- *Corresponding Author:
- Xi Feng
Department of Cardiology, Clinical Cardiovascular Center, Liyuan Hospital, Tongj Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430071, China
E-mail: hmz20913@163.com
| Date of Received | 04 February 2023 |
| Date of Revision | 21 September 2023 |
| Date of Accepted | 10 February 2024 |
| Indian J Pharm Sci 2024;86(1):266-271 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect and possible mechanism of Allium mongolicum Regel flavonoids on cardiomyocyte injury induced by hypoxia/reoxygenation. H9C2 cardiomyocytes were induced by hypoxia/reoxygenation and treated with different doses of Allium mongolicum Regel flavonoids. H9C2 cells were transfected with si-negative control/ si-TALNEC2 and treated with hypoxia/reoxygenation. Besides, cells transfected with plasmid cloning deoxyribonucleic acid/plasmid cloning deoxyribonucleic acid-TALNEC2 were treated with Allium mongolicum Regel flavonoids and induced with hypoxia/reoxygenation. Malondialdehyde, glutathione peroxidase and superoxide dismutase levels were tested to evaluate oxidative stress. Apoptosis rate was analyzed by flow cytometry. TALNEC2 expression was examined using quantitative reverse transcription-polymerase chain reaction, and cleaved caspase-3 and cleaved caspase-9 protein levels were tested by Western blot. Allium mongolicum Regel flavonoids could reduce malondialdehyde level, apoptosis rate, cleaved caspase-3 level, cleaved caspase-9 level, and TALNEC2 expression, while enhanced glutathione peroxidase and superoxide dismutase levels in hypoxia/reoxygenation-induced H9C2 cells in a dose-dependent manner. After transfection of si-TALNEC2, malondialdehyde level, apoptosis rate, cleaved caspase-3 level, and cleaved caspase-9 level were reduced, while superoxide dismutase and glutathione peroxidase levels were enhanced. Transfection of plasmid cloning deoxyribonucleic acid-TALNEC2 could abolish the effect of Allium mongolicum Regel flavonoids on cardiomyocyte injury. Allium mongolicum Regel flavonoids could inhibit hypoxia/reoxygenation-induced cardiomyocyte apoptosis and oxidative stress via reducing TALNEC2 expression.
Keywords
Allium mongolicum Regel flavonoids, hypoxia/reoxygenation, TALNEC2, cardiovascular disease, malondialdehyde
The mortality of cardiovascular disease is increasing year by year in China[1,2]. Although percutaneous coronary intervention and other treatments have achieved good results, reperfusion therapy can aggravate myocardial tissue damage and cause arrhythmia and other side effects[3,4]. Oxidative stress and apoptosis can cause myocardial Ischemia-Reperfusion (I/R) injury[5,6]. Active ingredients of Traditional Chinese Medicine (TCM) have anti-apoptosis and anti-oxidative stress effects, and can be used to alleviate myocardial I/R injury[7,8]. Therefore, it is of great significance to find effective TCM active ingredients and reveal their potential molecular mechanisms for improving myocardial I/R injury.
Allium mongolicum Regel, belongs to Allium genus of Liliaceae, contains many active ingredients and have certain medicinal value[9]. Studies have shown that Allium mongolicum Regel Flavonoids (AMRF) can promote the contraction of intestinal smooth muscle and improve constipation in mice[10]. Importantly, AMRF has been confirmed to have anti-oxidant, anti-apoptosis and anti-inflammatory properties[11-13]. However, whether AMRF can improve myocardial I/R injury by suppressing cardiomyocyte apoptosis and oxidative stress is still unknown.
Long noncoding RNA (lncRNA) has been confirmed to be involved in human diseases development[14,15]. Previous study suggested that lncRNA TALNEC2 knockdown alleviated cerebral I/R injury via inhibiting neuronal apoptosis and inflammation[16,17]. Moreover, TALNEC2 was overexpressed in myocardial ischemic patients, and its overexpression could promote Hypoxiainduced Cardiomyocytes (H9C2) injury[18]. Here, we found that AMRF exerted an inhibitory effect on TALNEC2 expression. However, whether AMRF can improve myocardial I/R injury through regulating TALNEC2 expression is unclear.
Based on the above, our study investigated whether AMRF affected myocardial I/R injury via regulating TALNEC2 using Hypoxia/Reoxygenation (H/R)- induced H9C2 cells.
Materials and Methods
Preparation of AMRF:
Allium mongolicum Regel (Sihehui Trading, Inner Mongolia, China) was extracted by 75 % ethanol for 2 h (70°), and then the supernatant was obtained by centrifugation. The supernatant was concentrated under the reduced pressure by a rotary evaporator. Sodium hydroxide reaction method was used to determine the composition of flavonoids in the extract (obtained 12.96 mg/g AMRF). AMRF was diluted by Dimethylsulfoxide (DMSO) to prepare different concentrations.
Cell culture and grouping:
H9C2 cells (Procell, Wuhan, China) were cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 10 % Fetal Bovine Serum (FBS). To construct H/R cell model, H9C2 cells were cultured under hypoxia condition (5 % Carbon dioxide (CO2), 95 % Nitrogen (N2) and 0.1 % Oxygen (O2)) for 6 h and then performed reoxygenation (5 % CO2 and 95 % air) for 12 h[19]. Normal cultured cells were used as control group. H9C2 cells were treated with different concentrations (25, 50, and 100 μg/ ml) of AMRF for 24 h and then induced with H/R, which were recorded as H/R+low-AMRF group, H/R+middle-AMRF group and H/R+high-AMRF group, respectively. H9C2 cells were transfected with si-NC/si-TALNEC2 using Lipofectamine 3000 (Invitrogen, Carlsbad, California, United states of America (USA)) and then induced with H/R, which were recorded as H/R+si-NC group and H/R+si-TALNEC2 group. Also, H9C2 cells transfected with plasmid cloning deoxyribonucleic acid (pcDNA)/pcDNA-TALNEC2 were treated with 100 μg/ml AMRF and induced with H/R, which were recorded as H/R+high-AMRF+pcDNA group and H/R+high-AMRF+pcDNA-TALNEC2 group.
Assessing of oxidative str ess:
H9C2 cells were collected and lysed by repeated freeze-thaw method. Malondialdehyde (MDA), Glutathione Peroxidase (GSh-Px) and Superoxide Dismutase (SOD) levels were detected by corresponding kits according to kit instructions.
Flow cytometry:
H9C2 cells were digested to collect cell suspensions. After suspended with binding buffer, cells were stained with Annexin V-Fluorescein Isothiocyanate (FITC) and Propidium Iodide (PI) (Beyotime, Shanghai, China), and cell apoptosis rate was detected by FACS Calibur flow cytometry.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR):
Total Ribonuclic Acid (RNA) was extracted and complementary DNA (cDNA) was synthesized. qRT-PCR was amplified using SYBR Green (Invitrogen), cDNA and specific primers of TALNEC2. Relative expression was calculated by 2−ΔΔCt method.
Western blot:
Radioimmunoprecipitation Assay (RIPA) buffer was used to extract total protein. Protein was taken for Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) reaction and Polyvinylidene Difluoride (PVDF) membrane transferring. Membrane was incubated with anti-cleaved caspase-3 (ab90437; 1:1000), anticleaved caspase-9 (1:1000), anti-Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (1:2500, ab9485), and secondary antibody (1:50000, ab205718). Protein bands were visualized by Enhanced Chemiluminescence (ECL) reagent (Beyotime) and quantitatively analyzed by Quantity One® software.
Statistical analysis:
Data were expressed as x̄ ±s and analyzed by Statistical Package for the Social Sciences (SPSS) 21.0 software. Student’s t-test and Analysis of Variance (ANOVA) were used for comparisons. p<0.05 was considered significant difference.
Results and Discussion
MDA level was enhanced, while GSH-Px and SOD levels were suppressed in the H/R group (Table 1). Furthermore, MDA level was reduced, while GSH-Px and SOD levels were increased in the H/R+low-AMRF, H/R+middle-AMRF and H/ R+high-AMRF groups (Table 1).
| Group | MDA (nmol/l) | SOD (U/ml) | GSH-Px (U/ml) |
|---|---|---|---|
| Control | 5.62±0.49 | 68.44±5.92 | 82.78±6.86 |
| H/R | 45.46±4.29* | 21.22±2.12* | 33.24±3.02* |
| H/R+low-AMRF | 31.61±3.13# | 34.25±3.37# | 45.03±4.08# |
| H/R+middle-AMRF | 19.91±1.77#& | 46.16±4.41#& | 60.02±4.09#& |
| H/R+high-AMRF | 9.87±0.86#&$ | 57.65±5.52#&$ | 74.42±6.65#&$ |
| F | 369.497 | 155.151 | 139.85 |
| p | 0.000 | 0.000 | 0.000 |
Table 1: Effects of AMRF on H/R-Induced Cell Oxidative Stress
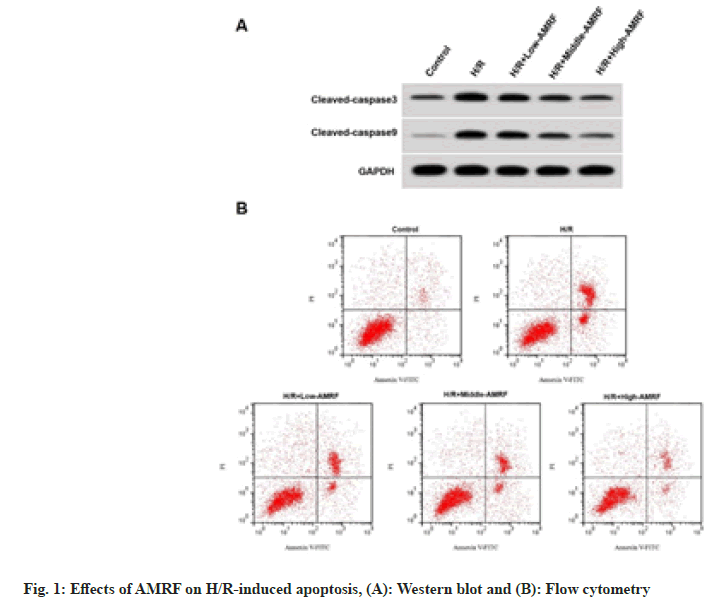
Apoptosis rate, cleaved caspase-3 and cleaved caspase-9 levels were enhanced in the H/R group (fig. 1A), while were reduced in the H/R+low- AMRF, H/R+middle-AMRF and H/R+high-AMRF groups (Table 2 and fig. 1B).
| Group | Apoptosis rate (%) | Cleaved caspase-3 | Cleaved caspase-9 |
|---|---|---|---|
| Control | 5.32±0.45 | 0.25±0.02 | 0.12±0.02 |
| H/R | 32.74±2.94* | 0.79±0.06* | 0.58±0.04* |
| H/R+low-AMRF | 23.34±2.34# | 0.64±0.05# | 0.42±0.03# |
| H/R+middle-AMRF | 16.15±1.42#& | 0.51±0.04#& | 0.31±0.03#& |
| H/R+high-AMRF | 8.61±0.74#&$ | 0.34±0.03#&$ | 0.18±0.02#&$ |
| F | 329.932 | 239.650 | 367.714 |
| p | 0.000 | 0.000 | 0.000 |
Table 2: Effects of AMRF on H/R-Induced Apoptosis
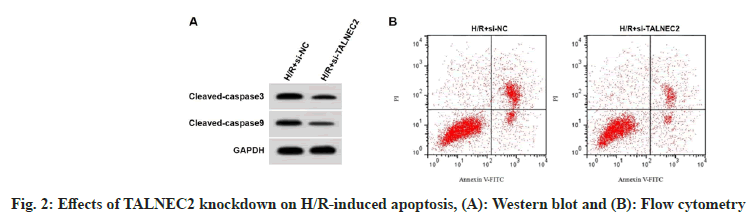
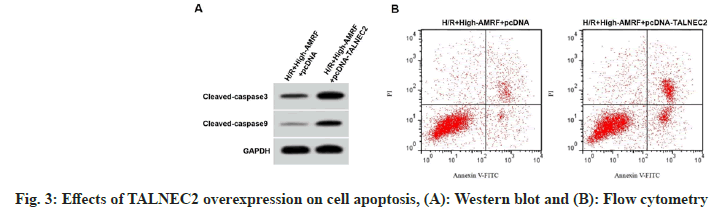
TALNEC2 level was enhanced in the H/R group, while was reduced in the H/R+low-AMRF, H/ R+middle-AMRF and H/R+high-AMRF groups (Table 3). MDA level was decreased, while GSH-Px and SOD levels were increased in H/ R+si-TALNEC2 group (Table 4). Apoptosis rate, cleaved caspase-3 and cleaved caspase-9 levels were reduced in H/R+si-TALNEC2 group (Table 5 and fig. 2). As shown in fig. 3 and Table 6, MDA level, apoptosis rate, cleaved caspase-3 and cleaved caspase-9 levels were enhanced, while GSH-Px and SOD levels were decreased in the H/ R+high-AMRF+pcDNA-TALNEC2 group.
| Group | TALNEC2 |
|---|---|
| Control | 1.00±0.00 |
| H/R | 3.54±0.27* |
| H/R+low-AMRF | 2.66±0.23# |
| H/R+middle-AMRF | 1.98±0.12#& |
| H/R+high-AMRF | 1.36±0.12#&$ |
| F | 302.635 |
| p | 0.000 |
Table 3: Effects of AMRF on TALNEC2 Expression
| Group | TALNEC2 | MDA (nmol/l) | SOD (U/ml) | GSH-Px (U/ml) |
|---|---|---|---|---|
| H/R+si-NC | 1.00±0.00 | 48.79±4.41 | 20.58±2.01 | 31.54±3.14 |
| H/R+si-TALNEC2 | 0.32±0.03* | 15.54±1.22* | 50.31±4.07* | 67.07±5.08* |
| t | 68.000 | 21.800 | 19.649 | 17.848 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Table 4: Effects of TALNEC2 Knockdown on H/R-Induced Oxidative Stress
| Group | Apoptosis rate (%) | Cleaved caspase-3 | Cleaved caspase-9 |
|---|---|---|---|
| H/R+si-NC | 34.23±3.02 | 0.77±0.04 | 0.57±0.05 |
| H/R+si-TALNEC2 | 12.69±1.26* | 0.40±0.04* | 0.24±0.02* |
| t | 19.748 | 19.622 | 18.384 |
| p | 0.000 | 0.000 | 0.000 |
Table 5: Effects of TALNEC2 Knockdown on H/R-Induced Apoptosis
| Group | TALNEC2 | MDA (nmol/l) | SOD (U/ml) | GSH-Px (U/ml) | Apoptosis rate (%) | Cleaved caspase-3 | Cleaved caspase-9 |
|---|---|---|---|---|---|---|---|
| H/R+high-AMRF+pcDNA | 1.00±0.00 | 9.51±0.83 | 59.09±4.71 | 76.29±6.92 | 8.26±0.62 | 0.31±0.03 | 0.17±0.02 |
| H/R+high-AMRF+pcDNA-TALNEC2 | 3.26±0.29* | 33.24±3.02* | 32.75±2.91* | 42.99±4.18* | 22.04±1.78* | 0.68±0.04* | 0.47±0.03* |
| t | 23.379 | 22.730 | 14.273 | 13.228 | 21.932 | 22.200 | 24.962 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Table 6: Effects of TALNEC2 Overexpression on Cell Injury
Cardiomyocyte ischemia causes oxidative stress, and reperfusion causes cardiomyocyte apoptosis[20,21]. Studies have shown that TCM can inhibit cardiomyocyte apoptosis and oxidative stress by regulating multiple targets[22,23]. lncRNA has been confirmed to be abnormally expressed in myocardial I/R injury[24,25]. However, whether lncRNA can be served as a potential target for TCM to alleviate myocardial I/R injury needs to be further explored.
The polysaccharides and flavonoids of Allium mongolicum Regel may slow down the progression of many diseases[11-13]. Similar to the reports of previous studies[26,27], we found that H/R induction elevated MDA level and decreased GSH-Px and SOD levels in cardiomyocytes, suggesting that H/R induction promoted oxidative stress in cardiomyocytes. Further studies revealed that AMRF reduced MDA level, while enhanced GSHPx and SOD levels in H/R-induced cardiomyocytes, indicating that AMRF could inhibit cardiomyocyte oxidative stress. Besides, H/R induced cardiomyocyte apoptosis, which were consistent with the previously studies[28,29]. Furthermore, H/R-induced apoptosis could be inhibited with the increasing of AMRF concentrations, revealing that AMRF repressed H/R-induced apoptosis in cardiomyocytes.
TALNEC2 was upregulated in cerebral I/R injury mouse models, which promoted neuronal apoptosis to facilitate cell injury[16,17]. Besides, inhibition of TALNEC2 attenuated hypoxia-induced injury in mouse embryonic osteoblasts[30]. Our study revealed that TALNEC2 expression was elevated in H/R-induced cardiomyocytes, and AMRF was able to reduce TALNEC2 expression in a concentration-dependent manner. Furthermore, TALNEC2 knockdown inhibited cardiomyocyte injury, whereas its upregulation attenuated the inhibitory effect of AMRF on cardiomyocyte injury. Here, AMRF mitigated myocardial I/R injury by decreasing TALNEC2 level.
In summary, AMRF inhibited H/R-induced apoptosis and oxidative stress in cardiomyocytes depending on reducing TALNEC2 expression. Our findings confirmed that TALNEC2 might serve as a potential target for AMRF in treating myocardial I/R injury.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat Rev Cardiol 2019;16(4):203-12.
[Crossref] [Google Scholar] [PubMed]
- Report TW, Sheng-Shou HU. Report on cardiovascular health and diseases in China 2021: An updated summary. J Geriatr Cardiol 2023;20(6):399.
- Zheng Y, Li C, Yang J, Seery S, Qi Y, Wang W, et al. Atherogenic index of plasma for non-diabetic, coronary artery disease patients after percutaneous coronary intervention: A prospective study of the long-term outcomes in China. Cardiovasc Diabetol 2022;21(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Suo W, Zhang X, Lv J, Liu Z, Liu R. Roles and mechanisms of quercetin on cardiac arrhythmia: A review. Biomed Pharmacother 2022;153:113447.
[Crossref] [Google Scholar] [PubMed]
- Xiang M, Lu Y, Xin L, Gao J, Shang C, Jiang Z, et al. Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid Med Cell Longev 2021;2021:6614009.
[Crossref] [Google Scholar] [PubMed]
- Korshunova AY, Blagonravov ML, Neborak EV, Syatkin SP, Sklifasovskaya AP, Semyatov SM et al. BCL2-regulated apoptotic process in myocardial ischemia-reperfusion injury. Int J Mol Med 2021;47(1):23-36.
[Crossref] [Google Scholar] [PubMed]
- Wang R, Wang M, Zhou J, Wu D, Ye J, Sun G, et al. Saponins in Chinese herbal medicine exerts protection in myocardial ischemia–reperfusion injury: Possible mechanism and target analysis. Front Pharmacol 2021;11:570867.
[Crossref] [Google Scholar] [PubMed]
- Xu S, Wu B, Zhong B, Lin L, Ding Y, Jin X, et al. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)/System xc-/glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered 2021;12(2):10924-34.
[Crossref] [Google Scholar] [PubMed]
- Qu H, Guo Z, Ma L, Zhang X, Ma H, Chen Y. Antifungal effects and active compounds of the leaf of Allium mongolicum Regel. Front Chem 2022;10:993893.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Ding Z, Wu Y, Chen Q, Liu M, Yu H, et al. Effects of Allium mongolicum Regel and its flavonoids on constipation. Biomolecules 2019;10(1):14.
[Crossref] [Google Scholar] [PubMed]
- Li MY, Guo WQ, Guo GL, Zhu XM, Niu XT, Shan XF, et al. Effect of sub-chronic exposure to selenium and Allium mongolicum Regel flavonoids on Channa argus: Bioaccumulation, oxidative stress, immune responses and immune-related signaling molecules. Fish Shellfish Immunol 2019;91:122-129.
[Crossref] [Google Scholar] [PubMed]
- Zhao L, Yuan BD, Zhao JL, Jiang N, Zhang AZ, Wang GQ, et al. Amelioration of hexavalent chromium-induced bioaccumulation, oxidative stress, tight junction proteins and immune-related signaling factors by Allium mongolicum Regel flavonoids in Ctenopharyngodon idella. Fish Shellfish Immunol 2020;106:993-1003.
[Crossref] [Google Scholar] [PubMed]
- Li MY, Zhu XM, Niu XT, Chen XM, Tian JX, Kong YD, et al. Effects of dietary Allium mongolicum Regel polysaccharide on growth, lipopolysaccharide-induced antioxidant responses and immune responses in Channa argus. Mol Biol Rep 2019;46(2):2221-30.
[Crossref] [Google Scholar] [PubMed]
- Tan YT, Lin JF, Li T, Li JJ, Xu RH, Ju HQ. lncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun (Lond) 2021;41(2):109-20.
[Crossref] [Google Scholar] [PubMed]
- Huang Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med 2018;22(12):5768-75.
[Crossref] [Google Scholar] [PubMed]
- Luan D, Jiang C. The mechanism of lncRNA TALNEC2 regulating miR-19a-3p/JNK to alleviate cerebral ischemia injury in rats with acute cerebral infarction. Cell Mol Biol 2022;68(6):17-24.
[Crossref] [Google Scholar] [PubMed]
- Cao Y, Gao W, Tang H, Wang T, You C. Long Non-coding RNA TALNEC2 aggravates cerebral Ischemia/Reperfusion injury via acting as a competing endogenous RNAs for miR-650 to target apoptotic peptidase activating factor 1. Neuroscience 2021;458:64-76.
[Crossref] [Google Scholar] [PubMed]
- Hao L, Wang J, Liu N. Long noncoding RNA TALNEC2 regulates myocardial ischemic injury in H9c2 cells by regulating miR-21/PDCD4-medited activation of Wnt/β-catenin pathway. J Cell Biochem 2019;120(8):12912-23.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Zhou M, Liu H, Liu W, Chen J. Protective effects of Shen Yuan Dan on myocardial ischemia-reperfusion injury via the regulation of mitochondrial quality control. Cardiovasc Diagn Ther 2023;13(2):395.
[Crossref] [Google Scholar] [PubMed]
- Gumpper-Fedus K, Park KH, Ma H, Zhou X, Bian Z, Krishnamurthy K, et al. MG53 preserves mitochondrial integrity of cardiomyocytes during ischemia reperfusion-induced oxidative stress. Redox Biol 2022;54:102357.
[Crossref] [Google Scholar] [PubMed]
- Zhu Q, Luo Y, Wen Y, Wang D, Li J, Fan Z. Semaglutide inhibits ischemia/reperfusion-induced cardiomyocyte apoptosis through activating PKG/PKCε/ERK1/2 pathway. Biochem Biophys Res Commun 2023;647:1-8.
[Crossref] [Google Scholar] [PubMed]
- Bai J, Wang X, Du S, Wang P, Wang Y, Quan L, et al. Study on the protective effects of Danshen-Honghua Herb Pair (DHHP) on Myocardial Ischaemia/Reperfusion Injury (MIRI) and potential mechanisms based on apoptosis and mitochondria. Pharm Biol 2021;59(1):333-44.
[Crossref] [Google Scholar] [PubMed]
- Song M, Cui X, Zhang J, Li Y, Li J, Zang Y, et al. Shenlian extract attenuates myocardial ischaemia-reperfusion injury via inhibiting M1 macrophage polarization by silencing miR-155. Pharm Biol 2022;60(1):2011-24.
[Crossref] [Google Scholar] [PubMed]
- Yu SY, Dong B, Fang ZF, Hu XQ, Tang L, Zhou SH. Knockdown of lncRNA AK139328 alleviates myocardial ischaemia/reperfusion injury in diabetic mice via modulating miR-204-3p and inhibiting autophagy. J Cell Mol Med 2018;22(10):4886-98.
[Crossref] [Google Scholar] [PubMed]
- Fu D, Gao T, Liu M, Li C, Li H, Jiang H, et al. LncRNA TUG1 aggravates cardiomyocyte apoptosis and myocardial ischemia/reperfusion injury. Histol Histopathol 2021;36(12):1261-72.
[Crossref] [Google Scholar] [PubMed]
- Zhu X, Wu Y, Zhang X, Gu W, Ning Z. Stachydrine ameliorates hypoxia reoxygenation injury of cardiomyocyte via enhancing SIRT1-Nrf2 pathway. J Cardiothor Surg 2023;18(1):265.
- Sun S, Mei X. Effect of CASC15 on apoptosis and oxidative stress of cardiomyocytes after hypoxia/reperfusion injury. Rev Port Cardiol 2023;2551(23):391-8.
[Crossref] [Google Scholar] [PubMed]
- Chai R, Ye Z, Xue W, Shi S, Wei Y, Hu Y, et al. Tanshinone IIA inhibits cardiomyocyte pyroptosis through TLR4/NF-κB p65 pathway after acute myocardial infarction. Front Cell Dev Biol 2023;11:1252942.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Zhang T, Zhai J, Wang Z, Wang Y, He L, et al. miR-21 attenuates FAS-mediated cardiomyocyte apoptosis by regulating HIPK3 expression. Biosci Rep 2023;43(9):BSR20230014.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Xin J, Chen W, Jing L, Zhang P. Icariin alleviates hypoxia-induced damage in MC3T3-E1 cells by downregulating TALNEC2. Biotechnol Appl Biochem 2020;67(6):1000-10.
[Crossref] [Google Scholar] [PubMed]