- *Corresponding Author:
- S. Q. Ma
Department of Orthopaedics, Qinzhou Second People's Hospital, Qinzhou, Guangxi 535000, China
E-mail: mashiqian168@163.com
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “22-29” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The main objective of this study is to explore the clinical efficacy of sitagliptin combined with recombinant bovine basic fibroblast growth factor gel in the treatment of chronic diabetic foot ulcers and its effects on inflammatory factors. 116 patients with chronic diabetic foot ulcers were selected, in which 58 patients were treated with routine treatment and set as the control group. While the remaining 58 patients were treated with sitagliptin+recombinant bovine basic fibroblast growth factor gel on the basis of the control group and set as the combined group. The efficacy and various clinical therapeutic indexes were comparatively analyzed. The combined group showed higher efficacy than the control group after treatment, with smaller ulcer area and shorter ulcer wound healing time. In addition, the granulation tissue formation time and granulation tissue coverage were more significantly increased in the combined group compared with the control group, while the granulation tissue growth thickness was statistically reduced. Moreover, the serum high-sensitivity C-reactive protein and interleukin-6 levels were significantly reduced in the combined group, and the quality of life was obviously improved. Sitagliptin+recombinant bovine basic fibroblast growth factor gel is highly effective in the treatment of chronic diabetic foot ulcers and is a feasible clinical treatment scheme for the disease.
Keywords
Diabetic foot, chronic ulcer, sitagliptin, recombinant bovine basic fibroblast growth factor gel
As a chronic complication of diabetes, Diabetic Foot Ulcers (DFUs) are the foot ulcers and foot gangrenous lesions generally caused by nutritional disorders of the foot skin and subcutaneous tissue due to diabetic peripheral neuropathy and microangiopathy[1]. The International Diabetes Federation reported that the global incidence of DFUs was about 19 % to 34 % in 2018. About 1 % of diabetic patients in China are disabled due to diabetic foot gangrene every year and the DFU-associated mortality risk death is 2.5 times more than that of diabetes[2]. Moreover, DFUs lead to reduced psychosocial adaptability and quality of life in patients. Early mild ulcers that are not actively treated can develop into incurable ulcers, endangering patients' lives. Currently, debridement, decompression, antibacterial and vascular reconstruction are mainly used to treat DFUs, but none of them contribute to satisfactory results[3]. Thus, exploring effective and safe treatments for DFUs remains an urgent issue for medical practitioners.
Sitagliptin (SIT) has a good effect on various difficult-to-heal wounds and has been applied in the treatment of injuries such as burns[4], scalds[5] and ulcers[6]. However, the research on its application in DFU wounds is limited. Recombinant bovine basic Fibroblast Growth Factor (rb-bFGF) gel can repair wounds, promote tissue regeneration and improve local microcirculation, thus accelerating wound healing[7]. Previous studies believed that during the pathological process, the lower extremity blood vessels of DFU patients would be in chronic inflammatory state and there would be secretion of inflammatory factors[8]. High-sensitivity C-Reactive Protein (hs-CRP) is an acute reactive protein that is strongly associated with the collective inflammatory response[9]. Previous studies have shown that hs-CRP is closely related to vascular lesions in the body[10].
Interleukin-6 (IL-6) is a cytokine involved in the body's inflammatory response, which can regulate the growth and differentiation of a variety of cells and has a pro-pathological effect on diabetic foot[11]. Based on the fact that there has been no study on the efficacy of SIT+rb-bFGF in the treatment of DFUs and its effect on inflammatory factors, we conducted relevant analysis to observe its clinical efficacy in DFUs and explore possible mechanisms.
Materials and Methods
General information:
This study was approved by the Ethics Committee of Qinzhou Second People's Hospital and carried out in accordance with the Declaration of Helsinki. Because the study was retrospective and the data was anonymized, informed consent was not required. Patients with chronic DFUs treated in our hospital from December 2020 to December 2022 were selected.
Inclusion criteria:
Diagnosis of DFU according to the “Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes”[12] and the “Guidelines for the Diagnosis and Treatment of Diabetic Foot”[13]. Based on the Wagner classification of DFUs by the health department, the patients with Wagner grade between 2-6[14] are included in this study.
Exclusion criteria:
Patients who are allergic to the drugs used in this study, patients with severe cardiovascular diseases and few patients who withdrawn from the study.
Finally, 116 patients with chronic DFUs were included and grouped as a control group and a combined group with 58 patients in each group. The two groups were comparable in baseline data detailed in Table 1 (p>0.05).
| Characteristics | Control group (n=58) | Combined group (n=58) | χ2/t/Z | p |
|---|---|---|---|---|
| Sex | ||||
| Male | 36 (62.07) | 40 (68.97) | 0.611 | 0.435 |
| Female | 22 (37.93) | 18 (31.03) | ||
| Age (years) | 60.31±5.59 | 60.31±5.59 | 0.065 | 0.948 |
| BMI (kg/m2) | 23.98±3.22 | 24.06±3.15 | 0.135 | 0.893 |
| Duration of diabetes (years) | 3.68±0.54 | 3.86±0.67 | 1.593 | 0.114 |
| Blood glucose level (mmol/l) | 6.13±0.82 | 5.86±0.93 | 1.658 | 0.1 |
| Ulcer area (cm2) | 12.13±3.06 | 11.68±3.12 | 0.784 | 0.435 |
| Ulcer below the ankle joint (days) | 45.46±8.23 | 42.89±7.72 | 1.735 | 0.086 |
| Wagner classification of DFUs | ||||
| Grade 2 | 21 (36.21) | 26 (44.83) | -0.962 | 0.336 |
| Grade 3 | 15 (25.86) | 14 (24.14) | ||
| Grade 4 | 12 (20.69) | 10 (17.24) | ||
| Grade 5 | 10 (17.24) | 8 (13.79) |
Table 1: Baseline Data of the two Groups of Patients
Treatment methods:
Control group: Blood glucose control was carried out first by subcutaneous injection of insulin or continuous subcutaneous pumping of 30 R insulin (30 R is a premixed insulin containing 30 % short-acting insulin and 70 % intermediate-acting insulin), as well as oral hypoglycemic drugs when necessary, were used to control blood glucose. Blood pressure, blood lipids and other accompanying diseases were also properly managed. During insulin application, blood glucose (including fasting blood glucose and 2 h postprandial blood glucose) were monitored and patients were informed of the symptoms of hypoglycemia, methods to prevent hypoglycemia and emergency treatment measures. Then, the foot skin ulcer wound was treated. First, the ulcerative wound was rinsed with sterile saline to thoroughly clean the secretions. Then, the clear necrotic tissue was removed with sterile scissors. Next, 0.1 % benzalkonium bromide disinfection solution was used for cleaning and disinfection once a day. Finally, sterile gauze soaked with gentamicin liquid was spread on the ulcer surface for dressing, maintaining seamless contact between the sterile gauze and the ulcerated surface to ensure complete drug absorption. At the same time, according to the bacterial culture results and drug sensitivity test of the ulcer surface, effective antibiotics were used for anti-infection treatment in the early stage and were discontinued after the development of granulation tissue and the decrease of exudation, depending on the situation. Furthermore, aspirin (Beijing Tianhui Pharmaceutical Co., Ltd, SFDA approval number: H43021776, 30 tablets×2 boards/box), vasodilator drugs, microcirculation-improving drugs, nutritional nerve medication and other comprehensive treatment were given as appropriate. During treatment whenever secretion was found, dressing need to be changed, long-term drooping and weight-bearing of the affected limb were avoided as much as possible. After cleaning the foot every night, safflower oil (Guangdong Taienkang Pharmaceutical Factory Co., Ltd., SFDA approval number: Z44023725) was applied to the whole foot skin and the foot was gradually massaged upward from the toes to promote blood circulation and the treatment lasted for 28 d.
Combined group: The recommended dose of bFGF was applied to the wound, followed by oral administration of SIT. Rb-bFGF gel (Zhuhai Yisheng Biopharmaceutical Co., Ltd., SFDA approval number: S20040001, 21 000 IU/5 g) was gently applied to the ulcer wound with a recommended dose of 300 IU/cm2, three times/day. SIT (Hangzhou MSD Pharmaceutical Co., Ltd., SFDA approval number: J20140095, 100 mg/tablet) was taken orally 100 mg once a day. The patient was required to rest in bed with the affected limb elevated by 30° and the treatment also lasted for 28 d.
Observation indicators and evaluation criteria:
Clinical efficacy: The clinical efficacy was evaluated by the following criteria[15] in which cure means the skin color around the ulcer surface was close to normal or became routine after treatment, with no swelling of the affected foot and basically healed ulcer surface. Markedly effective means the swelling of the affected foot and the skin color around the ulcer surface were significantly improved after treatment, and the area of the ulcer surface was reduced by >70 %. Effective means the swelling of the affected foot and the skin color around the ulcer surface were improved after treatment, and the area of the ulcer surface was reduced by 30 %-70 %. Ineffective means the patient needed to be transferred to the foot and ankle surgery department for local skin grafting due to no alleviation in swelling nor reduction in the area of the ulcerated surface of the affected foot after treatment.
Total effective rate=((Effective+markedly effective+cure)/Total number of patients)×100
Ulcer area: Before treatment, as well as on the 7th, 14th and 28th d of treatment, the outline of the ulcer wound was delineated with a transparent film and photographed under a unified standard to calculate the ulcer area using Image-Pro Plus software.
Total healing time: The total healing time of ulcers and the healing time of superficial, grade 2, grade 3 and grade 4 ulcers of both groups were counted. After 60 d of treatment, patients with unhealed ulcers were followed up in outpatient manner until the ulcer wound healed.
Granulation growth: The formation time of fresh granulation tissue and the healing time of the ulcer wounds were recorded. Before treatment and 7 d after treatment, the coverage rate of granulation tissue (granulation tissue coverage area/wound area×100 %) was calculated by Photoshop and the thickness of granulation tissue (granulation tissue thickness-thickness before treatment) was measured by a micrometre.
Detection of inflammatory factors: Before and after treatment, 5 ml of fasting venous blood was extracted and centrifuged for 10 min at 3000 rpm with a centrifugation radius of 3 cm. The collected serum was tested for hs-CRP and IL-6 levels with an automatic biochemical analyzer.
Quality of life: Before and after 28 d of treatment, patients were assessed for their quality of life using the Diabetes-Specific Quality of Life Scale (DSQOLS)[16] from the dimensions of physiological function, psychological or spiritual, social relationship and therapeutic effect. There were 27 entries on a scale of 1 to 5, with higher scores indicating poorer quality of life.
Statistical analysis:
We used Statistical Package for the Social Sciences (SPSS) version 17.0 software to analyze the data. The x?±s was used to indicate measurement data and the comparison between two sets of measurement data was conducted by the t test. The number of patients or percentage was used to express the count data and the comparison was made by the Chi-square (χ2) test. Data at different time points between the two groups were analyzed by repeated measurement of analysis of variance and p<0.05 was considered as statistically significant.
Results and Discussion
The therapeutic efficacy of the two groups of patients was shown in Table 2. The comparison of therapeutic efficacy revealed better overall efficacy (p<0.001) and higher total effective rate in the combined group compared with the control group (p=0.001).
| Group (n=58) | Ineffective | Effective | Markedly effective | Cure | Total effective |
|---|---|---|---|---|---|
| Control | 20 (34.48) | 7 (12.07) | 11 (18.97) | 20 (34.48) | 38 (65.52) |
| Combined | 5 (8.62) | 10 (17.24) | 3 (5.17) | 40 (68.97) | 53 (91.38) |
| Z/χ2 | -3.723 | 11.473 | |||
| p | ?0.001 | 0.001 |
Table 2: Comparison of Therapeutic Efficacy Between the two Groups of Patients, n (%)
Ulcer wound repair area of the two groups of patients was shown in Table 3. The combined group had a smaller ulcer area than the control group (p<0.001). The ulcer area of both groups decreased with time (p<0.001) and there was an interaction effect between grouping and time (p=0.001).
| Time | Control group (n=58) | Combined group (n=58) | t | p |
|---|---|---|---|---|
| Before treatment | 1.38±0.42 | 1.42±0.46 | 0.489 | 0.626 |
| Treatment of 7 d | 1.09±0.30 | 0.85±0.38 | 3.775 | <0.001 |
| Treatment of 14 d | 0.76±0.28 | 0.56±0.14 | 4.866 | <0.001 |
| Treatment of 28 d | 0.54±0.11 | 0.32±0.08 | 12.32 | <0.001 |
Table 3: Statistical Analysis of Ulcer Area in the two Groups of Patients (x?±s, cm)
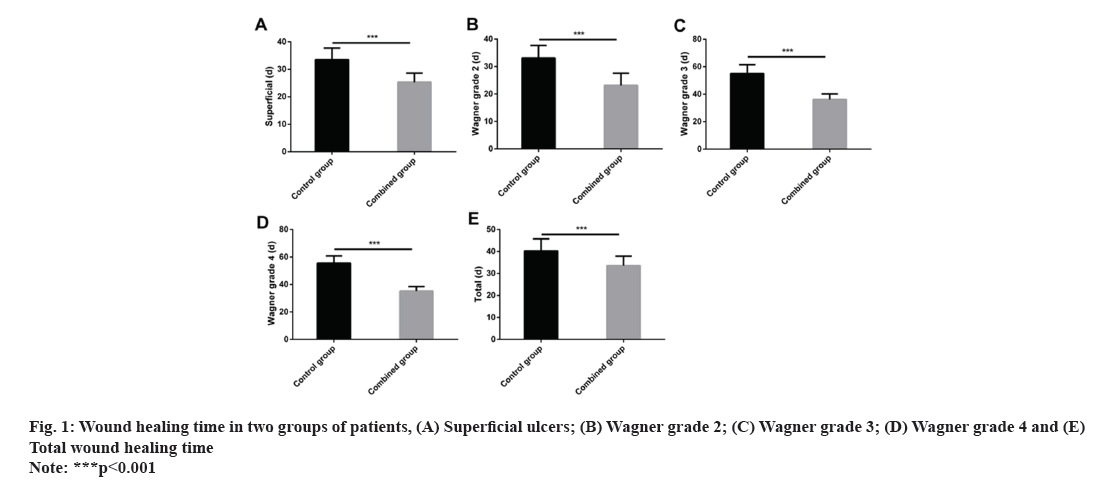
The wound healing time for ulcers of different parts or grades in the control group was follows as superficial (33.52±4.19) d, Wagner grade 2 (33.15±4.53) d, Wagner grade 3 (55.12±6.38) d, Wagner grade 4 (55.63±5.21) d and total wound healing time (40.26±5.52) d. The wound healing time for ulcers of different parts or grades in the combined group was follows as superficial (25.31±3.32) d, Wagner grade 2 (23.18±4.41) d, Wagner grade 3 (36.18±4.06) d, Wagner grade 4 (35.28±3.31) d and total wound healing time (33.61±4.29) d. The overall healing time and the healing time of superficial, Wagner grade 2, Wagner grade 3 and Wagner grade 4 ulcers were significantly lower in the combined group compared with the control group (p<0.001) (fig. 1).
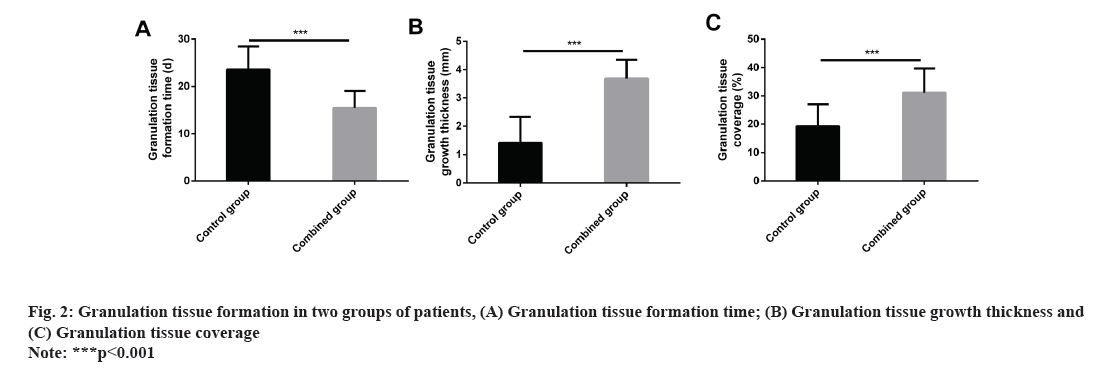
The granulation tissue formation time, granulation tissue growth thickness, and granulation tissue coverage in the control group were (23.59±4.86) d, (1.41±0.92) mm and (19.22±7.79) %, respectively, while those in the combined group were (15.48±3.55) d, (3.68±0.66) mm and (31.15±8.53) %, respectively. The above data showed that the granulation tissue formation time and granulation tissue coverage were more significantly increased in the combined group compared with the control group, while the granulation tissue growth thickness was statistically reduced (all p<0.001) (fig. 2).
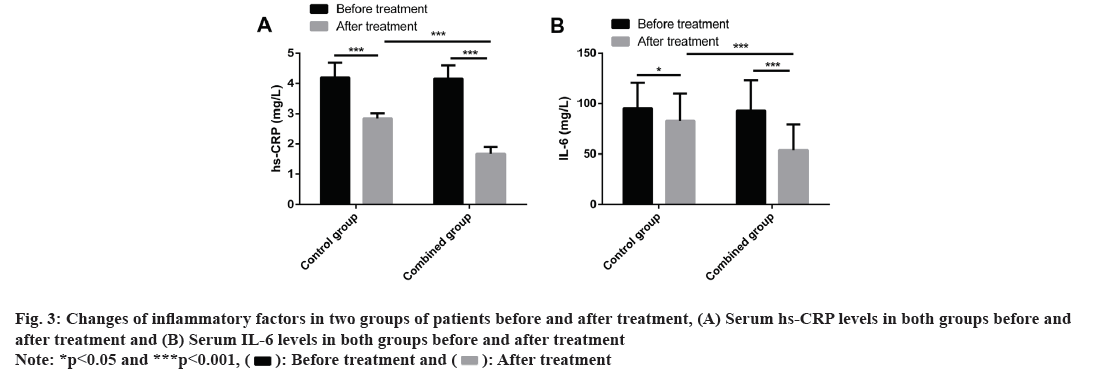
We compared inflammatory factors such as serum hs-CRP and IL-6 levels between the two groups before and after treatment. Before treatment, the two groups did not differ significantly in serum hs-CRP and IL-6 levels (p>0.05). After treatment, serum hs-CRP and IL-6 levels were significantly decreased in both groups (p<0.05) and the decrease was more significant in the combined group (p<0.001) (fig. 3).
After treatment, observing patients’ quality of life, it was found that the scores of physiological function, psychological or spiritual, social relations and treatment effects were decreased in the combined group compared with the control group (p<0.05) (Table 4).
| Factors | Treatment | Control group (n=58) | Combined group (n=58) | t | p |
|---|---|---|---|---|---|
| Physiological function | Before | 40.23±5.84 | 39.72±6.62 | 0.44 | 0.661 |
| After | 32.58±4.15* | 25.62±5.58* | 12.65 | <0.001 | |
| Psychological or spiritual | Before | 32.26±5.59 | 31.62±5.38 | 0.628 | 0.531 |
| After | 19.86±6.25* | 14.39±5.67* | 4.937 | <0.001 | |
| Social relationship | Before | 13.88±4.37 | 14.10±4.15 | 0.278 | 0.782 |
| After | 12.22±3.38* | 9.15±2.63* | 5.459 | <0.001 | |
| Therapeutic effect | Before | 12.25±2.61 | 11.76±2.74 | 0.986 | 0.326 |
| After | 8.12±2.37* | 6.14±2.26* | 4.605 | <0.001 |
Note: (*) indicates that compared with the data before treatment in the same group, p<0.05
Table 4: Statistical Analysis of Quality of Life in the two Groups of Patients (X?±S, Points)
The incidence of diabetes remains high, threatening public health. Poor blood glucose control in diabetics can lead to many complications in the long run. Diabetic foot lesions are a common condition associated with diabetes, manifested as stenosis or even complete occlusion of the distal vascular space of the affected limb due to poor blood circulation below the ankle joint and peripheral neuropathy[17]. When secondary infection of the foot and ischemic ulcers are developed, then the disease progresses to DFUs. At present, there is still insufficient understanding of the combination therapy for DFUs and inflammatory reactions in patients. Therefore, it is of clinical significance to explore a new and effective therapeutic regimen and its mechanism of action to improve the treatment outcomes of DFU patients.
The pathological mechanism of DFUs is related to impaired angiogenesis and over activation of inflammatory response, and its etiology is associated with infection, impaired immune function, fibrosis, neuropathy and ischemia, etc.[18-21]. Many scholars have conducted in-depth research on the treatment and related mechanisms of DFUs. For example, Huang et al. pointed out that hyperbaric oxygen can promote the proliferation of human skin fibroblasts and the angiogenesis of human umbilical vein endothelial cells by mediating the Hypoxia-Inducible Factor-1 alpha (HIF-1α) signaling pathway, thereby promoting wound healing in mouse models of DFUs[22]. Choudhury et al. suggested that silver nanoparticles, as a dressing treatment option, could activate the body’s cellular mechanisms and promote chronic wound healing through anti-inflammatory and antibacterial activities, but their toxicity is of concern[23]. This study focused on the therapeutic effect of SIT+rb-bFGF in DFUs. SIT, which is a Dipeptidyl Peptidase 4 (DDP4) inhibitor, has similar efficacy to linagliptin in the treatment of type 2 diabetes and is superior to empagliflozin in inhibiting glycated Haemoglobin (HbA1c) levels at 24 w[24,25]. The repair mechanism of SIT in skin wound healing is related to its pharmacological inhibition of Cluster of Differentiation 26 (CD26), which has an impact on the proliferation, migration and collagen synthesis of fibroblasts in vitro, thereby reducing scarring in vivo[4]. In addition, rb-bFGF has been indicated to simplify the treatment of the most common small and medium-sized burns and scalds, showing therapeutic action from the stage of tissue necrosis directly to the stage of tissue repair and significantly shorten the course of the disease, thus effectively avoiding or reducing the scar formation and pigmentation of burns, scalds and wounds and greatly enhancing the healing effect. This study confirmed that SIT combined with rb-bFGF can improve the therapeutic effect. Further study showed that the ulcer area was smaller and the ulcer wound healing time was shorter in the combined group compared with the control group; suggesting that SIT and rb-bFGF can promote ulcer wound healing and shorten ulcer surface healing time in DFU patients. After statistical analysis of the data, SIT was found to exert potent effects on facilitating the healing of Wagner grade 3 and grade 4 ulcers, and had similar efficacy in treating Wagner grade 2 and superficial ulcers. Along with that SIT+rb-bFGF dressing change significantly promoted overall ulcer healing compared with conventional dressing.
We also found that the application of SIT+rb-bFGF contributed to shorter granulation tissue formation time, thicker granulation tissue growth and higher granulation tissue coverage, suggesting that SIT+rb- bFGF can promote the formation and growth of granulation tissue in the treatment of DFUs. Granulation tissue hyperplasia is a critical stage in repairing and healing skin defects. Fibroblasts and capillaries proliferate in the defect wound and form a large amount of granulation tissue, which fills the defect and provides an excellent vascular bed for the growth of new epithelium of the surrounding skin to the center of the wound. In our study, hs-CRP and IL-6 levels were significantly reduced after the combination treatment. Rb-bFGF has multiple functions and can accelerate the repair and healing of skin defects to various degrees. There will be inflammatory responses in the body in the early stage of skin defects, while bFGF has obvious activity on inflammatory cells, which can stimulate fibroblasts, vascular endothelial cells and other cells to move to the wound surface[26,27], and promote wound healing by promoting granulation tissue hyperplasia.
DFU patients usually present with local skin itching below the ankle joint, cold foot of the affected limb and skin numbness symptoms in the early stage. While the disease progresses, persistent numbness, hypoalgesia and sensory disappearance of the affected foot was observed. Few patients feel different degrees of pain in the affected foot and the symptoms were aggravated by heat at night. Unsatisfactory early disease control may result in adverse events such as foot ulceration, infection, pus, wound non-healing and even amputation occurs. DFUs can effects patients' quality of daily life[28]. This study revealed lower scores of all dimensions of the factors that affect quality of life in the combined group compared with control group, indicating that SIT+rb-bFGF dressing change significantly improved patients' quality of life. The rapid repair effect of SIT+rb-bFGF on ulcer wounds enhanced patients' quality of life. However, due to the small number of patients included in this study and the failure to perform therapeutic classification statistics according to the diabetic foot classification, the sample size needs to be expanded for further study.
In summary, SIT combined with rb-bFGF has a significant clinical effect in the treatment of chronic DFUs without adverse reactions, which can significantly reduce inflammatory responses in patients and improve their quality of life.
Conflict of interests:
The authors declared no conflict of interests.
References
- Sharma R, Sharma SK, Mudgal SK, Jelly P, Thakur K. Efficacy of hyperbaric oxygen therapy for diabetic foot ulcer, a systematic review and meta-analysis of controlled clinical trials. Sci Rep 2021;11(1):1-12.
[Crossref] [Google scholar] [PubMed]
- Boyko EJ, Zelnick LR, Braffett BH, Pop-Busui R, Cowie CC, Lorenzi GM, et al. Risk of foot ulcer and lower-extremity amputation among participants in the diabetes control and complications trial/Epidemiology of diabetes interventions and complications study. Diabetes Care 2022;45(2):357-64.
[Crossref] [Google scholar] [PubMed]
- Rinkel WD, Fakkel TM, Cabezas MC, Birnie E, Coert JH. (Cost-) effectiveness of lower extremity nerve decompression surgery in subjects with diabetes: The DeCompression (DECO) trial-study protocol for a randomised controlled trial. BMJ Open 2020;10(4):1-10.
[Crossref] [Google scholar] [PubMed]
- Jiang Y, Yao Y, Li J, Wang Y, Cheng J, Zhu Y. Functional dissection of CD26 and its pharmacological inhibition by sitagliptin during skin wound healing. Med Sci Monit 2021;27:1-10.
[Crossref] [Google scholar] [PubMed]
- Grouzmann E, Bigliardi P, Appenzeller M, Pannatier A, Buclin T. Substance P-induced skin inflammation is not modulated by a single dose of sitagliptin in human volunteers. Biol Chem 2011;392(3):217-21.
[Crossref] [Google scholar] [PubMed]
- Shih CM, Huang CY, Huang CY, Wang KH, Wei PL, Chang YJ, et al. A dipeptidyl peptidase-4 inhibitor promotes wound healing in normoglycemic mice by modulating keratinocyte activity. Exp Dermatol 2018;27(10):1134-41.
[Crossref] [Google scholar] [PubMed]
- Zeng J, Li Z, Lin F, Fu S, Li J, Zhai Y. Hydrogel plus growth factors treatment after 2940 nm erbium: YAG lattice laser improves periorbital wrinkles and wound healing: A case report. J Int Med Res 2021;49(9):1-5.
[Crossref] [Google scholar] [PubMed]
- Wang Y, Lin S, Chen Z, Chen Q, Fu M. Effects of infrared combined with methylcobalamin on the vibratory sensory threshold and nerve conduction velocity of the lower extremity in patients with diabetic foot treatment. Dis Markers 2022;2022:1-5.
[Crossref] [Google scholar] [PubMed]
- Li Z, Yuan J, Luo Y, Chen Z, Mo C. Analysis of serum hepcidin levels and related factors in patients with diabetic lower extremity vascular disease and foot ulcer. J Chin Physician 2021;23(5):674-8.
- Xie P, Deng B, Zhang X, Li Y, Du C, Rui S, et al. Time in range in relation to amputation and all-cause mortality in hospitalized patients with diabetic foot ulcers. Diabetes Metab Res Rev 2022;38(2):1-10.
[Crossref] [Google scholar] [PubMed]
- Tuttolomondo A, La Placa S, di Raimondo D, Bellia C, Caruso A, Lo Sasso B, et al. Adiponectin, resistin and IL-6 plasma levels in subjects with diabetic foot and possible correlations with clinical variables and cardiovascular co-morbidity. Cardiovasc Diabetol 2010;9:1-7.
[Crossref] [Google scholar] [PubMed]
- Zhong HG, China Clinical Guidelines for the Prevention and Treatment of Elderly Diabetes' writing group. Chinese Clinical Guidelines for the Prevention and Treatment of Type 2 Diabetes in the Elderly (2022 Ed). Chin J Diabetes 2022;30(1):2-51.
- Zhong G, China Health Care International Exchange Promotion Association Diabetic Foot Disease Branch. Chinese guidelines for diagnosis and treatment of diabetic foot. Natl Med J China 2017;97(4):251-8.
- Shah P, Inturi R, Anne D, Jadhav D, Viswambharan V, Khadilkar R, et al. Wagner's classification as a tool for treating diabetic foot ulcers: Our observations at a suburban teaching hospital. Cureus 2022;14(1):1-11.
[Crossref] [Google scholar] [PubMed]
- Ma YY, Zhao G. Clinical observation on treatment of diabetic foot (Damp-heat poisonous type) by external application of QuanXie ointment. Chin J Surg Integr Tradit West Med 2020;26(2):296-9.
- Wu YY, Cheng LY, Guo HJ, Xu JH, Min J. Mapping diabetes specific quality of life scale to EQ-5 D utility scale. Chin J Health Stat 2022;39(4):499-503.
- Ndong A, Konta B, Tendeng JN, Dia DG, Dia AD, Diao ML, et al. Factors associated with foot lesions in diabetic patients at Saint-Louis Hospital (Senegal): A case-control study protocol. Int J Surg Protoc 2021;25(1):16-20.
[Crossref] [Google scholar] [PubMed]
- Zhu L, Qian J, Jiang Y, Yang T, Duan Q, Xiao X. PlGF reduction compromises angiogenesis in diabetic foot disease through macrophages. Front Immunol 2021;12:1-10.
[Crossref] [Google scholar] [PubMed]
- Ramirez HA, Pastar I, Jozic I, Stojadinovic O, Stone RC, Ojeh N, et al. Staphylococcus aureus triggers induction of miR-15B-5P to diminish DNA repair and deregulate inflammatory response in diabetic foot ulcers. J Invest Dermatol 2018;138(5):1187-96.
[Crossref] [Google scholar] [PubMed]
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med 2014;6(265):1-16.
[Crossref] [Google scholar] [PubMed]
- Sawaya AP, Stone RC, Brooks SR, Pastar I, Jozic I, Hasneen K, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun 2020;11(1):1-14.
[Crossref] [Google scholar] [PubMed]
- Huang X, Liang P, Jiang B, Zhang P, Yu W, Duan M, et al. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci 2020;259:1-11.
[Crossref] [Google scholar] [PubMed]
- Choudhury H, Pandey M, Lim YQ, Low CY, Lee CT, Marilyn TC, et al. Silver nanoparticles: Advanced and promising technology in diabetic wound therapy. Mater Sci Eng C Mater Biol Appl 2020;112:1-16.
[Crossref] [Google scholar] [PubMed]
- Keshavarz K, Lotfi F, Sanati E, Salesi M, Hashemi-Meshkini A, Jafari M, et al. Linagliptin vs. sitagliptin in patients with type 2 diabetes mellitus: A network meta-analysis of randomized clinical trials. Daru 2017;25(1):1-11.
[Crossref] [Google scholar] [PubMed]
- Kitazawa M, Katagiri T, Suzuki H, Matsunaga S, H Yamada M, Ikarashi T, et al. A 52?week randomized controlled trial of ipragliflozin or sitagliptin in type 2 diabetes combined with metformin: The N?ISM study. Diabetes Obes Metab 2021;23(3):811-21.
[Crossref] [Google scholar] [PubMed]
- Zhang X, Wu Y. Acupoint massage plus recombinant bovine basic fibroblast growth factor ophthalmic gel and limbal stem cell transplantation on visual quality, corneal refraction, and aesthetic outcome in patients with pterygium. Evid Based Complement Alternat Med 2022;2022:1-5.
[Crossref] [Google scholar] [PubMed]
- Luo Y, Luan XL, Sun YJ, Zhang L, Zhang JH. Effect of recombinant bovine basic fibroblast growth factor gel on repair of rosacea skin lesions: A randomized, single?blind and vehicle?controlled study. Exp Ther Med 2019;17(4):2725-33.
[Crossref] [Google scholar] [PubMed]
- Mader JK, Haas W, Aberer F, Boulgaropoulos B, Baumann P, Pandis M, et al. Patients with healed diabetic foot ulcer represent a cohort at highest risk for future fatal events. Sci Rep 2019;9(1):1-6.
[Crossref] [Google scholar] [PubMed]