet, Chennai - 600044, Tamilnadu, India
E-mail: drmadhubala2013@gmail.com
| Date of Received | 27 June 2023 |
| Date of Revision | 08 March 2024 |
| Date of Acceptance | 19 August 2024 |
| Indian J Pharm Sci 2024;86(4):1247-1254 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study focuses on the analysis of phytochemical constituents, antibacterial activity, and antifungal activity of ethanolic extract isolated from the seed coat of Borassus flabellifer. The ethanolic extract of Borassus flabellifer seed coat was prepared by cold extraction technique using sterile filter paper. The extract was subjected to qualitative phytochemical analysis preliminarily. Antibacterial activity against gram-positive strains of Bacillus cereus and Streptococcus mutans, gram-negative strains of Pseudomonas aeruginosa and Salmonella typhi, and antifungal activity against strains of Candida albicans and Aspergillus flavus were assessed using agar well diffusion method by observing the diameter of zone of inhibition. The qualitative phytochemical analysis of ethanolic extract of Borassus flabellifer seed coat revealed the presence of phytoconstituents like tannins, saponins, flavonoids, alkaloids, phenol. It was found to have exhibited good antibacterial effect against gram-positive strains of Bacillus cereus (14-21 mm) and Streptococcus mutans (10- 14 mm) at all concentrations but not exceeding standard antibiotic (16-26 mm) and also exhibited average antibacterial effect against Pseudomonas aeruginosa (12-16 mm) but ineffective against Salmonella typhi. There was limited antifungal activity against Candida albicans (14 mm) and nil antifungal activity against Aspergillus flavus. This study concludes that the seed coat of Borassus flabellifer has significant antibacterial activity against several human pathogens. The phytochemicals present are positively associated with antimicrobial properties. Hence, it can be used for treating a variety of infectious conditions.
Keywords
Antibacterial, antifungal, Borassus flabellifer, phytochemical, seedcoat extract
Since prehistoric times, numerous medicinal plants have been unveiled and are being used for treating several illnesses. Plants have different phytochemical substances that are found to be pharmacologically active and have the potential to be effective as drugs. The use of plant products in drug research has always resulted in the creation of potential compounds that are useful in treating a variety of ailments. Plant products are preferred over synthetic drugs as they are readily available, much cheaper and also cause minimal serious adverse effects. Moreover, antibiotic resistance has become a major public threat leading to longer hospital stays, higher healthcare costs, and increased mortality.
Hence, the development of drugs from plant products has become a prime necessity in recent days. Borassus flabellifer (Asian palmyra palm) commonly referred to as Ice apple, Nungu (Tamil), Tale hannu (Kannada), Tadgola (Hindi), most prevalent in areas of South India, Malaysia, Thailand, Cambodia, Myanmar, and Srilanka [1]. Borassus is the genus name and flabellifer is the species name belonging to the family, Arecaceae. Palmyra palm is a slow-growing, perennial, dioecious tree with a single stem that reaches 30 m in height and has big fan shaped leaves. The lifespan of the woody tree is estimated to be around 100 y [2]. It takes nearly 12-20 y to get mature enough to bear its first flower.
Different parts of this woody tree were found to have different pharmacological actions which help us in formulating a novel drug. The young roots and the male inflorescence of the palm tree were reported to possess anti-inflammatory activity [2]. The palm fruit was found to have antioxidant and antimicrobial properties. Similarly, the seed was found to have antioxidant property [3], antidiabetic property and also help in hydration. The sap obtained was reported to act as a diuretic, laxative, and anti-parasitic agent [1]. Toddy which is produced by fermenting the palm sap was found to treat gastric ulcers [1]. Several parts of this woody tree have been used by our ancestors and are being used in Ayurvedic medicines due to its enormous health benefits. It’s a common practice to eat only the scrumptious, fleshy palatable seed, and the seed coat (skin) is usually discarded (fig. 1). Hence, the present study is focused on investigating the less explored discarded seed coat of Borassus flabellifer for its phytochemical constituents present, its antibacterial & antifungal properties in vitro.
Materials and Methods
Plant material collection:
The tender seeds of Borassus flabellifer were procured from the palm grove situated in the southern part of Tamilnadu during the summer season. The seeds were washed under running water for 2 min and the seed coat was peeled with the help of a sterile knife. The peeled seed coat was dried under shade for 5 d. The shade dried peels were powdered and stored in an airtight container at room temperature for later use.
Plants extract preparation:
The plant extract was prepared using ethanol as solvent by cold extraction technique. About 10 g of powdered seed coat was made to get soaked in 100 ml of ethanol and allowed to stand for 72 h. Followed by, filtering it using a sterile filter paper. Then, the filtrate was collected and incubated at room temperature for evaporation. Finally, the ethanolic extract of Borassus flabellifer seed coat was stored in a sterile capped container under refrigeration at 4° for further studies.
Qualitative phytochemical screening:
The ethanolic extract of Borassus flabellifer seed coat was subjected to qualitative screening of phytochemical constituents present in it by diluting 10 mg of crude extract in 10 ml of ethanol according to the standard protocols [4,5,6] and modified methods [7,8,9,10].
Ferric chloride test-test for tannins: [4]
1 ml of seed coat extract was mixed with a few drops of 0.1 % ferric chloride. The presence of gallic tannins and catechol tannins was determined by looking for blackish blue and blackish green colours respectively.
Frothing test-test for saponins: [5]
3 ml of seed coat extract were added to ten ml of distilled water and strongly mixed for 5 min. Later, it was permitted to stand for 30 min. The presence of saponins is indicated by frothing or foaming appearance.
Shinoda test-test for flavonoids: [7]
A few drops of magnesium chloride and concentrated hydrochloric acid were added to 1 ml of seed coat extract and observed after 5 min. The presence of flavonoids is confirmed by observing red to pink colour.
Dragendorff test-test for alkaloids: [6]
Few drops of Dragendorff reagent were added to 2 ml of seed coat extract. The presence of alkaloids is indicated by orange-red precipitate.
Bradford protein assay-test for proteins: [4]
Few drops of Bradford’s reagent were mixed with 2 ml of seed coat extract and observed for colour shift from brown to blue indicating the existence of proteins.
Salkowski’s test-test for steroids: [8]
3 drops of 10 % concentrated sulphuric acid were added to 5 ml of seed coat extract and observed for red colour which indicates the activity of steroids.
Borntrager’s reaction-test for anthraquinones: [9]
10 ml of 10 % ammonia solution was mixed with 1 ml of seed coat extract and strongly mixed for 30 s. The activity of anthraquinones is confirmed by observing pink, violet or red colour.
Salkowski’s test-test for terpenoids: [10]
2 ml of chloroform was added along with little drops of concentrated sulphuric acid to 1 ml of seed coat extract. The formation of a reddish-brown ring at the interface confirms the activity of terpenoids.
Keller kiliani test-test for cardiac glycosides: [8]
1 ml of glacial acetic acid, 2 % ferric chloride solution in drops followed by 1 ml of concentrated sulphuric acid were added to 5 ml of seed coat extract. The formation of brown colour ring confirms the existence of cardiac glycosides.
Ferric chloride test-test for phenols: [9]
A few drops of 5 % ferric chloride were added to 1 ml of seed coat extract and noticed for dark green or bluish-black colour indicating the activity of phenols.
Microorganism strains used for analysing antibacterial activity:
The antibacterial activity of Borassus flabellifer seed coat extract was analysed using gram-positive bacterial strains of Bacillus cereus and Streptococcus mutans and gram-negative bacterial strains of Salmonella typhi and Pseudomonas aeruginosa.
Antibacterial properties of Borassus flabellifer seed coat extract:
The antibacterial activity was evaluated by the agar well diffusion method on Mueller Hinton Agar (MHA) medium [11]. Active cultures of the aforementioned microorganisms were prepared from stock cultures which were maintained at 4° on the nutrient agar slant. Test tubes with nutrient broth for bacterial culture were incubated at 37° for 24 h and kept ready. A loop of cells from stock cultures was added to test tubes of nutrient broth and sub-cultured overnight. 10 mg of crude seed coat extract was diluted with 10 ml of ethanolic solvent to prepare the stock solution. 125 ml of stock solution was diluted in 875 ml of ethanolic solvent to formulate 125 mg/ml of ethanolic seed coat extract concentration. Similarly, other concentrations from 250 mg, 500 mg to 1000 mg were prepared.
The sterile MHA medium was poured into sterile petri plates and permitted to solidify for 1 h. Following solidification, the inoculums were made to spread on the plates with the help of a swab with cultured bacterial suspension. Wells of 6 mm size were made using sterile cork borer and seed coat extract was added in it. Streptomycin was added to one of the wells since it was used as a positive control. Similarly, Dimethyl Sulfoxide (DMSO) was used as a negative control. The plates were incubated at 37° for 24 h. Then, the diameter of the zone of inhibition was measured in mm using a HiMedia zone reader to estimate the microbial growth [11]. Various concentrations of ethanolic seed coat extract (125 mg, 250 mg, 500 mg, 1000 mg) in respective wells of the inoculated petri plates were tested for each microorganism to analyse the zone of inhibition in accordance with the increasing concentration of seed coat extract.
Microorganisms used for analysing antifungal activity:
The antifungal activity of Borassus flabellifer seed coat extract was analysed using Candida albicans and Aspergillus flavus.
Antifungal properties of Borassus flabellifer seed coat extract:
The antifungal activity was evaluated by agar well diffusion method on Potato Dextrose Agar (PDA) medium [12]. Active cultures of the aforementioned microorganisms were prepared from stock cultures which were maintained at 4° on slant of nutrient agar. Test tubes of nutrient broth for bacterial culture were incubated at 37° for 24 h and kept ready. The overnight nutrient broth was sub-cultured by transferring a loop of cells from stock cultures to test tubes of nutrient broth. 10 mg of crude seed coat extract was diluted with 10 ml of ethanolic solvent to prepare the stock solution. 125 ml of stock solution was diluted in 875 ml of ethanolic solvent to formulate 125 mg/ml of ethanolic seed coat extract concentration. Similarly, other concentrations from 250 mg, 500 mg to 1000 mg were prepared.
The sterile PDA medium was poured into sterile petri plates and permitted to solidify for 1 h. Following solidification, the inoculums were made to spread on the plates with the help of a swab with microbial suspension. Wells of 6 mm size were made using sterile cork borer and seed coat extract was added in it. Ketoconazole was added to one of the wells since it was used as a positive control. Similarly, DMSO was used as a negative control. The plates were incubated at 37° for 24 h.
Then, the diameter of the zone of inhibition was measured in millimetres (mm) using a HiMedia zone reader to estimate the microbial growth [12]. Various concentrations of ethanolic seed coat extract from 125 mg, 250 mg, 500 mg to 1000 mg in respective wells of inoculated petri plates were tested for each microorganism to analyse the zone of inhibition in accordance with the increasing concentration of seed coat extract.
Results and Discussion
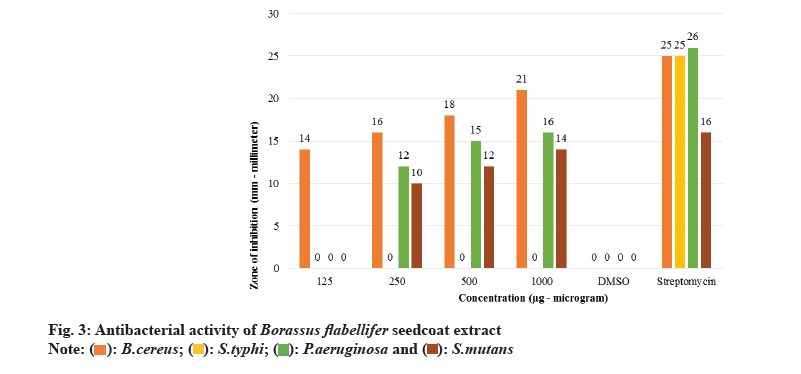
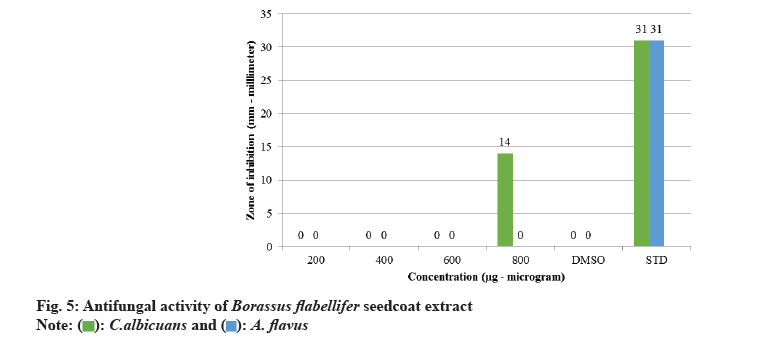
The screening of phytochemicals present in seed coat extract is considered the preliminary step to identify the bioactive substances present which might be possibly positively associated with several medicinal properties like anti-oxidant property, anti-inflammatory property, antimicrobial property, and anti-cancer property. The Phytochemicals present in Borassus flabellifer seed coat extract are listed in Table 1. Agar well diffusion method was employed to study the antibacterial activity (fig. 2) of Borassus flabellifer seed coat extract against gram-positive strains of Bacillus cereus and Streptococcus mutans and gram-negative strains of Salmonella typhi and Pseudomonas aeruginosa using MHA medium. As depicted in fig. 3, the seed coat extract exhibits strong antibacterial activity against Bacillus cereus with 14-21 mm zone of inhibition as the concentration of the extract increases. There is considerate antibacterial activity against Pseudomonas aeruginosa with 12-16 mm zone of inhibition at higher concentrations of seed coat extract. Limited antibacterial activity was observed against Streptococcus mutans with 10-14 mm zone of inhibition. And, it was noticed that seed coat extract was ineffective against Salmonella typhi even at its maximum amount concentration. Using PDA, the antifungal (fig. 4) activity of seed coat extract was evaluated using agar diffusion method against strains of Candida albicans and Aspergillus flavus. As shown in the fig. 5, there was minimal activity of seed coat extract against Candida albicans with 14mm zone of inhibition exhibited at the maximum concentration. Also, no antifungal activity was noticed even at the maximum of seed coat extract concentration against Aspergillus flavus. Although we have a wide class of modern medicines available for treating different bacterial infections, most of them come with adverse effects like anaphylactic reactions, gastro-intestinal disturbances, and toxicity along with their therapeutic value as chemicals are the source of many drugs. So, always there is an unending search for medicines from plants to minimise the adverse effects. Nature is the ultimate source available to combat several medical ailments.
| S.No | Phytochemicals | Seed coat extract |
|---|---|---|
| 1 | Tannins | + |
| 2 | Saponins | + |
| 3 | Flavonoids | + |
| 4 | Alkaloids | + |
| 5 | Proteins | + |
| 6 | Steroids | - |
| 7 | Quinones | - |
| 8 | Terpenoids | - |
| 9 | Cardiac glycosides | + |
| 10 | Phenols | + |
Table 1: Phytochemicals in Borassus flabellifer Seedcoat Extract
The screening of phytochemicals present in seed coat extract is considered to be the preliminary step, to identify the bioactive substances present in it which might be possibly positively associated with several medicinal properties like anti-oxidant property, anti-inflammatory property, antimicrobial property, anticancer property, anti-diabetic property. The presence of phytochemical constituents like tannins, saponins, alkaloids, and flavonoids has been reported already to be partly responsible for the antimicrobial activity [13]. Our study revealed the presence of tannins, saponins, alkaloids, flavonoids, proteins, cardiac glycosides, and phenols which are attributed to the antimicrobial properties of the seed coat extract. 2 gram-positive strains and 2 gram-negative strains were selected for analysing in the study to test them against different varied medical conditions. Bacillus cereus, gram-positive strain is known to cause food poisoning [14]. Streptococcus mutans, gram-positive strain colonises the dental surface resulting in dental caries [15]. Pseudomonas aeruginosa, gram-negative organism causes hospital-acquired infections like pneumonia, urinary tract infections as it always remains a concern to treat due to its resistance [16]. Salmonella typhi, gram-negative strain is typically associated with typhoid fever [17].
Candida albicans was selected as it is a widespread fungal infection affecting people of all ages causing oral thrush, genital infections like vulvovaginal candidiasis. It is also a normal commensal of our human body. Infection occurs when the fungus overgrows due to weakened immune system [18]. Aspergillus flavus, a common opportunistic pathogen causing aspergillosis, allergic reactions [19]. As the study implies, the seed coat extract can be tried effectively against food poisoning cases caused by Bacillus cereus, respiratory infections caused by Pseudomonas aeruginosa. A Study done by Rani et al. [10] reported that the aqueous fruit extract of Borassus flabellifer revealed zone of inhibition against pathogens like Bacillus cereus, Bacillus subtilis, and Staphylococcus aureus at the concentration of 30 μg/ml using azithromycin as control. In our study, pathogens like Bacillus cereus, Streptococcus mutans, Salmonella typhi, and Pseudomonas aeruginosa were analysed using Streptomycin as a positive control. It was reported by Jamkhande et al. [12] that the methanolic leaves extract of Borassus flabellifer had a significant antibacterial effect against Bacillus subtilis, Klebsiella pneumoniae, Pseudomonas aeruginosa where ciprofloxacin was used as standard. The same extract had nil effect against Aspergillus niger as our study concluded [12].
A study done by Sahni et al. [2] had observed that the methanolic dried root extract of Borassus flabellifer revealed zero activity against gram positive Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis and gram-negative Proteus mirabilis, Escherichia coli.
Sakande et al. [20] revealed that there was significant antifungal activity of dichloromethane-methanolic extract of Borassus aethiopum male inflorescences against Trichophyton rubrum, Trichophyton interdigitale, Microsporum langeronii and Epidermophyton floccosum. On the contrary, it was observed to have weak antibacterial activity against Escherichia coli, Enterobacter cloacae, Staphylococcus aureus. Ketoconazole and amoxicillin were used as standards respectively.
According to the study results of Duddukuri et al. [21] significant and consistent antibacterial effect of Borassus flabellifer’s methanolic seed coat extract was distinguished against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia. Our ethanolic seed coat extract expressed significant antibacterial activity against Bacillus cereus, Streptococcus mutans, Salmonella typhi, and Pseudomonas aeruginosa as the concentration of the seed coat extract increased.
Our current exploration on the ethanolic seed coat extract of Borassus flabellifer has revealed prominent antibacterial activity against Bacillus cereus, Streptococcus mutans (gram-positive strains) and Salmonella typhi, Pseudomonas aeruginosa (gram-negative strains). But, has only limited antifungal activity against Candida albicans. The emergence of antibiotic-resistant strains and their hazardous side effects has revived the search for novel antimicrobial agents from natural plant sources. The need of plant products as pharmacological agents is increasing day by day due to the increased cost of the available antibiotics and various serious adverse effects caused. Hence, natural antibiotics are preferred over other synthetic available drugs for a healthier quality of life. The present study paves the way for the potential utilization of Borassus flabellifer seed coat in pharmaceutical industries as a pertinent antibacterial agent against several infectious ailments like enteric fever, urinary tract infections, upper respiratory tract infections, food poisoning. Further in vitro, in vivo and clinical studies are necessary to provide more scientific evidence on consuming Borassus flabellifer seed as a whole along with the seed coat therapeutically to utilise the great medicinal benefits.
Acknowledgements:
The authors are thankful to the Department of Research and Development, SBMCH for providing necessary research and laboratory facilities related with the study.
Conflict of interest:
The authors declare no conflict of interest in this work.
References
- Krishnaveni TR, Arunachalam R, Chandrakumar M, Parthasarathi G, Nisha R. Potential review on palmyra (Borassus flabellifer L.). Adv Res 2020;21(9):29-40.
[Crossref ] [Google Scholar ]
- Sahni C, Shakil NA, Jha V, Gupta RK. Screening of nutritional, phytochemical, antioxidant and antibacterial activity of the roots of Borassus flabellifer (Asian palmyra palm). J Pharmacogn Phytochem 2014;3(4):58-68.
- Renuka KR, Devi VR, Subramanian SP. Phytochemical screening and evaluation of in vitro antioxidant potential of immature palmyra palm (Borassus flabellifer Linn.) fruits. Int J Pharm Sci 2018;10(8):77-83.
[Crossref ] [Google Scholar ]
- Harborne AJ. Phytochemical methods a guide to modern techniques of plant analysis. Springer science and business media 1998.
- Trease GE, Evans WC. Phenols and phenolic glycosides. Pharmacognosy 1996;14:218-54.
- Sofowora A. Phytochemical screening of medicinal plants and traditional medicine in Africa. Spectrum Books Limited. Nigeria. 1993:150-6.
- Saptarini NM, Herawati IE, Permatasari UY. Total flavonoids content in acidified extract of flowers and leaves of gardenia (Gardenia jasminoides Ellis). Asian J Pharm Clin Res 2016;9(1):213-5.
- Panchal P, Parvez N. Phytochemical analysis of medicinal herb (Ocimum sanctum). Int J Nanomater Nanotechnol Nanomed 2019;5(2):008-11.
- Shaikh JR, Patil M. Qualitative tests for preliminary phytochemical screening: An overview. Int J Chem Stud 2020;8(2):603-8.
- Rani VP, Mirabel LM, Priya KS, Nancy AA, Meena G. Phytochemical, antioxidant and antibacterial activity of aqueous extract of Borassus Flabellifer (L.). Int J Sci Res Sci Technol 2018;4(2):405-10.
- Kaladhar DS, Nandikolla SK. Antimicrobial studies, biochemical and image analysis in Mirabilis jalapa. Int J Pharm Technol 2010;2(3):683-93.
- Jamkhande PG, Suryawanshi VA, Kaylankar TM, Patwekar SL. Biological activities of leaves of ethnomedicinal plant, Borassus flabellifer Linn. (Palmyra palm): An antibacterial, antifungal and antioxidant evaluation. Bulletin of Faculty of Pharmacy, Cairo University 2016;54(1):59-66.
- Wintola OA, Afolayan AJ. The antibacterial, phytochemicals and antioxidants evaluation of the root extracts of Hydnora africana Thunb. used as antidysenteric in Eastern Cape Province, South Africa. BMC Complement Altern Med 2015;15:1-2.
[Crossref] [Google Scholar] [PubMed]
- Griffiths MW, Schraft H. Bacillus cereus food poisoning. InFoodborne diseases 2017: 395-405.
- Forssten SD, Björklund M, Ouwehand AC. Streptococcus mutans, caries and simulation models. Nutrients 2010;2(3):290-8.
[Crossref] [Google Scholar] [PubMed]
- Wagner S, Sommer R, Hinsberger S, Lu C, Hartmann RW, Empting M, et al. Novel strategies for the treatment of Pseudomonas aeruginosa infections. J Med Chem 2016;59(13):5929-69.
[Crossref] [Google Scholar] [PubMed]
- Rowe B, Ward LR, Threlfall EJ. Multidrug-resistant Salmonella typhi: A worldwide epidemic. Clin Infect Dis 1997;24:S106-9.
[Crossref] [Google Scholar] [PubMed]
- Nobile CJ, Johnson AD. Candida albicans biofilms and human disease. Annu Rev Microbiol 2015;69(1):71-92.
[Crossref] [Google Scholar] [PubMed]
- Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007;153(6):1677-92.
[Crossref] [Google Scholar] [PubMed]
- Sakande J, Nikiema A, Kabre E, Lompo M, Nikiema JB, Nacoulma OG, et al. In vitro assay of potential antifungal and antibacterial activities of extracts of Borassus aethiopum Mart. Biokemistri 2012;24(1):48-51.
- Duddukuri GR, Sastry YN, Kaladhar DS, Rao KK, Chaitanya KK. In vitro antibacterial activity of organic solvent seed coat extracts of Borassus flabellifer linn. Biosci Biotechnol Res Asia 2016;8(1):295-9.