- *Corresponding Author:
- H. L. Guo
Department of Stomatology, School and Hospital of Stomatology, Tianjin Medical University, Qixiangtai, Heping, Tianjin 300070, China
E-mail: hguo@tmu.edu.cn
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “36-43” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To better understand the functions of T-cell lymphoma invasion and metastasis-inducing factor 1 for oral squamous cell carcinoma patients with lymphoma metastasis, we performed this study. This study was a retrospective study using clinical recruited oral squamous cell carcinoma individuals. The differentially expressed messenger ribonucleic acid and micro ribonucleic acid for oral squamous cell carcinoma patients with lymphoma metastasis was generated compared with conventional oral squamous cell carcinoma. The primary differentially expressed messenger ribonucleic acids and differentially expressed micro ribonucleic acids were further investigated in human lymphomas cell line Rec-1. The oral squamous cell carcinoma patients with lymphoma metastasis demonstrated 268 differentially expressed messenger ribonucleic acids and 50 differentially expressed micro ribonucleic acids. The T-cell lymphoma invasion and metastasis-inducing factor 1 gene and micro ribonucleic acid-30a was the primary differentially expressed messenger ribonucleic acids and differentially expressed micro ribonucleic acids respectively. The concentration of T-cell lymphoma invasion and metastasis-inducing factor 1 in the peripheral blood of oral squamous cell carcinoma patients with lymphoma metastasis was dramatically increased to 83.2±19.8 ng/ml, compared with 34.8±11.6 ng/ml in conventional oral squamous cell carcinoma patients (p<0.05). The T-cell lymphoma invasion and metastasisinducing factor 1 was a direct target for micro ribonucleic acid-30a. T-cell lymphoma invasion and metastasisinducing factor 1 activated the Wingless-related integration site and c-Jun N-terminal kinase signaling pathways based on enrichment analysis and cellular functional experiments. Rec-1 cells transfected with T-cell lymphoma invasion and metastasis-inducing factor 1, small interfering ribonucleic acid significantly reduced the colony formation, cell proliferation and cell invasion while elevated cell apoptosis. This study hypothesized T-cell lymphoma invasion and metastasis-inducing factor 1 as a key regulator for oral squamous cell carcinoma with lymphoma metastasis, which was modulated by micro ribonucleic acid-30a.
Keywords
Lymphomas, micro ribonucleic acid-30a, oral squamous cell carcinoma, T-cell lymphoma invasion and metastasis-inducing factor 1
Lymphomas are considered as the most malignancy affecting almost any organ in the human body. Clinically, with the detection of primary care physicians and physicians from most specialties, lymphomas demonstrate a broad range of typical as well as atypical symptoms[1]. Lymphomas are subdivided into non-Hodgkin lymphoma (which accounts for about 90 % of all lymphomas) and Hodgkin lymphoma (which accounts for about 10 % of all lymphomas) types[2]. The lymphoma patients with B cell origin make up over 90 % of all lymphomas, as the rest are lymphoma patients with T cell or natural killer cell origin. Lymphomas originates from lymphocytes with multiple stages of development, meanwhile the characteristics of the specific lymphoma subtype reflect those of the cell from which they originated. Moreover, the lymphoma displays a wide spectrum of metastasis including lung[3], neurologic system[4], liver[5] and oral cavity. Oral Squamous Cell Carcinoma (OSCC) is currently suggested as the sixth leading malignancy worldwide, which comprises for more than 90 % of all oral malignancies as well as approximately
38 % of head and neck tumors[6]. As 500 000 new cases diagnosed every year, the administration for OSCC has become a worldwide medical issue[7]. The primary risk factors for OSCC include diabetes, alcoholism, smoking as well as dietary habits especially in developed regions[8-10]. With decades of efforts, multiple molecular mechanisms have been implied in the development and formation of OSCC. Importantly, in 2011, research revealed top 10 primary key alterations fundamental to OSCC development, which are sustaining proliferative signaling, evading growth suppressors, avoiding immune destruction, activating invasion and metastasis, tumor-promoting inflammation, enabling replicative immortality, inducing angiogenesis, genome instability and mutation, resisting cell death and deregulating energetics[11]. Among these, the activating invasion and metastasis from lymphomas is considered as a critical procedure. However, even with years of study, the knowledge of the genetic basis for OSCC with lymphoma metastasis is still far from satisfactions.
T-cell lymphoma invasion and metastasis-inducing factor 1 (Tiam1), which was originally recognized as an invasion-inducing gene by proviral insertion combined with in vitro selection of invasive mouse T-lymphoma variants[12]. The Tiam1 gene is a Wingless-related integration site (Wnt) signaling associated gene which drives cancerous cell selfrenewal and metastasis, the functions of which are modulated by Cancer Associated Fibroblasts (CAFs). In colorectal cancer, it has been shown that Tiam is functional as a key antagonist of Wnt signaling in colorectal cancer progression via inhibiting Transcriptional Coactivator with PDZbinding motif (TAZ) and Yes-Associated Protein (YAP)[13]. Moreover, the dysregulation of Tiam1 has been suggested in a wide range of cancers, including esophageal[14], renal carcinoma[15], thyroid[16] and nasopharyngeal[17]. At the same time, Tiam1 has been also approved to have close connections with various processes of tumor formation such as apoptosis, migration, as well as invasion[18,19]. However, the connections between Tiam1 and OSCC with lymphomas metastasis are still poorly understood.
To better understand the mechanisms underlined the OSCC with lymphoma metastasis, in this study, a comprehensive differential expression analysis for OSCC with lymphoma metastasis has been generated compared with conventional OSCC.
Tiam1 displayed a specific expression pattern for OSCC with lymphomas metastasis, which was a potential target for micro Ribonucleic acid (miR)- 30a for the process. The functions of the innovative signaling axis for lymphomas metastasis have been also in-depth studied based on both in vivo and in vitro analysis, which provided a beneficial guidance for future OSCC study.
Materials and Methods
Study subjects:
This study was a retrospective study using clinical recruited OSCC patients. 20 OSCC patients (including 10 conventional OSCC, named OSCC group and 10 OSCC with lymphoma metastasis, named OSCCL group) were randomly selected from School of Stomatology, Tianjin Medical University. The patients from two groups underwent tumor biopsy or resection. All procedure in this study was approved by the institutional review committee of Tianjin Medical University and all the informed consents have been obtained from the study participants prior to study commencement (Number: TMUhMEC20211209). There was no significant difference for clinical characteristics (age, sex etc.) between two groups.
The recruited OSCC patients were randomly selected based on the following inclusion criteria which includes, adults (age from 20 to 60 y); confirmed by imaging method such as Computed Tomography (CT) scanning; positive for other laboratory diagnosis and pathological examination. The exclusion criteria include presence of other malignancy except OSCC and presence of other chronic oral disorders. The reporting of this study conforms to Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines[20].
High-throughput sequencing:
The cancerous tissues from each individual were snap-frozen and stored in liquid nitrogen. The total RNA was extracted using RNAiso Plus (Takara, Beijing, China). The high-throughput sequencing was conducted and analyzed by Genewiz Co. Tianjin based on ABI solid sequencer platform.
Enzyme-Linked Immunosorbent Assay (ELISA) analysis of Tiam1:
The concentration of Tiam1 protein was determined by ELISA double antibody sandwich method in peripheral blood of OSCC patients. The specific operation was carried out in strict accordance with the instructions of the kit (Abcam company, US). The experimental results were repeated three times independently and were tested by statistical methods.
Prediction of miRNA target genes:
The target genes of miRNAs were predicted through the microRNA Target Prediction Database (miRDB) (http://mirdb.org/index.html, version 6.0) database. Meanwhile, the Cytoscape (https://cytoscape.org/, version 3.7.2) was performed to visualize the miRNAmessenger RNA (mRNA) regulatory network.
Cell culture:
The human lymphoblast cell line (Rec-1) was purchased from American Type Culture Collection (ATCC) Co. The base medium for this cell line was ATCC-formulated Roswell Park Memorial Institute (RPMI)-1640 Medium, Catalog No. 30-2001 with fetal bovine serum to a final concentration of 10 %.
The luciferase reporter assay:
The 3' non-coding region of Tiam1 was synthesized and inserted into the Xho1 and NotⅠ sites of the pCheck2 reporter luciferase vector downstream of the luciferase gene after annealing. The wild-type or mutant plasmid, pCheck2 plasmid and the same amount of negative control or miR-30a simulated plasmid were generated for co-transfection of cells. The luciferase analysis was performed by dual luciferase reporter analysis system (Promega).
Transfection of miR-30a mimics and inhibitors as well as Tiam1 small interfering RNA (siRNA):
The specific miR-30a mimic/inhibitor (Ambion) and an according negative control (Ambion) were transfected into the Rec-1 cells. For siRNA transfection, the Tiam1 siRNA (50 nmol/l) (OriGene) and corresponding negative control were transfected into the Rec-1 cells.
Cell proliferation, invasion and apoptosis measurement:
The cell proliferation of each group was evaluated using Cell Counting Kit-8 (CCK-8) (Fisher, China). At the same time, the cell apoptosis was examined based on flow cytometry analysis after Annexin V Fluorescein Isothiocyanate (FITC)/Propidium Iodide double staining. The cell invasion test was measured as previously described[21].
Functional enrichment analysis:
The “clusterProfiler” functions package in R language was developed for the enrichment analysis of Gene Ontology (GO) (including biological process, molecular function and cellular component) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway[22]. When p-value<0.05, we considered the corresponding entries to be significantly enriched.
Statistical analysis:
All the experiments were conducted triplicate independently. The Statistical Analysis System (SAS) 9.4 software was developed for data analysis. The continuous variables were tested for normal distribution and the student t-test in SAS 9.4 was used to analyze the difference between two groups, with p<0.05 as a significant difference.
Results and Discussion
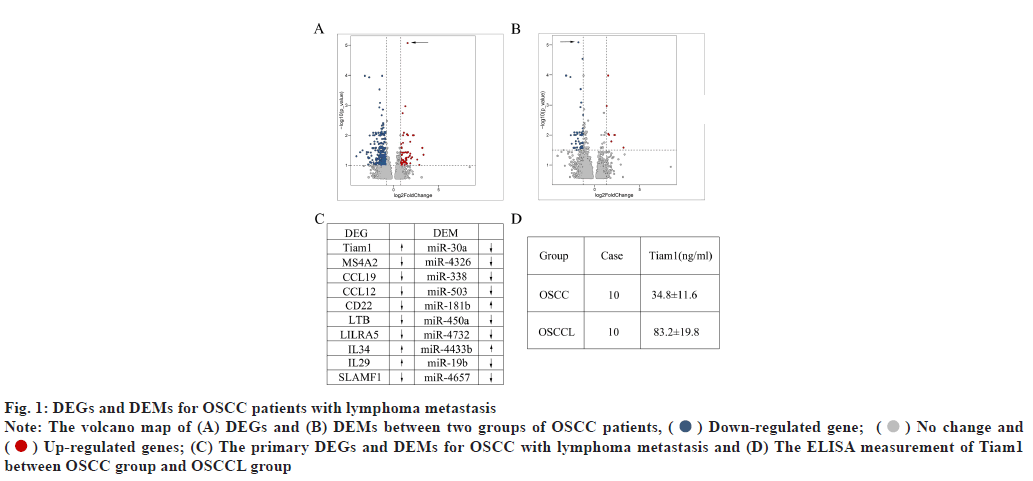
Differentially Expressed mRNA (DEGs) and Differentially Expressed miRNA (DEMs) for OSCC patients with lymphomas metastasis was shown in fig. 1A-fig. 1D. The volcano map of DEGs between two groups of OSCC patients were drawn where the horizontal axis represents the multiple of differential expression (Log2FC), the vertical axis represents -log10 False Discovery Rate (FDR). Compared with patients in OSCC group, patients in OSCCL group demonstrated 268 differentially expressed genes (DEGs) including 202 down-regulated ones and 46 up-regulated ones (fig. 1A). Among these, Tiam1, Membrane Spanning 4-Domains A2 (MS4A2), C-C Motif Chemokine Ligand 19 (CCL19), C-C motif Ligand 12 (CCL12), Cluster of Differentiation-22 (CD22), Lymphotoxin Beta (LTB), Leukocyte Immunoglobulin Like Receptor A5 (LILRA5), Interleukin 34 (IL34), Interleukin 29 (IL29) and Signaling Lymphocytic Activation Molecule Family member 1 (SLAMF1) were the primary DEGs, with Tiam1 was significantly enhanced in OSCCL (fig. 1C). The volcano map of DEMs between two groups of OSCC patients were plotted where the horizontal axis represents the multiple of differential expression (Log2FC), the vertical axis represents -log10 (FDR). At the same time, there were 50 DEMs between two groups, including 42 down-regulated ones and 8 upregulated ones (fig. 1B). The miR-30a, miR-4326, miR-338, miR-503, miR-181b, miR-450a, miR 4732, miR-4433b, miR-19b and miR-4657 were the top 10 DEMs, as the miR-30a significantly decreased in OSCCL patients (fig. 1C). As the most significantly different DEG for OSCC patients with lymphomas metastasis, the concentration of Tiam1 was further evaluated using ELISA assay. As shown in fig. 1D, the concentration of Tiam1 in OSCCL patients was dramatically increased to 83.2±19.8 ng/ml, compared with 34.8±11.6 ng/ml in OSCC patients (p<0.05).
Fig. 1: DEGs and DEMs for OSCC patients with lymphoma metastasis
Note: The volcano map of (A) DEGs and (B) DEMs between two groups of OSCC patients, ( ) Down-regulated gene; (
) Down-regulated gene; ( ) No change and
(
) No change and
( ) Up-regulated genes; (C) The primary DEGs and DEMs for OSCC with lymphoma metastasis and (D) The ELISA measurement of Tiam1
between OSCC group and OSCCL group
) Up-regulated genes; (C) The primary DEGs and DEMs for OSCC with lymphoma metastasis and (D) The ELISA measurement of Tiam1
between OSCC group and OSCCL group
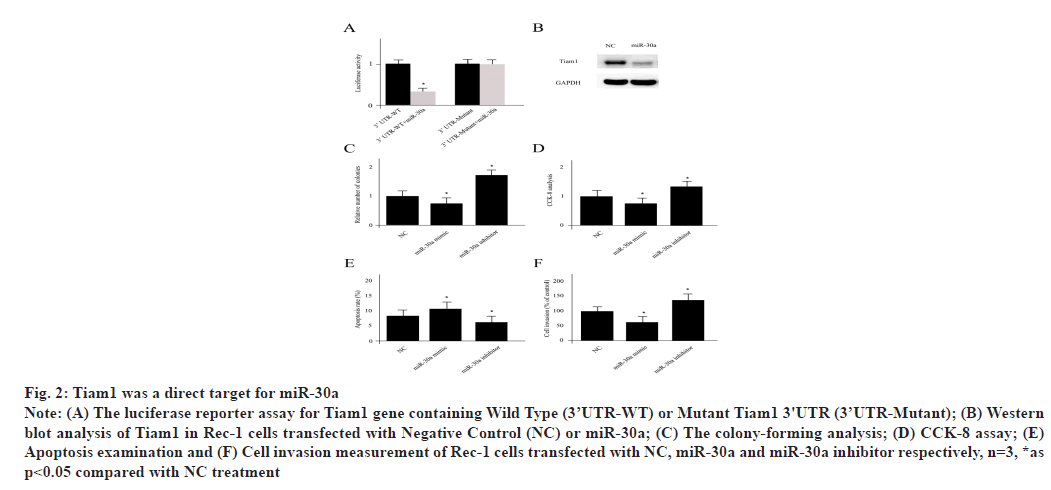
Tiam1 was a key target for miR-30a which was explained here. The luciferase reporter for Tiam1 gene containing Wild Type (3’UTR-WT) or Mutant Tiam1 3'UTR (3’UTR-Mutant) was shown in fig. 2A. The mRNA-miRNA interaction was generated by 3 programs including miRDB, TargetScan and miRTarBase, which suggested that Tiam1 was a potential target for miR-30a. In Rec-1 cells, miR-30a transfection significantly repressed the expression level of Tiam1 (fig. 2B). In order to verify whether Tiam1 was a direct target for miR-30a, a luciferase containing three miR-30a targeting sequences was co-transfected into Rec-1 cells and the decreased activity of luciferase was observed. However, the co-transfection of miR-30a with the mutant form of Tiam1 3′ Untranslated Regions (3'UTR) without all three binding sites significantly restored the luciferase activity. The miR-30a treated Rec-1 cells displayed decreased colony formation, low level of proliferation and invasion as well as elevated cell apoptosis, which were all attenuated by miR-30a inhibitor treatment (fig. 2C-fig. 2F).
Fig. 2: Tiam1 was a direct target for miR-30a
Note: (A) The luciferase reporter assay for Tiam1 gene containing Wild Type (3’UTR-WT) or Mutant Tiam1 3'UTR (3’UTR-Mutant); (B) Western
blot analysis of Tiam1 in Rec-1 cells transfected with Negative Control (NC) or miR-30a; (C) The colony-forming analysis; (D) CCK-8 assay; (E)
Apoptosis examination and (F) Cell invasion measurement of Rec-1 cells transfected with NC, miR-30a and miR-30a inhibitor respectively, n=3, *as
p<0.05 compared with NC treatment
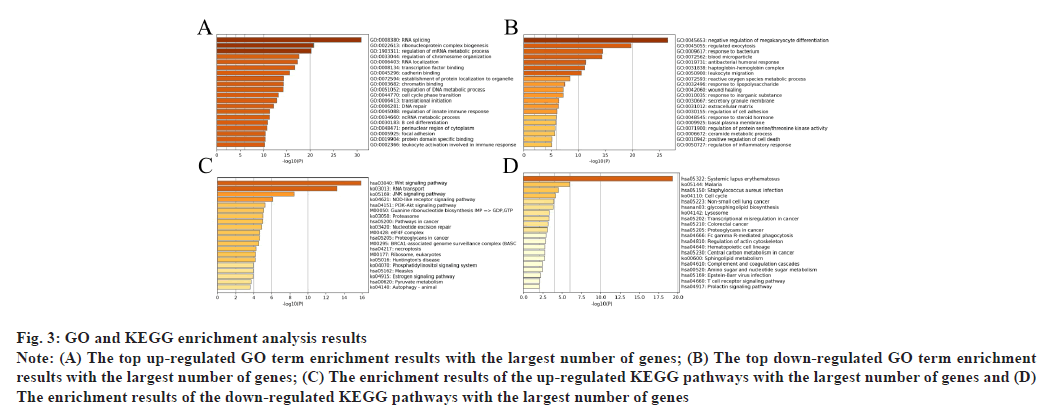
GO and KEGG enrichment analysis results were shown in fig. 3. Based on the GO and KEGG enrichment analysis on these 268 differentially expressed genes, it could be found that the DEGs were significantly enriched in GO terms related to the biological processes such as RNA splicing (up-regulation) and regulated exocytosis (downregulation) etc. In the figure, the horizontal axis represents the number of enriched genes and the vertical axis represents the name of each GO term and KEGG pathway respectively (fig. 3A and fig. 3B). At the same time, the Wnt signaling and c-Jun N-terminal kinase (JNK) signaling pathways were significantly enriched in KEGG pathway analysis (fig. 3C and fig. 3D).
Fig. 3: GO and KEGG enrichment analysis results
Note: (A) The top up-regulated GO term enrichment results with the largest number of genes; (B) The top down-regulated GO term enrichment
results with the largest number of genes; (C) The enrichment results of the up-regulated KEGG pathways with the largest number of genes and (D)
The enrichment results of the down-regulated KEGG pathways with the largest number of genes
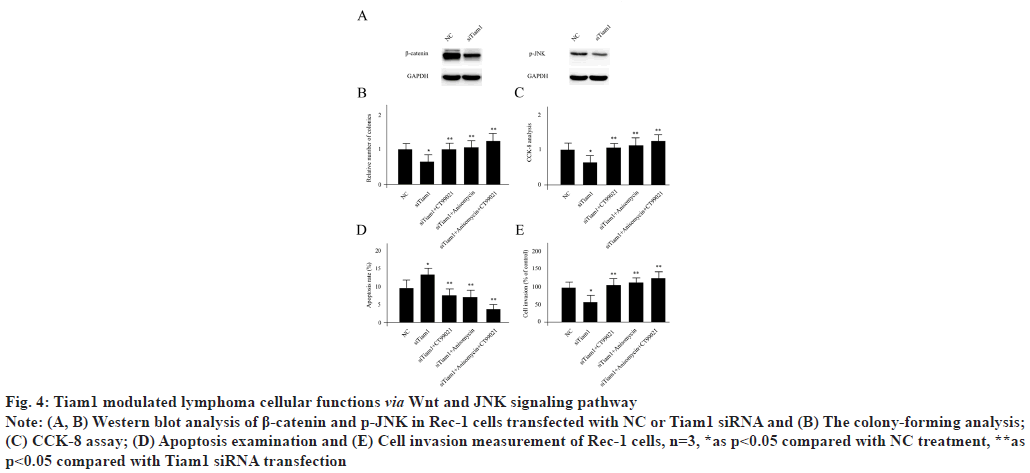
Tiam1 modulated lymphoma cellular functions via Wnt and JNK signaling pathway. Since Tiam1 was suggested to be closely associated with Wnt signaling and JNK signaling pathways by enrichment analysis, next we sought to explore the connections using functional experiments in Rec-1 cells. Knocking down of Tiam1 significantly reduced the expression level of beta (β)-catenin and slightly decreased the activity of p-JNK (fig. 4A). 10 nM of CT99021 monohydrochloride was used as a β-catenin specific activator and 0.233 μM anisomycin was utilized as a phosphorylated JNK (p-JNK) activator to treat Rec-1 cells. Tiam1 siRNA transfection significantly repressed the colony formation, cell proliferation and cell invasion which enhanced cell apoptosis. Meanwhile, these phenomenon’s were reversed by CT99021 monohydrochloride or anisomycin treatment individually or combined together respectively (fig. 4B-fig. 4E).
Fig. 4: Tiam1 modulated lymphoma cellular functions via Wnt and JNK signaling pathway
Note: (A, B) Western blot analysis of β-catenin and p-JNK in Rec-1 cells transfected with NC or Tiam1 siRNA and (B) The colony-forming analysis;
(C) CCK-8 assay; (D) Apoptosis examination and (E) Cell invasion measurement of Rec-1 cells, n=3, *as p<0.05 compared with NC treatment, **as
p<0.05 compared with Tiam1 siRNA transfection
Even with years of hard work for the breakthroughs in the prevention, early screening, diagnosis and treatments of OSCC, the disorder has brought many obstacles to clinical treatment based on the facts of high mortality[23]. With the circulatory characteristics of lymphoma, it has been shown to play a fundamental role in several carcinomas with short or distant metastasis[24,25]. The OSCC induced by lymphoma metastasis has been approved as a major risk factor. However, the underlined molecular mechanism is still poorly understood. Here, in this study, we systematically compared the conventional OSCC and OSCC with lymphoma metastasis patients and initiated Tiam1 as a primary DEG. Tiam1 was originally identified by Habets and his colleagues based on a proviral tagging in combination with in vitro selection for invasiveness[12]. Cell clones that were invasive in vitro could successively generate experimental metastases in nude mice and transfection of truncated Tiam-1 complementary Deoxyribonucleic Acid (cDNA) into noninvasive cells made these cells invasive, which supported the invasion functions of the gene. Structurally, the protein encoded by Tiam1 is similar to the Guanosine Diphosphate (GDP)-Guanosine Triphosphate (GTP) exchangers for Rho-like proteins that have been implicated in cytoskeletal organization. Later, Tiam1, as it is called as T-lymphoma invasion and metastasis 1, was suggested to be a specific activator for the Rho-like Guanosine Triphosphatases (GTPase) Rac. In vivo, Tiam1 deficiency protected against Ras-induced skin carcinogenesis, underscoring the consequences of deregulated signaling for the onset and progression of tumours[26]. Currently, Tiam1 has been shown to be closely associated with various cancers, including esophageal[14], renal carcinoma[15], thyroid[16] and nasopharyngeal[17]. However, the connection between Tiam1 and lymphoma especially OSCC with lymphoma metastasis has not been fully expanded. The B-cell lymphoma is the majority of lymphoma, which comprise over 90 % of all lymphomas. While, if Tiam1 is the primary DEG for OSCC with lymphoma metastasis, T-cell lymphoma might play a pivotal function in this process.
Previously, a report by Song et al. aimed to explore the expression of Tiam1 in OSCC patients and investigated its clinical significance[27]. They compared the activity of Tiam1 in cancerous tissues from 120 cases of OSCC and oral mucosa from 40 normal cases using immunohistochemistry method. Interesting, they claimed that the positive expression rate of Tiam1 in the OSCC tissues was dramatically higher than that in the normal oral mucosa (92.5 % vs. 0 %). Whereas, the expression level of Tiam1 was associated with Tumor Node Metastasis (TNM) stage and the occurrence of lymph node metastasis. However, they did not distinguish the conventional OSCC and OSCC with lymphoma metastasis. Based on our observation, Tiam1 was critical for the formation OSCC with lymphoma metastasis, which indicated that the OSCC caused by lymphoma invasion might make up a high percentage of total OSCC patients.
In addition to the key DEG in this study, we also suggested an innovative genetic network for OSCC with lymphoma metastasis. The miR-30a was demonstrated to be an important DEM for the disorder and also the modulator for Tiam1. Previously, the metastasis of ovarian cancer has been reported to be regulated by miR-30a. They claimed that miR-30a and mucin type O-glycan biosynthesis were closely connected to ovarian cancer. Meanwhile, miR-30a was significantly downregulated in ovarian cancer cells and the overexpression of miR-30a decreased cell viability and inhibited migration and invasion. The S phase Kinase-Associated Protein 2 (SKP2), B Cell Lymphoma 9 (BCL9) and Neurogenic Locus Notch Homolog Protein 1 (NOTCH 1) were the direct target genes for miR-30a in this process. There was no clue for the connection between Tiam1 and miR-30a before our study. While, in hepatocellular carcinoma, miR-377 was found to be markedly downregulated and inhibit cell growth and invasion, with Tiam1 was a direct and functional target[28]. Finally, miR-377 was inversely correlated with Tiam1 expression in human hepatocellular carcinoma tissues, suggesting miR-377 was functional as a tumor suppressor. We could not detect the miR-377 as a DEM in this study, which confirmed the organ specificity for these DEMs.
Accumulating evidences have found the close relationships between Tiam1 and Wnt signaling as well as JNK signaling pathways[29]. A study by Diamantopoulou et al. implied that Tiam1 localized to the destruction complex of β-catenin and promoted TAZ degradation by enhancing its interaction with β-Transducin repeat-containing Protein (βTrCP) in the cytoplasm[13]. At the same time, JNK/Activating Transcription Factor-2 (ATF-2) signaling pathway is closely associated with the invasion, metastasis, epithelial-mesenchymal transition and apoptosis of malignant tumors[30]. While the Tiam1, has been illustrated to be significantly correlated with laryngeal squamous cell carcinoma aggressive action and a poor outcome by regulating the expression of JNK/ATF-2 signaling pathway both in vitro and in vivo after radiation[31].
Collectively, to better understand the molecular mechanisms behind OSCC with lymphoma metastasis, we in-depth generated a differential expression profile for OSCC patients treated with lymphoma metastasis and provided miR-30a-Tiam1 signaling axis for the procedure. Even the majority of results were initiated from basic experiments, future perspectives of the study for clinical implications should be mentioned here. As discussed before, the patients with lymphomas barely demonstrated a typical symptom, which greatly hamper the diagnosis for the individuals in clinic. Furthermore, there is still missing a clinical biomarker to distinguish the conventional OSCC patients and OSCC patients with lymphoma metastasis. Here, we examined the concentration of Tiam1 protein using ELISA double antibody sandwich method in peripheral blood of OSCC patients from both groups. This may not only give a hint for future biomarker discovery but also be a first step for effective laboratory examination. All the work here may provide several new thoughts for the future OSCC patient’s research.
Several limitations have to be pointed out in the study. First, the signaling axis of the work was based on the observations of Rec-1 cell line, which is a human lymphoma cell line but not a specific line for OSCC patients with lymphoma metastasis. Based on the fact of lacking particular cell line, the results might introduce some deviations. Second, we focused on the most significant DEGs and miRNA here. However, other potential genes might be also crucial for tumor development. Third, the sample size was restricted in this study. To obtain the final translational value, increased sample size and multicenter recruited individuals may be required.
Author’s contributions:
Honglei Guo, Ning Wang Bo Xiao and Wantong Wu contributed equally to this work. Conception of the manuscript was done by Ning Wang; interpretation or analysis of data was done by Honglei Guo and Bo Xiao; preparation of the manuscript was done by Wantong Wu; revision for important intellectual content was done by Youbang Xie and supervision was carried out by Wantong Wu.
Conflict of interests:
The authors declared no conflict of interest.
References
- Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-hodgkin lymphoma. Lancet 2017;390(10091):298-310.
[Crossref] [Google scholar] [PubMed]
- Mugnaini EN, Ghosh N. Lymphoma. Prim Care 2016;43(4):661-75.
[Crossref] [Google scholar] [PubMed]
- Pisapia P, Malapelle U, Troncone G. Liquid biopsy and lung cancer. Acta Cytol 2019;63(6):489-96.
[Crossref] [Google scholar] [PubMed]
- Mauermann ML. Neurologic complications of lymphoma, leukemia and paraproteinemias. Continuum 2017;23(3):669-90.
[Crossref] [Google scholar] [PubMed]
- Wilcox RA. Cutaneous T‐cell lymphoma: 2017 update on diagnosis, risk‐stratification and management. Am J Hematol 2017;92(10):1085-102.
[Crossref] [Google scholar] [PubMed]
- Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol 2015;8(9):11884-94.
[Google scholar] [PubMed]
- Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer (Primer). Nat Rev Dis Primers 2017;3(1):17048.
[Crossref] [Google scholar] [PubMed]
- Alves AM, Diel LF, Lamers ML. Macrophages and prognosis of oral squamous cell carcinoma: A systematic review. J Oral Pathol Med 2018;47(5):460-7.
[Crossref] [Google scholar] [PubMed]
- Chakraborty D, Natarajan C, Mukherjee A. Advances in oral cancer detection. Adv Clin Chem 2019;91:181-200.
[Crossref] [Google scholar] [PubMed]
- Sasahira T, Kirita T. Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcinoma. Int J Mol Sci 2018;19(8):2413.
[Crossref] [Google scholar] [PubMed]
- Afzali P, Ward BB. Management of the neck in oral squamous cell carcinoma: Background, classification and current philosophy. Oral Maxillofac Surg Clin North Am 2019;31(1):69-84.
[Crossref] [Google scholar] [PubMed]
- Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, et al. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 1994;77(4):537-49.
[Crossref] [Google scholar] [PubMed]
- Diamantopoulou Z, White G, Fadlullah MZ, Dreger M, Pickering K, Maltas J, et al. TIAM1 antagonizes TAZ/YAP both in the destruction complex in the cytoplasm and in the nucleus to inhibit invasion of intestinal epithelial cells. Cancer Cell 2017;31(5):621-34.
[Crossref] [Google scholar] [PubMed]
- Wu QY, Wang Y, Tong JC, Zhang M, Zhang K. Expression and clinical significance of Tiam1 gene in esophageal carcinoma. Int J Clin Exp Med 2015;8(11):21229-34.
[Google scholar] [PubMed]
- Shan G, Tang T, Qian H, Xia Y. Expression of Tiam1 and Rac1 proteins in renal cell carcinoma and its clinical-pathological features. Int J Clin Exp Pathol 2017;10(11):11114-21.
[Google scholar] [PubMed]
- Liu L, Wu B, Cai H, Li D, Ma Y, Zhu X, et al. Tiam1 promotes thyroid carcinoma metastasis by modulating EMT viaWnt/β-catenin signaling. Exp Cell Res 2018;362(2):532-40.
[Crossref] [Google scholar] [PubMed]
- Ding Y, Chen B, Huang J, Zhang W, Yang H, Deng Y, et al. Overexpression of Tiam1 is associated with malignant phenotypes of nasopharyngeal carcinoma. Oncol Rep 2014;32(2):607-18.
[Crossref] [Google scholar] [PubMed]
- Boissier P, Huynh-Do U. The guanine nucleotide exchange factor Tiam1: A Janus-faced molecule in cellular signaling. Cell Signal 2014;26(3):483-91.
[Crossref] [Google scholar] [PubMed]
- Ding M, Li Y, Yang Y, Zhu K, Che S, Lin Z, et al. Elevated expression of Tiam1 is associated with poor prognosis and promotes tumor progression in pancreatic cancer. Onco Targets Ther 2018:4367-75.
[Crossref] [Google scholar] [PubMed]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007;370(9596):1453-7.
[Crossref] [Google scholar] [PubMed]
- van Doren SR. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol 2015;44:224-31.
[Crossref] [Google scholar] [PubMed]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102(43):15545-50.
[Crossref] [Google scholar] [PubMed]
- Moskovitz J, Moy J, Ferris RL. Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol Rep 2018;20(2):1-7.
[Crossref] [Google scholar] [PubMed]
- Jiang M, Bennani NN, Feldman AL. Lymphoma classification update: T-cell lymphomas, Hodgkin lymphomas and histiocytic/dendritic cell neoplasms. Expert Rev Hematol 2017;10(3):239-49.
[Crossref] [Google scholar] [PubMed]
- Filip PV, Cuciureanu D, Diaconu LS, Vladareanu AM, Pop CS. MALT lymphoma: Epidemiology, clinical diagnosis and treatment. J Med Life 2018;11(3):187-93.
[Crossref] [Google scholar] [PubMed]
- Mertens AE, Roovers RC, Collard JG. Regulation of Tiam1-Rac signalling. FEBS Lett 2003;546(1):11-6.
[Crossref] [Google scholar] [PubMed]
- Song B, Wang LF, Fan XH, Zuo JH, Huang YM. Expression of T-lymphoma invasion and metastasis factor on the occurrence of oral squamous cell carcinoma. J Biol Regul Homeost Agents 2017;31(2):289-95.
[Google scholar] [PubMed]
- Wang L, Zhao S, Yu M. Mechanism of low expression of miR-30a-5p on epithelial-mesenchymal transition and metastasis in ovarian cancer. DNA Cell Biol 2019;38(4):341-51.
[Crossref] [Google scholar] [PubMed]
- Chen LC, Chueh TC, Tuan YF, Chen CC, Chien CC, Lee HY, et al. Activation of MAPK pathways and downstream transcription factors in 2‐aminobiphenyl‐induced apoptosis. Environ Toxicol 2015;30(2):205-11.
[Crossref] [Google scholar] [PubMed]
- Hammouda MB, Ford AE, Liu Y, Zhang JY. The JNK signaling pathway in inflammatory skin disorders and cancer. Cells 2020;9(4):857.
[Crossref] [Google scholar] [PubMed]
- Wang S, Zhu W, Ouyang L, Li J, Li S, Yang X. Up-regulation of Tiam1 promotes the radioresistance of laryngeal squamous cell carcinoma through activation of the JNK/ATF-2 signaling pathway. Onco Targets Ther 2020;13:7065-74.
[Crossref] [Google scholar] [PubMed]